Abstract
In this work, a skutterudite-based compound, Yb0.25Co4Sb12, added with Al x (x = 0, 0.1, 0.2, 0.3) was synthesized with a simple mechanical alloying technique followed by spark plasma sintering. The microstructural properties and thermoelectric properties of the as-sintered samples were investigated. The Al atoms formed AlSb nano-inclusions in the grain boundaries instead of entering the Sb-icosahedral voids, introducing point defects in the matrix lattice. By scattering low-energy electrons, the grain boundaries acted as a potential barrier in simultaneously attaining low electrical resistivity and high Seebeck coefficient. Therefore, Al0.1Yb0.25Co4Sb12 exhibited a high power factor of 4.8 × 10−3 W/m K2 at 377 °C. AlSb of nanometer length enhanced interfacial phonon scattering, thereby significantly reducing the lattice thermal conductivity of Al0.3Yb0.25Co4Sb12 to 0.6 W/m K at 500 K. The Al0.3Yb0.25Co4Sb12 composite exhibited the highest figure of merit, ZT = 1.36, at 850 K.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermoelectric materials have attracted extensive research attention because of their environment-friendly applications in waste heat recovery with the Seebeck effect [1,2,3,4,5] and refrigeration with the Peltier effect [6,7,8,9,10]. The efficiency of thermoelectric materials is characterized by the dimensionless figure of merit, ZT = α 2 T/ρК [11], where α, T, ρ, and К are the Seebeck coefficient, absolute temperature, electrical resistivity, and thermal conductivity, respectively. Developing materials with high electrical conductivity, high Seebeck coefficient, and low thermal conductivity is a significant challenge in thermoelectric research [12,13,14]. The phonon-glass-electron crystal (PGEC) concept aims to synthesize the ultimate thermoelectric material that conducts electricity similar to a crystal but insulates heat like a glass [15]. The skutterudite system is a promising material that utilizes the PGEC concept. The skutterudite system relies on crystal “cage-like” structures with loosely bonded filler atoms, mostly from alkaline earth metal and lanthanide elements, to allow simultaneous high electrical and low thermal conductivities.
Numerous filling elements have been explored to enhance the thermoelectric performance of CoSb3-based materials [14, 16,17,18,19,20,21,22,23,24,25]. Among these various fillers, Yb is most promising candidate for improving the thermoelectric properties of CoSb3 skutterudite. The Yb0.2Co4Sb12 compound prepared by rapid solidification and spark plasma sintering (SPS) exhibited a maximum ZT of 0.91 at 800 K [20]. The same composition achieved ZT = 1 at 823 K when synthesized by encapsulated melting and consolidated by hot pressing [26]. A composite containing Yb0.25Co4Sb12 and well-distributed Yb2O3 particles in the grain boundaries attained a maximum ZT of 1.3 at 850 K; the composite was synthesized by in situ reaction method and SPS [27]. A composite of n-type-filled skutterudite Yb0.26Co4Sb12 containing p-type GaSb nano-structured inclusions possessed a ZT value of 1.45 at 850 K [28]. However, Yb0.2Co4Sb12 composite with PbTe inclusions had a relatively low ZT value of 0.78 at 700 K when prepared through simple ball milling and hot pressing [29]. Consequently, introducing nano-structured inclusions into the lattice may reduce the magnitude of thermal conductivity by threefold, hence increasing ZT. However, fabricating high-efficiency skutterudite requires many processes and long production times; as such, minimizing the duration of the fabrication of skutterudite materials remains a challenge.
Selection of appropriate fabrication process and dopant plays key roles in improving the thermoelectric properties of Yb-filled Co4Sb12 skutterudite. Al was added to the ternary skutterudite system Yb0.25Co4Sb12 through ball milling followed by SPS.
Experimental
Elemental powders of Al (320 mesh, >99.99 wt%), Yb (200 mesh, >99.9 wt%), Co (22 mesh, >99.998 wt%), and Sb (200 mesh, >99.99 wt %) were blended at an atomic ratio of x:0.25:4:12 (x = 0, 0.1, 0.2, 0.3). Excess amounts of 2 wt% Sb were added to the mixtures to overcome the high volatility of Sb. The mixed powders were subjected to mechanical alloying (MA) with a planetary ball mill (GOKIN Ltd., PLANET) for 10 h. Zirconium oxide vials and balls (φ = 5 mm) were used. The ball-to-powder weight ratio was maintained at 15:1, and the rotation speed was fixed at 400 rpm. The powders were loaded and unloaded in a glove box filled with an argon atmosphere to avoid contamination and oxidization. Powders pretreated with (MA) were pressed under a vacuum atmosphere by using a SPS system.
