Abstract
A facile, mild and low-cost approach of the fabrication of self-cleaning cotton textile, which showed excellent superhydrophobic and photocatalytic properties, was proposed. The surface was firstly coated with pure anatase TiO2 through a sol–gel method catalyzed by glacial acetic acid and then modified by (heptadecafluoro-1,1,2,2-tetrahydrodecyl) triethoxysilane (F-17). The hydrophilic cotton textile turned superhydrophobic with a water contact angle of 160.0°. The wettability, surface morphology and chemical composition of pristine and modified textile were investigated, respectively. Meanwhile, the robustness of chemical solutions, laundering treatment and water pressure of the textile were all verified by exposing to different pH solutions, organic solvents, washing and immersing. Importantly, the textile surface showed outstanding self-cleaning performance toward solid pollutant, daily liquids, oil and even organic contaminant, attributing to the synergetic function of superhydrophobicity and photocatalysis of TiO2 nanoparticles. Therefore, the self-cleaning textile is an expected material for daily and industrial applications in diverse conditions, even harsh environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Self-cleaning surfaces have triggered enormous attention around the globe for the diversity of potential application fields [1, 2]. Currently, there are two mechanisms to create the self-cleaning surfaces: One is superhydrophobicity and another is photocatalysis [3].
Superhydrophobic surfaces have the compelling competence of repelling water, which requires the WCA > 150° by definition [4, 5]. In this way, the attached stain on surface can be washed away immediately and efficiently by flowing water. Two crucial factors of developing the superhydrophobic property are extremely low surface energy and specific surface structures, especially hierarchical textures [6, 7]. Many strategies, including sputter coating [8], plasma treatment [9], chemical vapor deposition [10, 11] and sol–gel route [12, 13], have been adopted. Recently, one of the popular research fields is superhydrophobic textile, demonstrating promising applications in water proofing [14], corrosive resistance [15, 16], flame prevention [17] and self-cleaning [18]. Unfortunately, this type of surfaces would be immediately stained by oil as well as organic contaminants, resulting in the reduction or even loss of superhydrophobicity [19, 20].
Another self-cleaning surface is produced by photocatalytic coating which can chemically degrade organic stain to CO2 and H2O under light exposure [21]. Photocatalysis is considered as a clean process, because the degradation process can be accomplished without high temperature, intensive energy and waste residue [22, 23]. TiO2 has many superiorities compared with other nanoparticles as photocatalysts, such as higher photocatalytic efficiency, chemical stability, availability and competitive cost [24]. The preferable catalytic activity of TiO2 nanoparticle has been achieved with anatase phase [25]. It is fascinating if a material with both superhydrophobicity and photocatalysis is developed, for it can not only repel water, but also decompose organic stains simultaneously [26]. However, to develop the functional materials with both superhydrophobic and photocatalystic properties is still a challenge for the surface that would either lose its superhydrophobicity under light irradiation or simply show photocatalysis on superhydrophilic surface [27].
The sol–gel method is frequently used to build desired surface roughness [28] and can readily applied on various substrates including steel, wood, sponges, filter paper, glass and fabric, at relatively low or even room temperature [29]. During TiO2 sols preparation, toxic and corrosive chemicals, including hydrochloric acid and nitric acid, are generally employed as catalysts [30, 31]. These chemicals would cause environmental pollution and destroy the original properties of pristine textile. Moreover, the obtained TiO2 sols may be a mixture of anatase, rutile and brookite, not pure anatase [32]. Thus, the adoption of milder acid at low temperature is not merely cost-saving, but also favorable for materials such as cotton that are vulnerable to chemical or thermal condition.
Hitherto, many researchers have diligently devoted to investigate and fabricate self-cleaning surfaces. Afzal et al. [33] prepared a superhydrophobic fabric with TiO2 and fluorine-free silanes through multi-stepped deposition, showing photocatalytic property in visible light. Huang et al. [34] achieved a robust self-cleaning cotton by hierarchical TiO2 particles via a hydrothermal reaction and fluoroalkylsilane modification. Deng et al. [35] reported a facile and practical sol–gel method of forming TiO2–SiO2 @PDMS multi-functional hybrid films, which gained both superhydrophobicity and photocatalysis. Takashi et al. designed a versatile surface, possessing superhydrophobicity and photocatalytic self-cleaning ability, with polytetrafluoroethylene (PTFE) and TiO2 as photocatalyst [8]. Nevertheless, it still faces a big challenge for constructing self-cleaning surface exhibiting both superhydrophobic and photocatalytic abilities through a facile and mild process and can be available to large-scale production and application.
