Abstract
In the present work, a series of single phase Ni-doped skutterudite compounds NixCo4−xSb12 (x = 0.1, 0.2, 0.3, 0.4, 0.5) were successful synthesized by 5 min microwave heating for the first time. The resulting ingots was pulverized and sintered by spark plasma sintering to fabricate bulk samples, and their microstructure and thermoelectric properties have been investigated systematically. The results show that the matrix grain characteristics are influenced by Ni concentration and microwave irradiation. The Ni0.3Co3.7Sb12 bulk has the smallest and uniform grain size of about 1–2 µm. The maximum power factors of NixCo4−xSb12 are 2273, 2479, 2711, 2613 and 2526 µWm−1K−2 respectively. A highest ZT of 0.52 for Ni0.3Co3.7Sb12 is achieved at 773 K along with a total thermal conductivity of 3.8 Wm−1K−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thermoelectric (TE) conversion technology could directly convert waste heat into electricity based on the Seebeck effect, which can become an important part of the solution to today’s ever-increasing energy and environmental crisis [1]. The application of thermoelectric conversion technology depends on the development of thermoelectric materials which requires the optimization of thermal and electrical transportation to achieve a high thermoelectric figure of merit (ZT), defined as ZT = S2T/ρκ, where S, ρ and κ is the Seebeck coefficient, electrical conductivity and thermal conductivity, respectively. Excellent TE materials should have good electrical and thermal transport properties simultaneously, namely, as high as possible Seebeck coefficient and as small as possible electronic thermal conductivity and lattice thermal conductivity.
Skutterudites, body-centered cubic structure AB3 (A: Co, Rh, Ir; B: P, As, Sb) compounds, are widely recognized as promising medium-temperature thermoelectric materials because they can satisfy the phonon-glass and electron-crystal scattering concept [2,3,4]. In the family of skutterudite compounds, CoSb3-based material is of the most interest to researchers due to their good electronic properties and stable mechanical property. Nevertheless, pure CoSb3 cannot be used in thermoelectric applications because its electrical resistivity and especially thermal conductivity are very high [3]. Two approaches, i.e., filling impurity atom into intrinsic holes or doping treatment [5,6,7,8], could be used to improve the thermal and electrical transport property of CoSb3-based materials. It is well known that Ni-doping in CoSb3-based skutterudites not only controls the conduction type but also enhances ZT through reduction of both electrical resistivity and thermal conductivity [3, 9,10,11,12,13,14,15,16]. Kitagawa et al. [12], Il-Ho et al. [13], Soon-Chul et al. [14] and Qinyu et al. [15] reported ZT of 0.28 (600K), 0.2 (650K), 0.3 (650K) and 0.7 (823K) for Co0.95Ni0.05Sb3, Co0.9Ni0.1Sb3, Co0.93Ni0.07Sb3 and nanostructured Co0.91Ni0.09Sb3 enhanced by Ni-doped, respectively. Ahmad et al. [16] reported largest power factor value of 13.65 µW/cm-K2 for Co3.922Ni0.078Sb12 at 475 K, 25 times improvement over the non-doped CoSb3.
The skutterudite compounds powder or ingot is usually synthesized via smelting [12,13,14], mechanical alloying [15], wet synthesis [16], or solid-state reaction [17,18,19]. Then the resulting powder or ingot is quenched, annealed for several days and subsequently sintered with spark plasma sintering or hot press sintering to fabricate bulk thermoelectric materials. The conventional fabrication techniques are complex and inefficient, so it’s important to explore new synthetic method which could lead to the superior thermoelectric compounds with better structural and performance. Microwave heating techniques, possessing characteristics of instantaneous, volumetric and selective heating, might offer faster and cost-effective processes for synthesis reaction [20,21,22,23,24,25,26]. In the present work, a series of Ni-doped skutterudite NixCo4−xSb12 (x = 0.1, 0.2, 0.3, 0.4, 0.5) bulks were fabricated by combination of microwave synthesis and spark plasma sintering (SPS). The phase composition, grain growth characteristics and thermoelectric performance were investigated. The effects of the microwave heating and impurity on microstructure and thermoelectric properties were systematically studied.