Sintering was performed with a heating rate of 100 °C min−1 from room temperature to 600 °C. The temperature was then held at 600 °C for 10 min. The sample was left to cool naturally for 2 h. During sintering, the sample was pressed by 36 MPa via a graphite die with an inner diameter of 10 mm.
X-ray diffraction (XRD) analysis was performed to obtain structural information of the disk samples by using a Bruker AXS D8 Advance X-ray diffraction spectrometer with Cu–Kα radiation at a wavelength of 1.5406 Å. Patterns were recorded within the 2θ range of 10° and 90° at a rate of 0.0264 in step width. Imaging at high resolution and real phase structure determination were conducted by the FEI Helios 450HP Dual-Beam FIB-SEM. Seebeck coefficient and electrical resistivity were measured simultaneously on a ZEM-3 (Ulvac-Riko) apparatus. Thermal conductivity was measured on a TC-7000H (Ulvac-Riko) apparatus.
Results and discussion
Microstructure properties
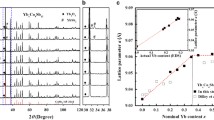
The XRD patterns of the as-sintered samples indicate that the mechanically alloyed powders were highly compacted to a composite polycrystalline skutterudite phase (Fig. 1). The main Yb0.25Co4Sb12 composite contained a small amount of Yb2O3 as a secondary phase. The Al-added Yb0.25Co4Sb12 composites contained AlSb as a secondary phase. The crystal structure was refined by JANA2006 [30], and quantitative analysis was conducted by High Score Plus software. The skutterudite phase contributed 99.6 wt% and the Yb2O3 secondary phase contributed 0.4 wt% of the Yb0.25Co4Sb12 sample. Therefore, a lattice parameter of 9.0394(1) Å was obtained. This result indicates that the Yb atoms did not fill the Co4Sb12 voids. AlSb was present in all Al-added Yb0.25Co4Sb12 ternary skutterudite systems. Additionally, the quantitative analysis of the XRD data indicated that less than 0.2 wt% of AlSb was present in all samples, and the major phase was skutterudite. Therefore, the lattice parameters of Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12 were 9.0470(1), 9.0503(2), and 9.0472(1) Å, respectively.
Figure 2a shows the SEM micrograph of the as-sintered Yb0.25Co4Sb12 sample. The elemental maps indicate that the SPS process on the mechanically alloyed powders did not introduce Yb atoms into the Co4Sb12 voids (Fig. 2b–g). As a result, Yb atoms were found in the grain boundaries beside the O atoms, forming the Yb2O3 secondary phase. The SEM micrographs of the as-sintered Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12 samples indicate two types of surface topographies (Figs. 3a, 4a, 5a), respectively. The first topography exhibits aggregated grains in the form of solid islands with very narrow grain boundaries. The second topography exhibits individual grains surrounded by relatively wide grain boundaries.
SEM/EDX verified the presence of an AlSb phase in the grain boundaries of the Al-added skutterudite samples. The microstructure and actual composition of the samples were further investigated with elemental mapping analysis. The elemental maps of the as-sintered Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12 samples clearly illustrate the distribution of the quaternary system elements (Figs. 3d–g, 4d–g, 5d–g), respectively. Yb, Co, and Sb were systemically distributed on the surfaces of the grains of the as-sintered samples; Al was less present inside the grains but was strongly present in the grain boundaries beside Sb. These results confirm that the skutterudite phase indicated by XRD belongs to the formation of a filled skutterudite phase in grains partially filled by Yb atoms. Moreover, the presence of Al beside Sb in the grain boundaries agrees with the AlSb peak indicated in the XRD graph. Inability of Al to fill the skutterudite cage can be explained by the theoretical predictions [31,32,33] that indicate that the electronegativities (X) of Sb and the filler must satisfy the relation XSb − Xfiller > 0.80. The Pauling electronegativity of aluminum is 1.61, merely 0.44 smaller than that of antimony, so aluminum should in fact not be able to fill the skutterudite voids.
