Abstract
Novel visible-light-driven WO3 nanorod arrays with oriented (200) facet were successfully synthesized via a facile solvothermal process using mixed ethanol and water as solvents. Subsequently, WS2 quantum dots (QDs) derived from bulk WS2 powder via ultrasonication were interspersed on WO3 nanorod arrays. The presence of suitable volume ratio of ethanol is a key factor to achieve greater exposure of the WO3 (200) facet. The morphologies, microstructures and optical properties of the prepared samples were systematically explored. The results illustrated that the preferential exposure on the (200) facet of WO3 nanorods as well as the efficient relative electrochemically active surface areas provided by WS2 QDs could facilitate the charge transfer between WS2 QDs and the (200) facet of WO3 nanorods. This study provides a promising approach to design high performance photoanodes for photoelectrochemical water splitting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conversion of solar energy to electric energy or other energies which can be long-term stored is an attractive and sustainable solution to the energy crisis and environmental pollution. It has been demonstrated in a number of studies that photoelectrochemical (PEC) water splitting is a viable way to convert solar energy to hydrogen fuel. Semiconductors, used as photocatalysts, provide the best known method for converting solar energy to electrical energy or chemical fuels [1]. Among various semiconductors, n-type metal oxide, such as WO3 [2], TiO2 [3] and α-Fe2O3 [4], are superior candidates for photoanodes in PEC water oxidation because of their high stability. Tungsten oxide is a visible-light-responsive photocatalyst, which exhibits a wide bandgap (2.5–2.8 eV) and the ability to absorb considerable portion (12%) of solar light to oxidize water, much higher than that of anatase TiO2 (4%) [5]. Compared with α-Fe2O3, WO3 photoanodes display a higher incident photon to current efficiency (IPCE) and provide an appropriate hole diffusion length (ca. 150 nm) while it is only 2–4 nm for α-Fe2O3 [6]. To further improve the PEC efficiency of WO3 photoanodes, several efforts have been devoted to surface modification [7, 8], morphology tuning [9, 10], transitional metal doping [11, 12] and building heterojunction structures [2, 13].
For a crystal, different facet-exposure will definitely influence the photocatalytic activity due to its unique electronic structures and surface dangling bonds [14]. So far, a few examples have been reported about the active crystal facet of WO3 arrays, and the number is even fewer on (200) facet in monoclinic WO3. Zhan and his colleagues have prepared monoclinic WO3 nanostructures with highly reactive (002) facet using boric acid as the guiding agent [15]. WO3 nanoplates were reported with IPCE as high as 67% at 350 nm, which was ascribed to the synergistic effect of the preferential exposure of (002) facet [16]. High photocurrent of 0.25 mA/cm2 at 1.23 V (vs. RHE) was achieved at an electrode based on monoclinic phase tungsten trioxide nanoplates along the (002) facet [17]. In another paper, monoclinic WO3 nanomultilayers thin film with dominant crystal facet of (002) showed a photocurrent density of ca. 0.88 mA/cm2 at 1.23 V (vs. RHE) [18]. In view of these, the (200) facet, as the main peak in monoclinic WO3 (JCPDS No. 43-1035), cannot be overlooked.
On the other hand, the introduction of smaller bandgap semiconductor quantum dots (QDs), such as WS2 [19], MoS2 [13], CdS [20], or Ag2S [21] to a wide bandgap photocatalyst, would be an effective way to restrain the recombination of photogenerated electron–hole pairs and to broaden the visible-light absorption region, leading to high photocatalytic efficiency for PEC water splitting. Significantly, tungsten disulfide and other transitional metal dichalcogenides have attracted great attention due to their physical properties [22,23,24].
In this work, WO3 nanorod arrays with oriented (200) facet were successfully synthesized via a facile solvothermal process, and then WS2 QDs prepared through ultrasonication process from bulk WS2 powder were interspersed on the WO3 nanorod arrays. The uniform morphology of WS2-QDs/WO3 nanorod arrays was confirmed, and high PEC performance was achieved at the electrodes. The optimized exposure of (200) facet and WS2 QDs loading generate 1.7 and 2.1-fold photocurrent density, respectively. This outstanding PEC performance benefits from the broadened light absorption, type II heterojunction energy band, enhanced separation and transfer efficiency of photogenerated electron–hole pairs, and increased electrochemically active surface areas.