2 Experimental
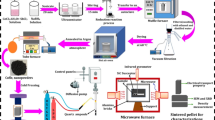
The starting metal powders are nickel (Ni, ~ 48 µm, 99.9%, Aladdin), cobalt (Co, ~ 48 µm, 99.99%, Aladdin) and antimony (Sb, ~ 20 µm, 99.9%, Aladdin). The three powders were weighed and mixed at nominal compositions of NixCo4−xSb12 (x = 0.1, 0.2, 0.3, 0.4, 0.5) and subsequently formed as button on powder compressing machine. The resulting button was sealed in vacuumed quartz tube and subsequently heated in microwave oven for 5 min (M1-L202B/700W, Midea/China). The synthesized ingot was pulverized and sintered by spark plasma sintering (SPS) (LABOX-100, Sinter Land INC./Japan) at 903 K for 5 min to fabricate bulk sample. More details concerning the process used are described in our previous papers [24,25,26].
The phase composition for bulk samples was detected by X-ray diffraction (XRD) on Bruker/D8-ADVANCE diffractometer with Cu-Kα radiation (λ = 1.54 Å) and scanning speed of 1 degree per min. The microstructure was observed by field emission scanning electron microscope (FE-SEM) on FEI/Nano-SEM430. The Seebeck coefficient and electrical resistivity were tested on JouleYacht/Namicro-3 test system at the range from room temperature to 773K with a step of 25 K. The thermal diffusivity was measured on Netzsch/LFA-427 laser flash thermal analyzer at the range from room temperature to 773K with a step of 100 K, then the thermal conductivity was calculated as κ = DdCp, where D is the thermal diffusivity, d is the sample density determined basing on the Archimedes’ method, Cp is the temperature dependence specific heat capacity, for CoSb3-based compounds whose value could be estimated as 0.24 J·g−1·K−1 [27].The electronic thermal conductivity was calculated as κel = LT/ρ, where ρ is electrical resistivity, L is Lorenz number estimated as 2.0 × 10−8V2K−2 [6]. The lattice thermal conductivity was calculated as κlat = κ-κel. The thermoelectric figure of merit was calculated as zT = T×(S2σ)/κ.
3 Results and discussion
3.1 Microstructures
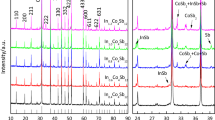
The XRD patterns of NixCo4−xSb12 compounds after SPS are shown in Fig. 1. All samples are single phase in accordance with the standard PDF card of CoSb3. Because the sintering temperature of 903K cannot meet the demand of alloying reaction, we vary the phase compositions of NixCo4−xSb12 through controlling the microwave synthesis process. Nearly single phase of different types of thermoelectric compounds, e.g., CoSb3, TiNiSn, CuSe, BiCuSeO, were achieved in 1–5 min microwave synthesis [24,25,26, 28,29,30,31]. As the inset in Fig. 1 shows that the XRD peak (310) of NixCo4−xSb12 compounds shift towards higher angles, indicating that Ni successfully enters into the Co site.
The lattice constants of NixCo4−xSb12 were calculated by using MDI Jade 5.0 software (DELL/Materials Date, Inc.) basing on the XRD patterns. It can be seen from Fig. 2 that the Ni doping causes a decrease of lattice parameter due to the relative smaller radius of nickel (~ 1.62 angstrom) atom than that of cobalt (~ 1.67 angstrom) atom. The lattice parameters of NixCo4−xSb12 basically decrease with the increase of Ni concentration. The lattice parameter depends on the real composition which is related to the reaction state of the synthesis process. The lattice parameters of Ni0.3Co3.7Sb12 and Ni0.4Co3.6Sb12 are not so different, meaning that the real compositions for them are close to each other. The possible reason is that the reaction states of different samples are not exactly the same due to the ultra-fast reaction rate of microwave synthesis. The lattice parameters calculated in this work are similar with those of samples obtained by conventional method [16].