In this study, a facile, mild and low-cost sol–gel method to prepare superhydrophobic and photocatalytic textile was proposed. The cotton surface was firstly coated with the anatase TiO2 sols using glacial acetic acid as catalyst and then modified by (Heptadecafluoro-1,1,2,2-tetrahydrodecyl)trimethoxysilane (F-17), which could render superhydrophobicity due to its low energy hydrophobic groups (–CF2, –CF3) [36]. The chemical stability, washing durability and stain resistance of the modified textile were also measured to evaluate the qualification of employing in practical occasions, particularly harsh conditions. This study was also verified its excellent photocatalytic self-cleaning activity by the degradation of colorant oil red.
Experimental
Materials
Titanium tetraisopropoxide (TTIP, 99.9%), anhydrous ethanol (EtOH), N,N-dimethylformamide (DMF), tetrahydrofuran (THF) and acetone (CP) were obtained from Aladdin. (Heptadecafluoro-1,1,2,2-tetrahydrodecyl)trimethoxysilane (F-17) was supplied by Jinan LangHua Chemical Co., Ltd. Commercial woven cotton textile (135 × 70 inch−1, 114 g m−2) was purchased from a textile supermarket. The textile was firstly cut into 4 cm × 4 cm pieces and cleaned with anhydrous ethanol and deionized water for three times to remove possible impurities and then thoroughly dried at 80 °C in an oven before experiments. All other chemical reagents were employed as supplied.
Preparation of TiO2 sols
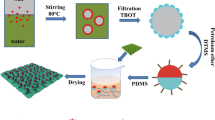
The method of generating TiO2 sols was revised moderately from the literature [37]. 2 g TTIP was added dropwise into 50 g deionized water involving 5 g glacial acetic acid with stirring to ensure sufficient reaction, and then, the mixture was kept stirring vigorously at 25 °C for 20 h. The newly prepared TiO2 solutions had rich precipitation, which turned into transparent sols after reserved without stirring at 25 °C within two weeks.
Fabrication of self-cleaning textiles
The clean cotton textiles were firstly immersed in the as-received TiO2 sols for 10 min at 25 °C and washed with deionized water for three cycles to remove superfluous TiO2 sols and then dried at 80 °C for 0.5 h. The modified textile was named as TiO2@tex. Next, the modified specimens were placed in 1, 2, 4, 6, 8, 10 wt% F-17 methanol solution (20 mL) under ultrasonic processing at 25 °C for 0.5 h. After treatment, the samples were cured at 110 °C for 0.5 h. Subsequently, the coated textiles were rinsed with absolute ethyl alcohol for three times to remove unattached F-17 and dried at 110 °C for 0.5 h. The obtained textiles were designated as TiO2@tex-F17.
Sample characterization
The crystal phase of TiO2 was revealed by X-ray diffraction (XRD, Philips X’Pert Pro MPD) with Cu-Kα radiation (0.154 nm), which conducted at 40 kV and 40 mA. The geometric morphologies of textile surfaces were obtained by scanning electron microscopy (SEM, Hitachi S-4800). Element information of surface was determined by an energy-dispersive spectroscopy (EDS, Horiba EX-250) and X-ray photoelectron spectra (XPS) using ESCALAB 250 Xi spectrometer and Al Kα X-ray radiation. The C 1 s peak (284.8 eV) was taken as reference of the binding energy. The water contact angle (WCA) of 4 μL water droplet was recorded with a contact angle meter (Shanghai Zhong Chen). Each of the WCA data were the average of five values at different positions on every specimen at room temperature and humidity.
Stability evaluation of TiO2@tex-F17
To study the chemical stability, the TiO2@tex-F17 was immersed in solutions of different pH values for 48 h. In addition, the chemical stability was further investigated by immersing in EtOH, DMF, deionized water (H2O), THF and CP under ultrasonic treatment for 2 h (25 °C, 40 Hz). After treatment, the specimens were rinsed with deionized water and dried to test the WCAs. The laundering test was performed using a revised method based on the literature [38]. The TiO2@tex-F17 was put into a homemade washing device containing water, 0.3 wt% detergent (Blue Moon detergent, obtained from Blue Moon company in China) and several balls with a rotate speed of 350 rpm at 40 °C. Moreover, 15 min was designated as a cycle. The laundered textile was rinsed thoroughly with enough water to remove the absorbed detergent and heated at 80 °C to dry. Then, the WCAs of laundered fabric were measured to investigate the washing durability of the coated textile.