Experimental section
Reagents
All of the reactants and solvents were of analytical grade and used without any further purification. Ammonium paratungstate (H42N10O42W12·xH2O), hydrogen peroxide (30 wt% aqueous solution), N-methyl-2-pyrrolidone (NMP) and hydrochloric acid (38%) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Tungsten disulfide powder (WS2) (< 2 μm diameter) was purchased from Aladdin (Shanghai, China). Fluorine-doped tin oxide (FTO)-coated glass (14 Ω/cm2) was obtained from Nippon Sheet Glass Company, Japan. The FTO glass was sliced to 30 mm × 10 mm per piece, followed by ultrasonic in acetone, ethanol and deionized water for 15 min, respectively. Deionized (DI) water was produced from a GWA-UN pure water system (Beijing, China).
Synthesis of WO3 arrays with different morphologies on FTO glass
0.08 mmol H42N10O42W12·xH2O was dissolved in 25 mL ethanol/water mixed solution with ethanol volume fractions of 0, 10, 30, 50%, and stirred for 30 min, then 1 mL hydrochloric acid (38%) was added, followed by stirring for 30 min. A faint yellow precursor solution was formed. After that, 0.7 mL hydrogen peroxide (30 wt% aqueous solution) was added and stirred for 30 min to obtain clear solution. The mixture was transferred into a 45 mL Teflon-lined autoclave, which contained FTO glasses against the wall and kept at 160 °C for 4 h. After the autoclave was cooled down to room temperature, the films were washed thoroughly with DI water, dried in air at 60 °C, and then calcined at 500 °C in a temperature-programmed furnace for 2 h in air. The films were stored in petri dish before being used, and the obtained samples were labeled as W-0, W-10, W-30 and W-50.
Synthesis of WS2 QDs
WS2 quantum dots were synthesized using solvent exfoliation method [25]. Typically, 100 mg WS2 powders (< 2 μm diameter) were dispersed in 100 mL NMP at a concentration of 1 mg/mL under stirring at 25 °C for 12 h. Secondly, the mixture was sonicated for several hours until the suspension solution was obtained. Afterward, the exfoliated WS2 solution was centrifuged at 10000 rpm for 20 min, and the upper one-quarter of supernatant was collected. After centrifugation for several times, yellowish transparent solution was obtained.
Synthesis of WS2 QDs/WO3 NRAs
Dipping-annealing process was used to deposit WS2 QDs onto the surface of WO3 film. The as-prepared W-30 film was vertically dipped into the above exfoliated WS2 solution overnight. Then, the sample was calcined at 200 °C in a temperature-programmed furnace for 30 min in Ar atmosphere. After being washed with ethanol to remove the impurity and dried naturally in air, the obtained sample was labeled as WS2-QDs/W-30.
Characterization of the samples
X-ray diffraction (XRD) was conducted on a Rigaku Smart Lab diffractometer configured with parallel beam optics. Scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDS) were performed with a field emission scanning electron microscope (FE-SEM, Merlin Compact, Carl Zeiss). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) images were taken with a JEM-2100f microscope. X-ray photoelectron spectroscopy (XPS) was performed on an Axis Ultra DLD X-ray photoelectron spectrometer employing a monochromated Al-Kα X-ray source (hν = 51486.6 eV). The optical properties of the samples were measured by UV-vis spectra (Hitachi U3010) and room temperature photoluminescence (PL, He-Cd laser, 325 nm).
Electrochemical measurements
All electrochemical experiments were conducted on an electrochemical workstation (CHI660C, Chenghua, Shanghai) at room temperature (25 ± 1 °C), using a three-electrode electrochemical cell in aqueous solution containing 0.50 M H2SO4 (pH = 0.31), which was degassed by bubbling pure N2 for 30 min prior to measurements. The prepared samples, a Pt plate and an Ag/AgCl electrode (3.0 M KCl) served as working electrode, counter electrode and reference electrode, respectively. The linear sweep voltammograms were typically carried out in the potential range from 0.2 to 1.8 V at a scan rate of 10 mV/s under chopped illumination of AM 1.5G solar simulator. The electrochemical impedance spectra (EIS) were conducted in a frequency range of 10−2–105 Hz at 0.8 V (vs Ag/AgCl). The Mott–Schottky plots were obtained at an AC frequency of 1 kHz.