The real densities of NixCo4−xSb12 compounds (x = 0.1, 0.2, 0.3, 0.4, 0.5) are measured as 7.554, 7.550 7.540, 7.557 and 7.597 respectively based on Archimedes’ method. The FE-SEM morphologies of cross section of NixCo4−xSb12 bulks are shown in Fig. 3a–e. The matrix grain and the grain boundaries are clearly visible in the field of view. The matrix grain sizes of NixCo4−xSb12 are about 1 to several micrometers with varying degrees of unevenness. The insets show the details of matrix grain for NixCo4−xSb12 bulks. The Ni0.1Co3.9Sb12 sample and Ni0.3Co3.7Sb12 sample obviously have the maximum and minimum grain size, respectively. The decreasing order of grain sizes for NixCo4−xSb12 (0.1, 0.2, 0.3, 0.4, 0.5) are Ni0.1Co3.9Sb12 (maximum), Ni0.2Co3.8Sb12 and Ni0.4Co3.6Sb12, Ni0.5Co3.5Sb12, Ni0.3Co3.7Sb12 (minimum). From the above, it can be deduced that the grain sizes for NixCo4−xSb12 depend on not only the point defects introduced by Ni doping, but also the microwave synthesis process. It is generally believed that the higher the heating rate the finer the grain size, however, it’s hard to control the grain size precisely in microwave field. The EDS elemental distribution maps of Ni0.3Co3.7Sb12 bulk are shown in Fig. 4. The results show that the concentration and distribution of Ni, Co and Sb coincide with the nominal composition of Ni0.3Co3.7Sb12.
3.2 Electrical transport properties
The electrical resistivity, Seebeck coefficient and power factor of NixCo4−xSb12 compounds are shown in Fig. 5a–c. The most significant trend is that the electrical resistivity decreases with the increased Ni concentration due to the increase of free electron. The electrical resistivity of Ni0.1Co3.9Sb12 decreases with the increased temperature due to the valence electrons constantly excited, exhibiting typical semiconductor behaviors. With the increase of Ni concentration, NixCo4−xSb12 gradually shows hybrid natures of semimetal and semiconductor behaviors, the electrical conductivity is very little affected by temperature, resulting in the very little variation of electrical resistivity. Similar trends can be seen in the previous works [15, 16].
The absolute value of Seebeck coefficients of NixCo4−xSb12 strictly decreases with the increased Ni concentration due to increase of electrical conductivity. There are maxima in the Seebeck coefficient vs temperature plots. The absolute value of Seebeck coefficient are firstly increase due to the reduction of chemical potential, which is a common phenomenon among degenerate semiconductors. With the increase of temperature, the lattice vibration will increase and resulting in significant enhancement of intrinsic excitation, and consequently cause the rapid increase of carrier concentration and decrease of Seebeck coefficient. Because of the bipolar effect, when at lower temperatures, the intrinsic excitation can be ignored and the carrier is mainly provided by the ionization of impurities (Se doping); on the opposite, when at higher temperatures, the intrinsic carriers dominate. The maximum Seebeck coefficients of NixCo4−xSb12, where x is 0.1, 0.2 ,0.3 0.4 and 0.5, are − 254, − 211, − 191, − 188 and −160 µVK−1, respectively.
The variations of power factors for different Ni-doped samples are consistent, which firstly increase and then decrease with the increased temperature. The variations of power factors with the increase of Ni concentration for different samples are not regular. The main reason is that the power factors are proportional to the Seebeck coefficient and inversely proportional to the conductivity synchronously, high power factor are required for moderate electrical conductivity and relative high Seebeck coefficient. The maximum power factors of NixCo4−xSb12 are 2273, 2479, 2711, 2613 and 2526 µWm−1K−2 respectively.