However, studies of In-doped Co4Sb12 reported that In formed InSb inclusions as a secondary phase instead of entering the Sb-icosahedral voids. Furthermore, these inclusions may potentially reduce lattice thermal conductivity without significantly decreasing the power factor [34]. The In0.4Co4Sb12 composite prepared by Mallik et al. [35] showed a low thermal conductivity of 1.9 W/m K, most likely because a small amount of InSb secondary phase was finely dispersed at the grain boundaries. Thus, regardless of the non-single-phase morphology, a ZT value of 0.96 at 673 K was obtained.
Thermoelectric properties
Figure 6 shows that the electrical resistivity of the Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) samples is temperature dependent. The Yb0.25Co4Sb12 sample had a very high electrical resistivity. However, the Al-added samples demonstrated very low electrical resistivity values over the entire temperature range compared with Yb0.25Co4Sb12. Al-added samples also had very low electrical resistivity values compared with the single-phase Yb0.2Co4Sb12 reported in [20], which had a resistivity of 11.4 µΩm at room temperature. The electrical resistivity of the Yb0.25Co4Sb12 sample decreased as temperature increased, indicating semiconducting behavior. Conversely, the electrical resistivity of the Al-added compositions increased as temperature increased, indicating metal-like behavior over the entire temperature range. This behavior is similar to the behavior of InxYb0.2Co4Sb12 compositions discussed by Peng et al. [36]. The electrical resistivity range was very narrow for all Al-added samples, i.e., minimum electrical resistivity values of 5.5, 5.3, and 7.6 µΩm at room temperature and maximum electrical resistivity values of 10.9, 9.3, and 10.5 µΩm at 577 °C were recorded for Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12, respectively. Therefore, the average value of electrical resistivity decreased with increasing amounts of AlSb nano-inclusions in the composite. Referring back to the XRD patterns, the Al0.2Yb0.25Co4Sb12 composite had the maximum AlSb content and the minimum electrical resistivity among all composites. This correlation disagrees with previous observations of YbxCo4Sb12/yGaSb [28] and Yb0.2Co4Sb12/xPbTe composites [29], in which nano-inclusions increased electrical resistivity.
Figure 7 shows the temperature dependence of the Seebeck coefficients for Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) samples. The Yb0.25Co4Sb12 sample showed p-type behavior. This behavior resulted from an insufficient number of cations in the Co4Sb12 skutterudite voids. Meanwhile, Al-added samples demonstrated n-type behavior. However, the absolute values of the Seebeck coefficient for all samples showed insignificant differences. The Yb0.25Co4Sb12 sample had a high Seebeck coefficient of 211 µV/K at 127 °C. Subsequently, Seebeck coefficients of 217, 207, and 212 µV/K were obtained for Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12 at 577 °C, respectively. The Seebeck coefficient is strongly associated with the mean carrier energy near the Fermi level [29]. Therefore, high Seebeck coefficients are speculated for all Al-added compositions despite their low electrical resistivity values. High Seebeck coefficients may be attributed to the scattering of low-energy electrons by the grain boundaries potential barrier, the dominant scattering mechanism. Martin et al. [37] proposed that carrier trapping in grain boundaries forms energy barriers that impede the conduction of carriers between grains, essentially filtering charge carriers with energy lower than the barrier height.
Figure 8 illustrates the temperature dependence of the power factors of the Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) samples. The Yb0.25Co4Sb12 sample had a relatively high Seebeck coefficient; however, it had a very low power factor of 0.44 × 10−3 W/m K2 at 277 °C as a result of high electrical resistivity. Conversely, the Al-added samples had a very high and stable operating range of power factors. Power factors of 4.9 × 10−3, 4.7 × 10−3, and 4.3 × 10−3 W/m K2, at 377, 327, and 577 °C were obtained for Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12 samples, respectively.