Results and discussion
The as-synthesized WS2 QDs were characterized by TEM (Fig. 1a). The WS2 QDs display a uniform distribution with an average size of 4.6 ± 0.9 nm. The high crystallinity can be observed from the HRTEM, with clear lattice spacing of 0.206 nm, which corresponds to the (006) of 2H-WS2 (JCPDS 08-0237) [25]. Figure S1a shows the absorption spectrum of WS2 QDs, and the peak of 432 nm reflects the strong quantum confinement of ultrasmall-sized WS2. Photoluminescence properties of the prepared WS2 QDs dispersion were investigated at different excitation wavelengths from 325 to 505 nm, as shown in Fig. S1b. The emission maximum shifted from 392 to 595 nm as the excitation wavelength increased from 325 to 505 nm. This excitation wavelength-dependent PL is attributed to various defect states of WS2 QDs as well as their different sizes [26].
The magnified image of a WS2 QD was shown as an inset in Fig. 1a. The WS2 QDs exhibited a honeycomb-like atomic arrangement with a minor contrast between the W and S sites, corresponding to the atomic structure of 2H-phase WS2 [22]. All of the aforementioned results reveal that the as-synthesized WS2 QDs are ascribed to the semiconducting (2H) phases [27].
Figure 2 shows surface morphologies of all the samples (W-0, W-10, W-30, W-50 and WS2-QDs/W-30). It is observed that the volume fraction of ethanol has a great influence on the morphological characters of WO3 arrays. At the initial stage, chaotic bulks and fragmental rods were formed in aqueous solution. With the addition of ethanol, bulks within it almost disappeared while rods with various sizes were deposited (Fig. 2b). As the volume fraction increased to 30%, relatively uniform WO3 nanorods were formed and they displayed the potential to assemble into nanosheets. Ultimately, the morphology of WO3 changed to nanosheet arrays when the content of ethanol reached 50%, as shown in Fig. 2d. After introducing ethanol in the precursor solutions, the cross sections of the WO3 became orderly with a thickness of 1.4 μm (Fig. S2). Moreover, after decorating with WS2 QDs, the WS2-QDs/W-30 arrays showed similar morphology to that of the W-30 (Fig. 2e), due to the ultrasmall size of WS2 QDs. In addition, the elemental content of the WS2-QDs/W-30 was examined by EDX (Fig. S3), which clearly confirmed the existence of O, W and S elements.
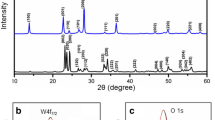
In order to investigate the influence of ethanol on the crystal structures, all samples were characterized by XRD. All the obtained samples are monoclinic WO3, which is in good agreement with JCPDS No. 43-1035. The superiority of triplet (002) (020) and (200) peaks in the patterns also presents the monoclinic phase [28]. It is noted that the intensity of these triplet peaks was changed by different volume ratio of ethanol. As the volume fraction of ethanol increased to 30%, the (002) peaks were no longer distinguishable, as shown in waterfall diagrams (Fig. 3b). Obviously, suitable volume ratio of ethanol inhibited the growth of (002) direction.
The d-spacing value (d) and crystallite size (D) for the (002), (020) and (200) plane diffraction peaks of WO3 nanocrystals were calculated by XRD line broadening of the most intense peak using Bragg’s [29] and Scherrer’s formula [30], respectively:
where κ is the shape factor, λ is the wavelength of X-ray, θ is Bragg’s angle, and β is the full width and half maxima. Here, κ and λ are taken as 0.9 and 0.15405 nm, respectively. This has been found to be in agreement with the HRTEM result (Fig. 4e) where d-spacing of the (200) lattice planes in the WO3 decreases upon the addition of ethanol, as listed in Table 1. In addition, the patterns of all samples show a relatively diminishing full width at half maximum (FWHM) with increasing ethanol content, suggesting that the average crystallite size of WO3 increased with more ethanol in the solvent [31]. The XRD patterns are consistent with the SEM images in Fig. 2.