Overall, the electrical transport properties for un-filled CoSb3 are sensitive to the Ni-doped concentration, which indicates that Ni atoms act as electron donors could significantly improve electrical conductivity at the cost of part of Seebeck coefficient, making the achieved power factors significantly higher than those of samples reported in previous works [16].
3.3 Thermal transport properties
The temperature dependences of electronic thermal conductivity, lattice thermal conductivity and total thermal conductivity of NixCo4−xSb12 compounds are shown in Fig. 6a–c respectively. The electronic thermal conductivities increase with the increased Ni concentration due to the increase of electrical conductivity if the Lorenz is fixed. The lattice thermal conductivities of NixCo4−xSb12 depend on the Ni concentration and the grain boundary characteristic. The lattice thermal conductivities at room temperature basically increase with the increased Ni concentration due to the increase of point defect. The lattice thermal conductivities are also influenced by the average grain size especially at relative higher temperature. The Ni0.3Co3.7Sb12 bulk has the smallest lattice thermal conductivity of 2.73Wm−1K−1 at 773K, which is significantly smaller than those of un-filled samples synthesized by conventional ways [10, 12,13,14, 16]. The lattice thermal conductivity makes major contribution to the total thermal conductivity. The variation of total thermal conductivity is similar to that of lattice thermal conductivity due to the ambipolar diffusion. The total thermal conductivity of Ni0.3Co3.7Sb12 is 3.89–5.56 Wm−1K−1.
3.4 Thermoelectric figure of merit, ZT
The thermoelectric figures of merit of NixCo4−xSb12 bulks are shown in Fig. 7. The thermoelectric figures of merit increase with the increased temperature. The Ni0.3Co3.7Sb12 has the maximum ZT of 0.52 at 773 K and that the values larger might be realized at a bit higher temperature. The maximum ZT of 0.52 for the present sample synthesized via 5 min microwave heating is slightly smaller than that of nanostructured sample synthesized via 20–50 h mechanical alloying [15], but significantly greater than those of samples synthesized via smelting [12,13,14] and solid-state reaction [10].
4 Conclusions
In summary, a series of single phase Ni-doped skutterudite compounds NixCo4−xSb12 (x = 0.1, 0.2, 0.3, 0.4, 0.5) were successful synthesized by 5 min microwave heating for the first time. The Ni0.3Co3.7Sb12 bulk has the smallest and uniform grain size of about 1–2 µm, such grain characteristic may beneficial to reduce the lattice thermal conductivity through enhance the phonon scattering. The electrical transport property has been enhanced by Ni doping. The Ni0.3Co3.7Sb12 has the highest ZT of 0.52 at 773 K. The new preparation route shows highly competitive compared with the conventional ways both in terms of efficiency and thermoelectric performance.
References
G. Mahan, B. Sales, J. Sharp, Phys. Today 50, 42 (1997)
G.A. Slack, V.G. Tsoukala, J. Appl. Phys. 76, 1665 (1994)
T. Caillat, A. Borshchevsky, J.P. Fleurial, J. Appl. Phys. 80, 4442 (1996)