Figure 9 shows the temperature dependence of the thermal conductivity (К) values of Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) samples. The minimum К for all samples was recorded at room temperature. The temperature dependence of К for The Yb0.25Co4Sb12 indicates a relatively low thermal conductivity of 2.5 W/m K. Yb2O3 formation in the grain boundaries increased phonon scattering, hence decreasing the sample’s overall thermal conductivity. The Al0.1Yb0.25Co4Sb12 sample had a minimum К value of 3.3 W/m K. The minimum К decreased to 3.0 W/m K for the Al0.2Yb0.25Co4Sb12 sample. A significant reduction in К (1.9 W/m K) was observed in the Al0.3Yb0.25Co4Sb12, sample with high Al content. The gradual increase in the thermal conductivities as a function of temperature for the Al0.1Yb0.25Co4Sb12 and Al0.3Yb0.25Co4Sb12 samples revealed a maximum К of 4.1 and 2.7 W/m k at 850 K, respectively. The thermal conductivity of the Al0.2Yb0.25Co4Sb12 sample at low temperatures gradually increased to 500 K and rapidly increased after 500 K, resulting in a relatively high thermal conductivity at elevated temperatures.
Figure 10 shows the temperature dependence of the lattice thermal conductivity (К L ) for Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) samples. К L can be estimated by subtracting the electronic contribution of the Wiedemann–Franz law, К e = LT/ρ, from the total thermal conductivity, where L = 2.45 × 10−8 V2K2 is the Lorenz number [22]. Results show that electron thermal conductivity negligibly contributed to the total thermal conductivity of the Yb0.25Co4Sb12 composite. On the contrary, the К L of Al-added composites was significantly suppressed compared with the К L of the Yb0.25Co4Sb12 composite. Therefore, Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12 had minimum К L values of 1.9, 1.3, and 0.6 W/m K at 400, 500, and 500 K, respectively. К e contributed more than 60% of the total thermal conductivity in this system. This contribution indicates high electrical transport properties.
The low К L of the Al0.3Yb0.25Co4Sb12 sample might be a result of the interaction between the localized modes of the two different dopants with sufficiently different frequencies and the heat-carrying acoustic phonon modes, which might strongly scatter a broader spectrum of heat-carrying phonons. In general, the significant decline in thermal conductivity in the Al-added samples mainly results from the oscillation of the loose Yb cation bond. The anharmonicity of the oscillation of the guest atom can increase phonon scattering. Likewise, the significant drop in thermal conductivity might result from point defects in the lattice microstructure caused by aggregated AlSb nano-inclusions in the grain boundaries and the large area fraction of the grain boundaries [34].
Figure 11 illustrates the dimensionless figure of merit, ZT, for Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) samples. The Yb0.25Co4Sb12 composite had high electrical resistivity, high Seebeck coefficient, and relatively low lattice thermal conductivity. Therefore, the Yb0.25Co4Sb12 composite also demonstrated a low ZT = 0.09 at 600 K. The Al x Yb0.25Co4Sb12 composites containing small amounts of AlSb had low electrical resistivity, high Seebeck coefficient, and low lattice thermal conductivity. These characteristics could be attributed to the phenomenological properties of the nanometer-length AlSb scales, including enhanced interfacial phonon scattering and charge carrier filtering. Therefore, high ZTs of 0.93, 0.87, and 1.36 at different operating temperatures of 800, 850, and 850 K were obtained for Al0.1Y0.25Co4Sb12, Al0.2Y0.25Co4Sb12, and Al0.3Y0.25Co4Sb12, respectively.
Remarkably, the Al x Yb0.25Co4Sb12 composites containing AlSb nano-inclusions yielded excellent results in terms of a fast fabrication process compared with Yb0.2Co4Sb12 composites containing PbTe inclusions, which showed a lower ZT value of 0.78 at 700 K [29]. The main composite of Yb0.2Co4Sb12 was prepared with a lengthy fabrication process. The PbTe inclusions were then introduced into the grain boundaries by the simple process of ball milling and hot pressing. Ballikaya et al. [38] prepared a skutterudite sample with a long fabrication process of melting–annealing followed by SPS. Their synthesized Yb0.2Ce0.15In0.2Co4Sb12/Yb2O3/Sb composite had a high ZT of 1.43 at 800 K.
Conclusions
In this work, we demonstrated that producing the Yb0.25Co4Sb12 sample with the simple fabrication process of MA followed by SPS could not introduce Yb atoms into Co4Sb12 skutterudite voids. On the other hand, the same preparation process produced Al x Yb0.25Co4Sb12 (x = 0.1, 0.2, 0.3) composites containing small amounts of AlSb and with high thermoelectric properties resulting from the addition of Al. The highest figure of merit, ZT = 1.36, was recorded at 850 K for the Al0.3Yb0.25Co4Sb12 composite. The high ZT resulted from a significantly decreased lattice thermal conductivity К L and a moderately increased electrical conductivity with the addition of 0.3 Al. Therefore, adding Al improves overall TE properties and significantly reduces the duration of the fabrication process. Moreover, the addition of Al to the Yb0.25Co4Sb12 composite induces Yb atoms to fill the Co4Sb12 voids.