Results and discussion
Preparation of the modified textile
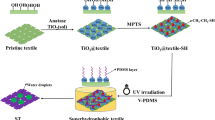
The preparation of the modified textile via a facile and mild sol–gel method is displayed in Fig. 1. TiO2 sol, which owned numerous of –Ti–OH groups, was firstly acquired by the hydrolysis and condensation reactions of TTIP. During the preparation process, high temperature, toxic and corrosive reagents were deliberately avoided. Thus, this means it could protect the safety of operators, save energy and apply in various materials, especially majority of biomaterials with relative poor thermal resistance.
Generally, TiO2 owns three crystalline phases, designated as brookite, anatase and rutile [39]. Among these phases, anatase TiO2 possesses more efficient photocatalytic activity than that of others. The XRD pattern of the acquired TiO2 was explored and is presented in Fig. 2. The peaks appeared at scattering angles of 25.3°, 37.8°, 48.0° and 54.2°, which corresponded to the (101), (004), (200) and (211) planes featured anatase TiO2 phase, respectively [40]. All diffraction peaks demonstrated the as-received TiO2 nanoparticles were pure anatase phase. Subsequently, the F-17 was coated and cured to endow the TiO2@tex with desirable superhydrophobicity via a facile and mild dip-coating method.
Figure 3 shows the influence of the F-17 concentration on the WCAs of TiO2@tex-F17. The TiO2@tex had a WCA of 0o, exhibiting hydrophilic character caused by the existence of plentiful hydroxyl groups on its surface. With the increase in F-17 concentration from 1 to 6 wt%, the WCA of TiO2@tex-F17 was enhanced sharply from 146.6° to 160.0° to generate excellent superhydrophobicity. In contrast, the WCA of the pristine textile just modified by F-17 was approximately 120°. However, for TiO2@tex-F17 modified with 10 wt% F-17, in spite of more fluorine content, the WCA was reduced to 154.2°. This result might be caused by partly destruction of the multi-scaled roughness [41]. Taking the WCA and economy into consideration, 6 wt% F-17 solution has been selected and adopted in the following test specimens. These results verified that the TiO2 nanoparticles and F-17 were both crucial for superhydrophobicity.
Surface morphology of the modified textile
To investigate the surface morphology of the original cotton, TiO2@tex and TiO2@tex-F17, the SEM images of fabric specimens are displayed in Fig. 4. The representative longitudinal fibril surface with natural furrows and veins, which furnished an inherent microscale roughness, could be evidently observed on the pristine fiber (Fig. 4a, b). After being coated by TiO2 sol, the TiO2@tex surface exhibited a compact and high-density TiO2 nanoparticle layer (Fig. 4c–e). In such a case, the roughness of the cotton surface was considerably improved. Figure 4f–h shows SEM images of TiO2@tex-F17 surface. The constructed surface preserved the original fiber morphology and formed a newly nano-scaled rough structure created by the TiO2 nanoparticles and F-17, resulting in the appropriate hierarchical roughness for preparing superhydrophobic fabric. Owing to the synergistic effect of the micro-/nano-binary surface and the materials of low surface energy, the TiO2@tex-F17 exhibited superhydrophobicity with a WCA of 160.0o. The results demonstrated that the TiO2 nanoparticle and F-17 had been successfully coated on cotton fibers, which was further verified by EDS analysis. EDS mapping images in Fig. 5a–f revealed that TiO2 and F-17 were not agglomerated, but uniformly distributed on the TiO2@tex-F17 surface. Only C and O elements were found on the pristine textile (Fig. 5g). However, the F, Si and Ti elements were also detected on TiO2@tex-F17 surface (Fig. 5h), implying the presence of the TiO2 nanoparticles and F-17 on cotton fibers.