G.A. Slack, CRC Handbook of Thermoelectrics (CRC Press, Florida, 1995), p. 407
B.C. Sales, D. Mandrus, R.K. Williams, Science 272, 1325 (1996)
B.C. Sales, D. Mandrus, B.C. Chakoumakos, Phys. Rev. B 56, 15081 (1997)
J. Yang, D.T. Morelli, G.P. Meisner, W. Chen, J.S. Dyck, C. Uher, Phys. Rev. B 67, 165207 (2003)
X.Y. Li, L.D. Chen, J.F. Fan, W.B. Zhang, T. Kawahara, T. Hirai, J. Appl. Phys. 98, 4442 (2005)
L.D. Dudkin, N.Kh. Abrikosov, Sov. J. Inorg. Chem. 2, 212 (1957)
H. Anno, K. Matsubara, Y. Notohara, T. Sakakibara, H. Tashiro, J. Appl. Phys. 86, 3780 (1999)
J.S. Dyck, W. Chen, J. Yang, G.P. Meisner, C. Uher, Phys. Rev. B 65, 115204 (2002)
H. Kitagawa, M. Wakatsuki, H. Nagaoka, H. Noguchi, Y. Isoda, K. Hasezaki, Y. Noda, J. Phys. Chem. Solids 66, 1635 (2005)
K. Il-Ho, U. Soon-Chul, Met. Mater. Int. 13, 53 (2007)
U. Soon-Chul, K. Il-Ho, J. Korean Phys. Soc. 55, 942 (2009)
H. Qinyu, H. Qing, W. Xiaowei, Y. Jian, L. Yucheng, Y. Xiao, Y. Bo, M. Yi, P. Bed, J. Giri, W. Dezhi, C. Gang, R. Zhifeng, J. Nanosci. Nanotechnol. 8, 4003 (2008)
A. Gharleghi, C.J. Liu, J. Alloy. Compd. 592, 277 (2014)
T. Dahal, H.S. Kim, S. Gahlawat, Acta Mate 117, 13 (2016)
G. Tan, H. Chi, W. Liu, J. Mater. Chem. C 3, 8372 (2015)
S. Wang, J.R. Salvador, J. Yang, NPG Asia Mate 8, e285 (2016)
W.Y. Lai, Q.Q. Chen, Q.Y. He, Q.L. Fan, W. Huang, Chem. Commun. 18(2006)1959
Y. He, H.T. Lu, L.M. Sai, W.Y. Lai, Q.L. Fan, L.H. Wang, J. Phys. Chem. B 110, 13352 (2006)
K. Biswas, S. Muir, M.A. Subramanian, Mater. Res. Bull. 46, 2288 (2011)
H.J. Kitchen, S.R. Vallance, J.L. Kennedy, N. Tapia-Ruiz, L. Carassiti, A. Harrison, A.G. Whittaker, T.D. Drysdale, S.W. Kingman, D.H. Gregory, Chem. Rev. 114, 1170 (2014)
Y. Lei, W.S. Gao, Y. Li, R.D. Wan, W. Chen, R. Zheng, L.Q. Ma, H.W. Zhou, Mater. Lett. 233, 166 (2018)
Y. Li, C. Cheng, Y. Lei, M. Wang, R.D. Wan, Dalton Trans. 46, 33 (2016)
Y. Lei, Y. Li, L. Xu, J.Y. Yang, R.D. Wan, H.M. Long, J. Alloy. Compd. 660, 166 (2016)
X. Shi, J. Yang, J.R. Salvador, M. Chi, J.Y. Cho, H. Wang, S.Q. Bai, J. Yang, W.Q. Zhang, L.D. Chen, J. Am. Chem. Soc. 133, 7837 (2011)
Y. Lei, C. Cheng, Y. Li, R.D. Wan, M. Wang, Ceram. Int. 43, 9343 (2017)
Y. Lei, M. Wang, Y. Li, W.S. Gao, R.D. Wan, C. Cheng, Mater. Lett. 201, 189 (2017)
Y. Li, R. Zheng, Y. Lei, W.S. Gao, L.Q. Ma, R.D. Wan, Chin. Pat., 201811362898.1
Y. Lei, L.Q. Ma, Y. Li, R.D. Wan, W.S. Gao, R. Zheng, Chin. Pat., 201810812316.9
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 51574134, No. 51574042), Joint fund between Shenyang National Laboratory for Materials Science and State Key Laboratory of Advanced Processing and Recycling of Nonferrous Metals (Grant No. 18LHPY016) and Anhui university outstanding young talent support program (key project) (Grant No. gxyqZD2017039).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, Y., Ma, L., Zheng, R. et al. Thermoelectric performance of skutterudite NixCo4−xSb12 rapidly synthesized by microwave heating. J Mater Sci: Mater Electron 30, 5929–5935 (2019). https://doi.org/10.1007/s10854-019-00892-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00892-2