References
Chen W-H, Liao C-Y, Hung C-I, Huang W-L (2012) Experimental study on thermoelectric modules for power generation at various operating conditions. Energy 45:874–881
Gou XL, Yang SW, Xiao H, Ou Q (2013) A dynamic model for thermoelectric generator applied in waste heat recovery. Energy 52:201–209
Jang J-Y, Tsai Y-C (2013) Optimization of thermoelectric generator module spacing and spreader thickness used in a waste heat recovery system. Appl Therm Eng 51:677–689
Lesage FJ, Pagé-Potvin N (2013) Experimental analysis of peak power output of a thermoelectric liquid-to-liquid generator under an increasing electrical load resistance. Energy Convers Manag 66:98–105
Lu H, Wu T, Bai S, Xu K, Huang Y, Gao W, Yin X, Chen L (2013) Experiment on thermal uniformity and pressure drop of exhaust heat exchanger for automotive thermoelectric generator. Energy 54:372–377
Shen LM, Xiao F, Chen HX, Wang SW (2013) Investigation of a novel thermoelectric radiant air-conditioning system. Energy Build 59:123–132
Luo TT, Wang SY, Li H, Tang XF (2013) Low temperature thermoelectric properties of melt spun Bi85Sb15 alloys. Intermetallics 32:96–102
Cherkez R (2012) Theoretical studies on the efficiency of air conditioner based on permeable thermoelectric converter. Appl Therm Eng 38:7–13
Chen LG, Meng FK, Sun FR (2012) Effect of heat transfer on the performance of thermoelectric generator-driven thermoelectric refrigerator system. Cryogenics 52:58–65
Meng F, Chen L, Sun F (2011) Performance prediction and irreversibility analysis of a thermoelectric refrigerator with finned heat exchanger. Acta Phys Pol A 120:397–406
Zhu YG, Shen HL, Guan H (2012) Microwave-assisted synthesis and thermoelelectric properties of CoSb3 compounds. J Mater Sci Mater Electron 23:2210–2215
Elsheikh MH, Shnawah DA, Sabri MFM, Said SBM, Hassan MH, Bashir MBA, Mohamad M (2014) A review on thermoelectric renewable energy: principle parameters that affect their performance. Renew Sustain Energy Rev 30:337–355
Bashir MBA, Said SM, Sabri MFM, Shnawah DA, Elsheikh MH (2014) Recent advances on Mg2Si1−xSnx materials for thermoelectric generation. Renew Sustain Energy Rev 37:569–584
Wan S, Huang X, Qiu P, Bai S, Chen L (2015) The effect of short carbon fibers on the thermoelectric and mechanical properties of p-type CeFe4Sb12 skutterudite composites. Mater Design 67:379–384
Glen AS (1995) New materials and performance limits for thermoelectric cooling. CRC handbook of thermoelectrics. CRC Press, Florida
Dyck JS, Chen W, Uher C, Chen L, Tang X, Hirai T (2002) Thermoelectric properties of the n-type filled skutterudite Ba0.3Co4Sb12 doped with Ni. J Appl Phys 91:3698–3705
Song XL, Yang JY, Peng JY, Chen YH, Zhu W, Zhang TJ (2005) Thermoelectric properties of La filled skutterudite prepared by mechanical alloying and hot pressing. J Alloy Compd 399:276–279
Mallik RC, Jung JY, Das VD, Ur SC, Kim IH (2007) Thermoelectric properties of SnzCo8Sb24 skutterudites. Solid State Commun 141:233–237
Pei YZ, Bai SQ, Zhao XY, Zhang W, Chen LD (2008) Thermoelectric properties of EuyCo4Sb12 filled skutterudites. Solid State Sci 10:1422–1428
Li H, Tang X, Zhang Q (2009) Microstructure and thermoelectric properties of Yb-filled skutterudites prepared by rapid solidification. J Electron Mater 38:1224–1228
Jiang YP, Jia XP, Su TC et al (2010) Thermoelectric properties of SmxCo4Sb12 prepared by high pressure and high temperature. J Alloy Compd 493:535–538