To gain a better understanding of the distribution of WS2 QDs, STEM mapping was used to characterize the WS2-QDs/W-30 arrays, which showed that tungsten and sulfur were evenly distributed throughout the nanorods (Fig. 4a, c). The detailed microscopic information of the complicated structure of WS2-QDs/W-30 arrays was observed by HRTEM, which was crucial in understanding how the WS2 QDs were deposited, as shown in Fig. 4e. The fringe spacings are measured to be 0.365 and 0.206 nm, which corresponded to the (200) of monoclinic WO3 (JCPDS No. 43-1035) and (006) of 2H-WS2 (JCPDS 08-0237), respectively. It can be observed from Fig. S4a, for the WS2-QDs/W-30 arrays, the bare surface of WO3 appears to be covered by a large quantity of quantum dots. Simultaneously, as shown in Fig. S4b, WO3 nanorods grow preferentially along the (200) facet. This is consistent with the XRD results. Furthermore, surface chemical compositions and electronic structure were characterized by XPS, as shown in Fig. S5. Clearly, the binding energy of W 4f7/2 and W 4f5/2 for W-30 and WS2-QDs/W-30 arrays negatively shifted by 0.1 and 0.3 eV compared with the W-0, respectively. The shift of these peaks toward lower binding energy in WS2-QDs/W-30 arrays can be attributed to photoelectrons emitted from lower oxidation states of tungsten, influenced by the introduction of WS2 QDs [28, 32, 33]. Based on the above characterization results, it can be verified that WS2-QDs/W-30 arrays were successfully prepared.
The UV-vis absorption spectra (DRS) of the samples are presented in Fig. 5a. It exhibits that the absorption edge of W-0, W-10, W-30, W-50 and WS2-QDs/W-30 is 438, 448, 462, 461 and 464 nm, respectively. The inset of Fig. 5a shows that the color of WO3 arrays gradually deepens with increasing volume fraction of ethanol. (The high-resolution version digital images are shown in Fig. S6.) Tungsten trioxide is an indirect bandgap semiconductor [28]. Thus, the bandgaps of W-0, W-10, W-30 and W-50 can be calculated to be 2.83, 2.77, 2.68 and 2.69 eV, respectively. Figure S7 shows the Mott–Schottky plots of all the electrodes at frequencies of 1000 Hz. The W-30 and WS2-QDs/W-30 electrodes show smaller slopes, suggesting significantly increased donor densities based on the Mott–Schottky equation [34, 35]:
where Nd is the carrier density and C is the space charge capacitance. Then, ε, ε0 represent the dielectric constant of WO3 and permittivity of vacuum, q is the electron charge, and V is the potential applied at the electrode. Notably, all the photoelectrodes exhibit an n-type characteristic with the positive slope, which indicates that electrons served as the majority carriers [36]. Therefore, the electron densities of W-0, W-10, W-30 and W-50 WO3 are calculated to be 1.39 × 1021, 3.06 × 1021, 3.51 × 1021 and 1.42 × 1021 cm−3, respectively. The enhanced donor density for the W-30 electrode facilitates the separation of electron–hole pairs and will contribute to its higher photocurrent response.
In order to analyze the photogenerated carrier separation mechanism of WO3 synthesized with different degrees of volume fraction of ethanol in the solvents, the bandgaps (Eg), conduction band (CB) and valence band (VB) positions of W-0 and W-30 were examined. As can be seen from Fig S7, the flat band potentials of W-0 and W-30 samples were 0.163 and 0.133 V (vs. Ag/AgCl), respectively, which corresponded to 0.36 and 0.33 V (vs. NHE). This was demonstrated in a number of studies that the flat band potential is approximately equivalent to the Fermi level (Ef) of n-type semiconductors [37, 38]. It can be found from Fig. 5c that the energy gap between Ef and VB was 2.45 and 2.25 V for W-0 and W-30, respectively. Hence, the VB positions can be calculated for W-0 and W-30 to be 2.09 and 1.92 V (vs. NHE), respectively. Simultaneously, based on the Eg (2.83 eV for W-0 and 2.68 eV for W-30), the CB positions were calculated to be − 0.74 and − 0.76 eV, respectively. The energy band diagrams of the W-0 and WS2-QDs/W-30 are schematically illustrated in Fig. 5d.