Chemical composition of TiO2@tex-F17 surface
The chemical composition changes of cotton specimens before and after treated by TiO2 nanoparticles and F-17 were further analyzed by XPS. As shown in Fig. 6a, it was obviously observed that pure cotton was composed just by C and O elements. The F, O, Ti, C and Si elements on TiO2@tex-F17 surface were detected and assigned to five peaks at binding energies of 688.6, 532.8, 291.3, 459.3 and 102.7 eV, respectively [42, 43]. The result was in well accordance with the previous EDS results, which confirmed that TiO2 nanoparticles and F-17 had been successfully covered on fibers. According to the XPS data (Table 1), the atomic ratios of C/O/Ti/F/Si of original cotton and TiO2@tex-F17 were calculated to be 64.31:35.69:0:0:0 and 11.52:7.83:1.72:78.01:0.93:1.47, respectively. The presence of Ti, F and Si of coated textile suggested the successful coating of TiO2 nanoparticles and F-17. Figure 6b shows three peak components curve-fitted by the high-resolution C 1 s peak of pristine textile: C–C (284.7 eV), C–O–H/C–O–C (285.7 eV) and C=O (288.8 eV) [44]. Compared with the pristine textile, the TiO2@tex-F17 displayed two new peaks of C-F2 (291.3 eV) and C-F3 (293.5 eV) (Fig. 6c), indicating the existence of F-17 [45]. The narrow scan of the Ti 2p XPS spectrum is presented in Fig. 6d. Two distinct peaks of Ti 2p were shown at 459.2 and 464.9 eV, which are assigned to Ti 2p1/2 and Ti 2p3/2 peaks. Moreover, the spin–orbital gap between the two doublets was 5.7 eV, which indicated the normal state of Ti4+ [34].
Based on the above results, it could be further verified that the TiO2 nanoparticles and F-17 which provided low surface energy had indeed coated on fibers. Such combination of nano- and microscaled structure and low surface energy material was favorable to fabricate the superhydrophobic materials, and this was in well accordance with the high WCA of the TiO2@tex-F17.
Durability
Considering the complexity and variety of application situations, the chemical durability of the textile undoubtedly should be tested. Herein, the resistance of TiO2@tex-F17 to solution with different pH values was also investigated. As shown in Fig. 7a, it was obvious that the TiO2@tex-F17 retained superhydrophobicity in different pH values liquids because the WCA was all greater than 150°. The phenomenon may be attributed to the trapped air layer formed on the surface, which could prevent the harm of acid or alkali. This indicated that the TiO2@tex-F17 has excellent stability in both acidic and alkaline conditions.
Besides, the WCAs of the TiO2@tex-F17 decreased slightly after ultrasonication in condition of immersing in a variety of organic solutions, such as DMF, H2O, THF, CP and EtOH (Fig. 7b). This result suggested that the TiO2 nanocomposites and F-17 could be covalently bonded to fibers.
As shown in Fig. 7c, the laundering durability of the TiO2@tex-F17 was also evaluated. The laundering test is a comprehensive indicator of the mechanical durability of fabric. After 30 washing cycles, the WCA of TiO2@tex-F17 decreased from 160.0° to 150.1°, which still remained decent hydrophobic performance. The surface element component of TiO2@tex-F17 after various stability tests was measured by XPS (Fig. 7d). The result indicated that no obvious changes occurred on these textile samples after chemical and mechanical treatments. Furthermore, the SEM images showed the compact layer coated on TiO2@tex-F17 was still intact after immersing in acid,alkali solutions and organic solvent (Fig. 7e–g), while the surface appeared slightly damaged after laundering for 30 times (Fig. 7h). In summary, the TiO2@tex-F17 dominates superior resistance against a variety of organic solvents, acid and alkali solutions, as well as washing. The verified desirable durability would undoubtedly expand its application in various fields, even harsh conditions.
Furthermore, to evaluate the performance of bearing a high liquid pressure, TiO2@tex-F17 was forced to immerse into water. A mirror-like surface was apparently visible due to the light reflection of residual air trapped between water and fabrics (Fig. 8b). In contrast, the pristine textile started to absorb water as long as immersed in water due to its hydrophilic nature (Fig. 8a) and immediately sunk in the bottom of the beaker without additional force (Fig. 8c). More interestingly, the TiO2@tex-F17 can readily keep floating on water, even though bearing several water droplets on top (Fig. 8d). This indicated that this series of superhydrophobic materials could be employed in shipbuilding industry [46].