Park K-H, Kim I-H (2010) Thermoelectric properties of Ca-filled CoSb3-based skutterudites synthesized by mechanical alloying. J Electron Mater 40:493–498
Deng L, Jia XP, Su TC, Zheng SZ, Guo X, Jie K, Ma HA (2011) The thermoelectric properties of InxCo4Sb12 alloys prepared by HPHT. Mater Lett 65:2927–2929
Mallik RC, Anbalagan R, Raut KK, Bali A, Royanian E, Bauer E, Rogl G, Rogl P (2013) Thermoelectric properties of Bi-added Co4Sb12 skutterudites. J Phys Condens Matter 25:105701
Zhang Q, Chen C, Kang Y, Li X, Zhang L, Yu D, Tian Y, Xu B (2015) Structural and thermoelectric characterizations of samarium filled CoSb3 skutterudites. Mater Lett 143:41–43
Park KH, Seo WS, Shin DK, Kim IH (2014) Thermoelectric properties of Yb-filled CoSb3 skutterudites. J Korean Phys Soc 65:491–495
Zhao XY, Shi X, Chen LD, Zhang WQ, Bai SQ, Pei YZ, Li XY, Goto T (2006) Synthesis of YbyCo4Sb12/Yb2O3 composites and their thermoelectric properties. Appl Phys Lett 89:092121
Xiong Z, Chen XH, Huang XY, Bai SQ, Chen LD (2010) High thermoelectric performance of Yb0.26Co4Sb12/yGaSb nanocomposites originating from scattering electrons of low energy. Acta Mater 58:3995–4002
Liu HQ, Zhou G, Sun Q, Gu YJ, Zhao XB (2011) Thermoelectric properties of xPbTe/Yb0.2Co4Sb12 hot-pressed samples. J Inorg Organomet Polym 21:858–861
Petricek V, Dusek M, Palatinus L (2014) Crystallographic computing system JANA2006: general features. Z Kristallogr 229:345–352
Ballikaya S, Wang G, Sun K, Uher C (2011) Thermoelectric properties of triple-filled BaxYbyInzCo4Sb12 skutterudites. J Electron Mater 40:570–576
Shi X, Zhang W, Chen LD, Yang J (2005) Filling fraction limit for intrinsic voids in crystals: doping in skutterudites. Phys Rev Lett 95:185503
Uher C (2000) Recent trends in thermoelectric materials research I. In: Tritt TM (ed) Semiconductors and semimetals. Academic Press, San Diego, pp 139–253
Graff J, Zhu S, Holgate T, Peng J, He J, Tritt TM (2011) High-temperature thermoelectric properties of Co4Sb12-based skutterudites with multiple filler atoms: Ce0.1InxYbyCo4Sb12. J Electron Mater 40:696–701
Mallik RC, Stiewe C, Karpinski G, Hassdorf R, Muller E (2009) Thermoelectric properties of Co4Sb12 skutterudite materials with partial in filling and excess in additions. J Electron Mater 38:1337–1343
Peng JY, He J, Alboni PN, Tritt TM (2009) Synthesis and thermoelectric properties of the double-filled skutterudite Yb0.2In(y)Co4Sb12. J Electron Mater 38:981–984
Martin J, Wang L, Chen L, Nolas GS (2009) Enhanced seebeck coefficient through energy-barrier scattering in PbTe nanocomposites. Phys Rev B 79:115311–115315
Ballikaya S, Uzar N, Yildirim S, Salvador JR, Uher C (2012) High thermoelectric performance of In, Yb, Ce multiple filled CoSb3 based skutterudite compounds. J Solid State Chem 193:31–35
Acknowledgements
This work was supported by UMRG (Grant Nos. RP023B-13AET and RP023C/13AET), Science Fund (Grant No. SF020-2013) and FRGS (Grant No. FP022/2014B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elsheikh, M.H., Sabri, M.F.M., Said, S.M. et al. Rapid preparation of bulk Al x Yb0.25Co4Sb12 (x = 0, 0.1, 0.2, 0.3) skutterudite thermoelectric materials with high figure of merit ZT = 1.36. J Mater Sci 52, 5324–5332 (2017). https://doi.org/10.1007/s10853-017-0772-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0772-8