To explore the PEC performance of the W-0, W-10, W-30, W-50 and WS2-QDs/W-30 arrays, all of them were measured as photoanodes in a 0.5 M H2SO4 electrolyte solution using a three-electrode electrochemical cell under AM 1.5G solar illumination at 100 mW/cm2. The W-0 film has a photocurrent density of 0.51 mA/cm2 at 1.23 V (vs. RHE). After adding ethanol in the precursor solution, the photocurrent obviously changed. As the volume fraction of ethanol increased from 10 to 30% in the solvents, the photocurrent density increased from 0.64 to 0.89 mA/cm2 at 1.23 V (vs. RHE). Then, the photocurrent decreases to 0.54 mA/cm2 when the volume fraction of ethanol is 50%. Ultimately, after decorating with WS2 QDs, the onset potential decreased, which indicates that WS2 QDs facilitated the water oxide reaction occurring at the WO3 electrolyte interface, and the photocurrent density of WS2-QDs/W-30 increased to 1.08 mA/cm2 at 1.23 V (vs. RHE). This is attributed to the enlarged light absorption range and enhanced absorption ability of WS2 QDs, as shown in Fig. 6c. The enhanced PEC property is consistent with the observation from the UV-vis results. Figure 6b shows the I-t curves of all samples at 1.23 V versus RHE under the chopped light illumination. From the beginning of irradiation to the end, the photocurrents of W-0, W-10, W-30 and W-50 decreased 32.7% (from 0.52 to 0.35 mA/cm2), 22.6% (from 0.62 to 0.48 mA/cm2), 20.9% (from 0.86 to 0.68 mA/cm2) and 26.8% (from 0.56 to 0.41 mA/cm2), respectively. The WS2-QDs/W-30 exhibited the highest photocurrent among these photoanodes under irradiation for five cycles. The photocurrent decreased 19.8% (from 1.01 to 0.81 mA/cm2), which shows the highest stability due to the efficient interfacial charge transfer induced by WS2 QDs.
To explore the photoactivity of the photoanodes under monochromatic light, the IPCE was measured at 1.23 V (vs. RHE) and the values are calculated by Equation [4]:
where Iph is the photocurrent density (mA/cm2), λ is the wavelength of the incident light (nm) and Jlight is the measured incident light power density (mW/cm2) at the wavelength λ. As shown in Fig. 6c, the IPCE curves of all samples are similar in shape to those of the absorption spectra in Fig. 5a. The magnified image in the wavelength region of 420–470 nm is shown as an inset in Fig. 6c. In other words, the addition of ethanol in precursor solution and the interspersing of WS2 QDs both affect the absorption range of visible light, leading to the remarkable photocurrent response in Fig. 6a.
To gain a clear idea of the charge-transfer process, the EIS of the electrodes were evaluated under visible light illumination, as shown in Fig. 7a. The Nyquist plots of W-30 and WS2-QDs/W-30 both show decreased EIS radiuses, indicating that the photogenerated charges could easily transfer to electrolyte. The data can be fitted to an equivalent circuit (Fig. S8) consisting of constant phase element (CPE1), solution resistance (Rs) and charge-transfer resistance (Rct). The charge-transfer resistance of W-0, W-10, W-30, W-50 and WS2-QDs/W-30 obtained from the fitting circuits are 1423, 796.5, 470.3, 884.8 and 258.0 Ω, respectively. Moreover, the lifetime of electrons for recombination with a time constant (τe) can be correlated with the frequency peak (fmax) position in the Bode plots using the formula [13]:
The corresponding τ e of W-0, W-10, W-30, W-50 and WS2-QDs/W-30 are 1.63, 1.36, 1.36, 1.63 and 2.28 ms. This indicates that electrons have longer lifetime and faster diffusion rate in WS2-QDs/W-30.