Self-cleaning
The superhydrophobic materials with low water adhesion generally possess the capacity of self-cleaning. On account of the unique wettability, water droplets would readily roll off the surface and passingly take away the subsistent contaminants on its surface. The surface should conform with various demands in practical application situations and provide satisfied performance in severe environments. To attest the self-cleaning performance of the TiO2@tex-F17, hydrophilic carbon powder was modeled as pollutant. As shown in Fig. 9, the water droplet hanging on the needle was capable to carry off the carbon powder through touching and moving actions. Meanwhile, the droplet was unable absorbed by surface owing to low adhesive force. Then, the pollutant readily attached on the surface of water droplet, verifying a self-cleaning ability similar to the lotus leaf.
Furthermore, a variety of domestic liquid pollutants, including tea, juice, coffee, cola, milk and even soybean oil, were chosen to further evaluate the self-cleaning performance of the TiO2@tex-F17. As shown in Fig. 10, these liquid droplets presented fairly standard spherical shape on the surface of TiO2@tex-F17, demonstrating excellent repellency and anti-fouling property against various daily liquids. It was pleased that these liquids immediately rolled off the coated surface as expected rather than adhere to it, and the surface kept clean as the original textile surface. According to above comprehensive tests, the well-pleasing anti-fouling ability of TiO2@tex-F17 was definitely confirmed. Therefore, the coating can be extensively applied in other daily application fields, such as household products and wall decoration, which expected anti-fouling ability.
The photocatalytic performance of the TiO2@tex-F17 under UV irradiation (20 mW cm−2) was also investigated. The photo-induced degradation of the oil red on cotton under UV irradiation is shown in Fig. 11, which displays the efficient photocatalytic activity of both TiO2@tex and TiO2@tex-F17. The stain of oil red on the TiO2@tex-F17 almost disappeared after UV irradiation for merely 1 h, as quickly as TiO2@tex. However, the red stain of oil red on pristine cotton and 17F@tex was still clearly visible even exposed to UV light for 5 h. Importantly, the TiO2@tex-F17 still maintained excellent photocatalytic performance even after treated with acid and basic solution, organic solvent and laundering test. As shown in Fig. 12, the stained red color in these treated samples nearly disappeared after 2 h UV irradiation, which was in accordance with the original TiO2@tex-F17 sample (Fig. 11b). Therefore, the self-cleaning performance for stain removal is comparably desirable.
All in all, the coated textile was prepared via a facile and low-cost procedure, which is comparable and even superior in superhydrophobicity, stability and self-cleaning property than previous reported research [33,34,35]. And the superhydrophobic coating has great potential in application of other substrates to fabricate novel multi-functional materials.
Conclusions
In this study, we proposed a facile, mild and low-cost sol–gel method for the preparation of superhydrophobic and photocatalytic self-cleaning cotton fabric. The surface was firstly coated by the TiO2 sols using acetic acid as catalyst and then hydrophobilized by F-17. The WCA of the coated fabric could reach as high as 160o. Furthermore, due to the excellent durability and stain resistance of TiO2@tex-F17, the resultant fabric could be employed in practical occasions, particularly harsh conditions. The as-received textile has attracting self-cleaning for superhydrophobicity and photocatalysis, which endowed the material potential application in broad filed.
References
Ahmad I, Kan CW (2016) A review on development and applications of bio-inspired superhydrophobic textiles. Materials 9(11):892
Cho EC, Chang-Jian CW, Chen HC, Chuang KS, Zheng JH, Hsiao YS, Lee KC, Huang JH (2017) Robust multifunctional superhydrophobic coatings with enhanced water/oil separation, self-cleaning, anti-corrosion, and anti-biological adhesion. Chem Eng J 314:347–357