It is generally accepted that PL emission reveals the migration, transfer and recombination process of the photogenerated electrons and holes in a semiconductor [39, 40]. As shown in Fig. S9, all samples were excited by light with wavelength of 325 nm, and the spectra shown an apparent characteristic emission peak from 420 to 500 nm. The strongest peak located at 469 nm could be attributed to the radiative recombination process of self-trapped excitations [41]. Compared with W-0, others have a weaker PL intensity and the WS2-QDs/W-30 shows the lowest emission peak. It demonstrates that lower recombination probability of free charges occurs by adding ethanol in the precursor solution and interspersing of WS2 QDs, coinciding with that obtained by EIS studies.
In order to further disclose the origin of the different PEC performance of the samples, their relative electrochemically active surface areas (ECSA) were estimated using cyclic voltammetry (CV) measurements by extracting the double-layer capacitance (CDL). The CDL can be determined by measuring the non-faradaic capacitive current from the scan-rate dependence of CVs (0.35–0.45 V vs. Ag/AgCl). The non-faradaic region is typically a 0.1 V potential window which centers at open-circuit potential (OCP), and all measured current in this region is assumed to be caused by double-layer charging [42].
As shown in Eq. 6, the capacitive current (ic) is equal to the product of the scan rate (v) and the CDL. Hence, a plot of ic as a function of v led to a straight line with a slope corresponding to CDL. Finally, the ECSA can be calculated from the double-layer capacitance according to Eq. 7, where Cs is the intrinsic specific capacitance. Figure 8a and b shows the capacitive currents of the W-30 and WS2-QDs/W-30 films with different scan rates. Assuming that the Cs is the same for all photoanodes, the ECSA could be determined by the ratio of the capacitive current, which is shown in Fig. 8c. It is observed that the WS2-QDs/W-30 film has the largest ECSA, which will provide more active sites for the surface reaction, thus the higher current density [43].
Cyclic voltammograms showing the capacitive currents for the W-30 WO3 (a) and WS2-QDs/W-30 arrays (b) at five different scan rates from 0.025 to 0.3 V/s. The linear relationship between the capacitive current at 0.40 V versus Ag/AgCl and the scan rate for the as-prepared films (c). The relatively electrochemically active surface areas (using the value of W-0 film as the baseline) are compiled in the inset
Conclusions
In summary, novel visible-light-driven WO3 nanorod arrays with oriented (200) facet were successfully synthesized via a facile ethanol/water hydrothermal method by adjusting the volume ratio of ethanol. The preferential exposure of (200) facet and WS2 QDs loading generate 1.7 and 2.1-fold photocurrent density, respectively. This outstanding PEC performance benefits from the broadened light absorption, type II heterojunction energy band, enhanced separation and transfer efficiency of photogenerated electron–hole pairs, and increased electrochemically active surface areas. The finding will broaden the applications of WO3-based nanomaterials for photoelectric devices.
References
Wang Q, Hisatomi T, Jia QX, Tokudome H, Zhong M, Wang CZ et al (2016) Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat Mater 15:611–615
Zhang J, Ma HP, Liu ZF (2017) Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Appl Catal B Environ 201:84–91
Wen W, Yao JC, Gu YJ, Sun TL, Tian H, Zhou QL et al (2017) Balsam-pear-like rutile/anatase core/shell titania nanorod arrays for photoelectrochemical water splitting. Nanotechnology 28:465602–465610
Liu Y, Su FY, Yu YX, Zhang WD (2016) Nano g-C3N4 modified Ti-Fe2O3 vertically arrays for efficient photoelectrochemical generation of hydrogen under visible light. Int J Hydrog Eng 41:7270–7279
Prevot MS, Sivula K (2013) Photoelectrochemical tandem cells for solar water splitting. J Phys Chem C 117:17879–17893
Hilliard S, Baldinozzi G, Friedrich D, Kressman S, Strub H, Artero V et al (2017) Mesoporous thin film WO3 photoanode for photoelectrochemical water splitting: a sol–gel dip coating approach. Sustain Ener Fuel 1:145–153
Ho GW, Chua KJ, Siow DR (2012) Metal loaded WO3 particles for comparative studies of photocatalysis and electrolysis solar hydrogen production. Chem Eng J 181:661–666
Hu DY, Diao P, Xu D, Wu QY (2016) Gold/WO3 nanocomposite photoanodes for plasmonic solar water splitting. Nano Res 9:1735–1751
Zheng F, Man WK, Guo M, Zhang M, Zhen Q (2015) Effects of morphology, size and crystallinity on the electrochromic properties of nanostructured WO3 films. CrystEngComm 17:5440–5450
Fernandez-Dornene RM, Sanchez-Tovar R, Segura-Sanchis E, Garcia-Anton J (2016) Novel tree-like WO3 nanoplatelets with very high surface area synthesized by anodization under controlled hydrodynamic conditions. Chem Eng J 286:59–67
Xiao YH, Xu CQ, Zhang WD (2017) Facile synthesis of Ni-doped WO3 nanoplate arrays for effective photoelectrochemical water splitting. J Solid State Electrochem 21:3355–3364
Kalanur SS, Yoo IH, Seo H (2017) Fundamental investigation of Ti doped WO3 photoanode and their influence on photoelectrochemical water splitting activity. Electrochim Acta 254:348–357
Xiao YH, Zhang WD (2017) MoS2 quantum dots interspersed WO3 nanoplatelet arrays with enhanced photoelectrochemical activity. Electrochim Acta 252:416–423
Ding J, Zhang L, Liu QQ, Dai WL, Guan GF (2017) Synergistic effects of electronic structure of WO3 nanorods with the dominant 001 exposed facets combined with silver size-dependent on the visible-light photocatalytic activity. Appl Catal B Environ 203:335–342
Zhan FQ, Liu WH, Li WZ, Li J, Yang YH, Liu Q et al (2017) Boric acid assisted synthesis of WO3 nanostructures with highly reactive (002) facet and enhanced photoelectrocatalytic activity. J Mater Sci Mater 28:13836–13845
Wang SC, Chen HJ, Gao GP, Butburee T, Lyu MQ, Thaweesak S et al (2016) Synergistic crystal facet engineering and structural control of WO3 films exhibiting unprecedented photoelectrochemical performance. Nano Energy 24:94–102
Zheng JY, Song G, Hong JS, Van TK, Pawar AU, Kim DY et al (2014) Facile fabrication of WO3 nanoplates thin films with dominant crystal facet of (002) for water splitting. Cryst Growth Des 14:6057–6066
Zhang JJ, Zhang P, Wang T, Gong JL (2015) Monoclinic WO3 nanomultilayers with preferentially exposed (002) facets for photoelectrochemical water splitting. Nano Energy 11:189–195
Ghorai A, Bayan S, Gogurla N, Midya A, Ray SK (2017) Highly luminescent WS2 quantum dots/ZnO heterojunctions for light emitting devices. Acs Appl Mater Inter 9:558–565
Liu ZF, Wu JY, Zhang L (2016) Quantum dots and plasmonic Ag decorated WO3 nanorod photoanodes with enhanced photoelectrochemical performances. Int J Hydrog Energy 41:20529–20535
Tubtimtae A, Cheng KY, Lee MW (2014) Ag2S quantum dot-sensitized WO3 photoelectrodes for solar cells. J Solid State Electrochem 18:1627–1633
Kim MJ, Jeon SJ, Kang TW, Ju JM, Yim D, Kim HI et al (2017) 2H-WS2 Quantum dots produced by modulating the dimension and phase of 1T-nanosheets for antibody-free optical sensing of neurotransmitters. Acs Appl Mater Inter 9:12316–12323
Zhou LY, Yan SC, Wu H, Song HZ, Shi Y (2017) Facile sonication synthesis of WS2 quantum dots for photoelectrochemical performance. Catalysts 7:18–26
Ahn SH, Manthiram A (2016) Edge-oriented tungsten disulfide catalyst produced from mesoporous WO3 for highly efficient dye-sensitized solar cells. Adv Energy Mater 6:1501814–1501820