Xu B, Ding J, Feng L, Ding Y, Ge F, Cai Z (2015) Self-cleaning cotton fabrics via combination of photocatalytic TiO2 and superhydrophobic SiO2. Surf Coat Technol 262:70–76
Golovin K, Boban M, Mabry JM, Tuteja A (2017) Designing self-healing superhydrophobic surfaces with exceptional mechanical durability. ACS Appl Mater Interfaces 9(12):11212–11223
Wang J, Han F, Liang B, Geng G (2017) Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions. J Ind Eng Chem 54:174–183
Zou HL, Lin SD, Tu YY, Liu GJ, Hu JW, Li F, Miao L, Zhang GW, Luo HS, Liu F, Hou CM, Hu ML (2013) Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J Mater Chem A 1(37):11246–11260
Zhi J-H, Zhang L-Z, Yan Y, Zhu J (2017) Mechanical durability of superhydrophobic surfaces: the role of surface modification technologies. Appl Surf Sci 392:286–296
Kamegawa T, Irikawa K, Yamashita H (2017) Multifunctional surface designed by nanocomposite coating of polytetrafluoroethylene and TiO2 photocatalyst: self-cleaning and superhydrophobicity. Sci Rep 7(1):13628
Cai L, Dai L, Yuan YH, Liu AQ, Li ZX (2016) Synthesis of novel polymethacrylates with siloxyl bridging perfluoroalkyl side-chains for hydrophobic application on cotton fabrics. Appl Surf Sci 371:453–467
Bao B, Sun J, Gao M, Zhang X, Jiang L, Song Y (2016) Patterning liquids on inkjet-imprinted surfaces with highly adhesive superhydrophobicity. Nanoscale 8(18):9556–9562
Gao XY, Guo ZG (2017) Biomimetic superhydrophobic surfaces with transition metals and their oxides: a review. J Bionic Eng 14(3):401–439
Sung JY, Ryu B-K, Lee D-H (2018) Structure and transmittance behavior of sol–gel silica nanoparticles synthesized using pH-stable alkanolamines. J Sol–Gel Sci Technol 85(2):495–503
Jiang Z, Fang S, Wang C, Wang H, Ji C (2016) Durable polyorganosiloxane superhydrophobic films with a hierarchical structure by sol–gel and heat treatment method. Appl Surf Sci 390:993–1001
Das I, De G (2015) Zirconia based superhydrophobic coatings on cotton fabrics exhibiting excellent durability for versatile use. Sci Rep 5:18503
Li X, Li Y, Guan T, Xu F, Sun J (2018) Durable, highly electrically conductive cotton fabrics with healable superamphiphobicity. ACS Appl Mater Interfaces 10(14):12042–12050
Zhang M, Wang C, Wang S, Li J (2013) Fabrication of superhydrophobic cotton textiles for water-oil separation based on drop-coating route. Carbohydr Polym 97(1):59–64
Zhang D, Williams BL, Shrestha SB, Nasir Z, Becher EM, Lofink BJ, Santos VH, Patel H, Peng X, Sun L (2017) Flame retardant and hydrophobic coatings on cotton fabrics via sol-gel and self-assembly techniques. J Colloid Interface Sci 505:892–899
Fu Y, Jin B, Zhang Q, Zhan X, Chen F (2017) pH-induced switchable superwettability of efficient antibacterial fabrics for durable selective oil/water separation. ACS Appl Mater Interfaces 9(35):30161–30170
Baba EM, Cansoy CE, Zayim EO (2016) Investigation of wettability and optical properties of superhydrophobic polystyrene-SiO2 composite surfaces. Prog Org Coat 99:378–385
Celia E, Darmanin T, Taffin de Givenchy E, Amigoni S, Guittard F (2013) Recent advances in designing superhydrophobic surfaces. J Colloid Interface Sci 402:1–18
Sidaraviciute R, Krugly E, Dabasinskaite L, Valatka E, Martuzevicius D (2017) Surface-deposited nanofibrous TiO2 for photocatalytic degradation of organic pollutants. J Sol–Gel Sci Technol 84(2):306–315
Jiang C, Liu W, Yang M, Liu C, He S, Xie Y, Wang Z (2019) Robust multifunctional superhydrophobic fabric with UV induced reversible wettability, photocatalytic self-cleaning property, and oil-water separation via thiol-ene click chemistry. Appl Surf Sci 463:34–44
Banerjee S, Dionysiou DD, Pillai SC (2015) Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl Catal B-Environ 176:396–428
Baiju KV, Shukla S, Sandhya KS, James J, Warrier KGK (2007) Photocatalytic activity of sol–gel-derived nanocrystalline titania. J Phys Chem C 111(21):7612–7622
Hu Y, Yuan C (2005) Low-temperature preparation of photocatalytic thin films from anatase sols. J Cryst Growth 274(3–4):563–568