Xu SJ, Li D, Wu PY (2015) One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv Funct Mater 25:1127–1136
Valappil MO, Anil A, Shaijumon M, Pillai VK, Alwarappan S (2017) A single-step electrochemical synthesis of luminescent WS2 quantum dots. Chem Eur J 23:9144–9148
Long H, Tao LL, Chiu CP, Tang CY, Fung KH, Chai Y et al (2016) The WS2 quantum dot: preparation, characterization and its optical limiting effect in polymethylmethacrylate. Nanotechnology 27:414005–414011
Zeng QY, Li JH, Bai J, Li XJ, Xia LG, Zhou BX (2017) Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance. Appl Catal B Environ 202:388–396
Zhang WY, Zhao JG, Liu ZZ, Liu ZJ, Fu ZX (2010) Influence of growth temperature of TiO2 buffer on structure and PL properties of ZnO films. Appl Surf Sci 256:4423–4425
Xiao YH, Pan ZC, Tian XL, Zhang HC, Zeng XF, Xiao CM et al (2014) Time controlled synthesis of urchin-like zinc oxide and characterization of its optical properties. Mater Lett 131:94–96
Upadhyay SB, Mishra RK, Sahay PP (2016) Cr-doped WO3 nanosheets: structural, optical and formaldehyde sensing properties. Ceram Int 42:15301–15310
Wang YR, Liu B, Xiao SH, Wang XH, Sun LM, Li H et al (2016) Low-temperature H2S detection with hierarchical Cr-doped WO3 microspheres. Acs Appl Mater Inter 8:9674–9683
Li Y, Wang C, Zheng H, Wan F, Yu F, Zhang X et al (2017) Surface oxygen vacancies on WO3 contributed to enhanced photothermo-synergistic effect. Appl Surf Sci 391:654–661
Xu YF, Rao HS, Chen BX, Lin Y, Chen HY, Kuang DB et al (2015) Achieving highly efficient photoelectrochemical water oxidation with a TiCl4 treated 3D antimony-doped SnO2 macropore/branched alpha-Fe2O3 nanorod heterojunction photoanode. Adv Sci 2:150049–150057
Lee MG, Kim DH, Sohn W, Moon CW, Park H, Lee S et al (2016) Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation. Nano Energy 28:250–260
Yang L, Xiong YL, Guo WL, Guo JN, Gao D, Zhang YH et al (2017) Mo6+ doped BiVO4 with improved charge separation and oxidation kinetics for photoelectrochemical water splitting. Electrochim Acta 256:268–277
Hong SJ, Lee S, Jang JS, Lee JS (2011) Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ Sci 4:1781–1787
Gao EP, Wang WZ, Shang M, Xu JH (2011) Synthesis and enhanced photocatalytic performance of graphene-Bi2WO6 composite. Phys Chem Chem Phys 13:2887–2893
Wei YJ, Chang XX, Wang T, Li CC, Gong JL (2017) A low-cost NiO hole transfer layer for ohmic back contact to Cu2O for photoelectrochemical water splitting. Small 13:1702007–1702013
Wang H, Qiu XQ, Liu WF, Yang DJ (2017) Facile preparation of well-combined lignin-based carbon/ZnO hybrid composite with excellent photocatalytic activity. Appl Surf Sci 426:206–216
Zhu ZF, Yan Y, Li JQ (2016) One-step synthesis of flower-like WO3/Bi2WO6 heterojunction with enhanced visible light photocatalytic activity. J Mater Sci 51:2112–2120. https://doi.org/10.1007/s10853-015-9521-z
McCrory CCL, Jung S, Ferrer IM, Chatman SM, Peters JC, Jaramillo TF (2015) Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc 137:4347–4357
Feng XY, Chen YB, Qin ZX, Wang ML, Guo LJ (2016) Facile fabrication of sandwich structured WO3 nanoplate arrays for efficient photoelectrochemical water splitting. Acs Appl Mater Inter 8:18089–18096
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21773074) and Guangdong Natural Science Foundation (2014A030311039).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiao, YH., Yu, YX. & Zhang, WD. Composite structures for enhanced photoelectrochemical activity: WS2 quantum dots with oriented WO3 arrays. J Mater Sci 53, 10338–10350 (2018). https://doi.org/10.1007/s10853-018-2303-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2303-7