Singh AK, Singh JK (2017) Fabrication of durable superhydrophobic coatings on cotton fabrics with photocatalytic activity by fluorine-free chemical modification for dual-functional water purification. New J Chem 41(11):4618–4628
Tung WS, Daoud WA (2009) Photocatalytic self-cleaning keratins: a feasibility study. Acta Biomater 5(1):50–56
Xue CH, Jia ST, Chen HZ, Wang M (2008) Superhydrophobic cotton fabrics prepared by sol–gel coating of TiO2 and surface hydrophobization. Sci Technol Adv Mater 9(3):035001
Petcu C, Purcar V, Ianchis R, Spataru CI, Ghiurea M, Nicolae CA, Stroescu H, Atanase LI, Frone AN, Trica B, Donescu D (2016) Synthesis and characterization of polymer-silica hybrid latexes and sol–gel-derived films. Appl Surf Sci 389:666–672
Petronella F, Truppi A, Ingrosso C, Placido T, Striccoli M, Curri ML, Agostiano A, Comparelli R (2017) Nanocomposite materials for photocatalytic degradation of pollutants. Catal Today 281:85–100
Macwan DP, Dave PN, Chaturvedi S (2011) A review on nano-TiO2 sol–gel type syntheses and its applications. J Mater Sci 46(11):3669–3686. https://doi.org/10.1007/s10853-011-5378-y
Pakdel E, Daoud WA, Wang X (2013) Self-cleaning and superhydrophilic wool by TiO2/SiO2 nanocomposite. Appl Surf Sci 275:397–402
Afzal S, Daoud WA, Langford SJ (2014) Superhydrophobic and photocatalytic self-cleaning cotton. J Mater Chem A 2(42):18005–18011
Huang JY, Li SH, Ge MZ, Wang LN, Xing TL, Chen GQ, Liu XF, Al-Deyab SS, Zhang KQ, Chen T, Lai YK (2015) Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil–water separation. J Mater Chem A 3(6):2825–2832
Deng Z-Y, Wang W, Mao L-H, Wang C-F, Chen S (2014) Versatile superhydrophobic and photocatalytic films generated from TiO2–SiO2@PDMS and their applications on fabrics. J Mater Chem A 2(12):4178–4184
Guo X, Lai C, Jiang X, Mi W, Yin Y, Li X, Shu Y (2018) Remarkably facile fabrication of extremely superhydrophobic high-energy binary composite with ultralong lifespan. Chem Eng J 335:843–854
Qi K, Xin JH (2010) Room-temperature synthesis of single-phase anatase TiO2 by aging and its self-cleaning properties. ACS Appl Mater Interfaces 2(12):3479–3485
Guo X-J, Xue C-H, Jia S-T, Ma J-Z (2017) Mechanically durable superamphiphobic surfaces via synergistic hydrophobization and fluorination. Chem Eng J 320:330–341
Junqi L, Defang W, Hui L, Zuoli H, Zhenfeng Z (2011) Synthesis of fluorinated TiO2 hollow microspheres and their photocatalytic activity under visible light. Appl Surf Sci 257(13):5879–5884
Fujishima A, Zhang XT, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep 63:515–582
Xiong D, Liu G, Duncan EJ (2012) Diblock-copolymer-coated water- and oil-repellent cotton fabrics. Langmuir 28(17):6911–6918
Chung C, Lee M, Choe E (2004) Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr Polym 58(4):417–420
Li J, Lu Y, Wu Z, Bao Y, Xiao R, Yu H, Chen Y (2016) Durable, self-cleaning and superhydrophobic bamboo timber surfaces based on TiO2 films combined with fluoroalkylsilane. Ceram Int 42(8):9621–9629
Li YW, Zheng XW, Xia ZY, Lu MG (2016) Synthesis of fluorinated block copolymer and superhydrophobic cotton fabrics preparation. Prog Org Coat 97:122–132
Shen Y, Liu S, Zhu C, Tao J, Wang G (2017) Facile fabrication of hierarchical structured superhydrophobic surface and its ultra dynamic water repellency. Chem Eng J 313:47–55
Gao S, Dong X, Huang J, Li S, Li Y, Chen Z, Lai Y (2018) Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil-water separation. Chem Eng J 333:621–629
Acknowledgements
This study was funded by the Key Laboratory of Cellulose and Lignocellulosics, Guangzhou Institute of Chemistry, Chinese Academy of Sciences and Provincial Science and technology project of Guangdong Province (No. 2015B090925019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, M., Liu, W., Jiang, C. et al. Robust fabrication of superhydrophobic and photocatalytic self-cleaning cotton textile based on TiO2 and fluoroalkylsilane. J Mater Sci 54, 2079–2092 (2019). https://doi.org/10.1007/s10853-018-3001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-3001-1