Abstract
The interest in studying cellulose especially bacterial cellulose (BC) and BC-based composites has increased dramatically, due to their outstanding properties. Among them, BC-based electroconductive composites seem to capture more attention because of their perfect structure and controllable synthesis as well as potential values. Meanwhile, the development of carbon fibers is becoming a hot spot in recent years. Here, we concentrate on describing their numerous approaches, and some improvements in the process, which are discussed in greater details with an emphasis on their functional properties and potential applications. The challenges in commercial scale applications are discussed and the efficiencies of various electroconductive composites are compared, in order to exploit its far-reaching application value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
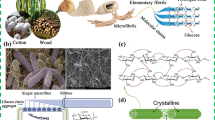
As a ubiquitous natural polysaccharide, cellulose has been extensively applied in commercialized markets, including paper, textile, food, medical, reinforcement agent, and electronic industries. While cellulose is the primary structural material of cell walls in most plants, it is also synthesized from several types of bacterial species [1]. Bacterial cellulose (BC), or microbial cellulose discovered by A.J. Brown, was initially produced by Acetobacter xylinum in 1886 [2]. These fibers undergo the process of crystallizing, forming self-assembled bundles and further tightly aggregate to form a fibrillar ribbon(<100 nm wide), which is an ultrafine fiber (1/10 to 1/1000 compared with pulp fiber) [3]. Finally, these ribbon-like fibrils transform into a three-dimensional (3D) reticulate structure (Fig. 1). The formation of 3D hierarchical structure can be related to the bacteria cell division and random motion during the cellulose synthesis process [4–6].
SEM image of 3D BC network contained Acetobacter xylinum (left) and Schematic diagram of the structure of BC nanocomposites (right). Reproduced from [6] with permission from Yang et al. (2012)
Apart from its ideal structure, BC has some other excellent properties superior to the cellulose produced by plants. It possesses higher chemical purity without pectin, hemicelluloses, or lignin. The ultrafine interconnected structure endows BC with ample porosity and specific surface area, which can serve as a matrix to support other functionalized materials. Furthermore, abundant hydrogen bonds and the 3D structure make BC possess high hydrophilicity and water-holding capability, which is characterized by the water-retention of 1000 % (while just 60 % for plant-derived cellulose) [7]. Moreover, BC also bears the merits of high crystallinity (60–90 %) and higher degree of polymerization (2000–8000), which lead to high Young’s modulus, great tensile strength [8, 9], and stable chemical properties [10].
On account of all these meritorious properties, great efforts have been dedicated toward developing BC-based materials, especially conducting or optical materials [11–14]. Numerous materials have been involved, as shown in Fig. 2, including conducting polymers, graphene and graphene oxide, carbon nanotubes, and carbon nanofibers. Advantages such as high hydration, excellent strength, and flexibility are combined with their intrinsic conducting properties, and the limitations are ameliorated [15, 16]. Today, also owing to their low cost, renewability, biocompatibility as well as biodegradability, BC membranes have attracted great attention in industrial scale production via the microbial fermentation process for the versatile application especially in fuel cell, ion battery, flexible supercapacitors, and other electrochemical devices.
Overall, the main focus of this review is not only to summarize current knowledge of BC-based conductive composites in recent 5 years but also to provide a comprehensive comparison of various conducting nanomaterials decorated by BC, which generally focus on their properties as well as potential value in the electrochemistry application. Owing to the rapidly expanding nature of this interesting field, we hope that this review can provide a helpful overview and insights to readers in this exciting research area.
Electronic and catalytic applications
Combination with conducting polymers
Since 1970s, electronically conducting polymers (ECPs) have received intensive interest for flexible electrodes due to their excellent conductivity, controllable synthesis process, and low density [17, 18]. Their wide applications involve electrical and optical devices, chemical and biological sensors, electromagnetic shielding materials, and even solar cells [19]. The growing attention of using bacterial cellulose as a suitable matrix is mainly attributed to the improvement of mechanical strength [20, 21]. Among ECPs, polypyrrole(PPy) is considered as a hopeful conducting polymer in electronics, optical, biological, and medical application areas for its direct polymerization, biocompatibility, and electrical conductivity with controllable synthesis process [22, 23]. Shi et al. [24] used regenerated nanoporous cellulose gels (NCG) as the template, which were prepared from aqueous alkali/urea solutions, and employed in situ vapor-phase polymerization of pyrrole monomers in the NCG matrix (Fig. 3a). The NCG/PPy composite hydrogels demonstrated good mechanical stability (Fig. 3c, d, e), sufficient electrical conductivity (up to 0.08 S/cm), and even good biocompatibility. Wang et al. obtained ordered core–sheath nanostructured BC/PPy nanocomposites which showed outstanding electrical conductivity as high as 77 S/cm [25]. This result is far optimized than other research results [9, 26–28]; because of its excellent electric conductivity, stable thermal stability, and well-controlled microstructure, this composite extends the applications of new conductive materials based on BC/PPy [29].
(a) Preparation of NCG/PPy composite aerogels. (i) The NCG hydrogel with interconnected nanofibrillar network is solvent exchanged with ethanol, and then impregnated with a solution of FeCl3 in ethanol to form the NCG/FeCl3 alcogel. (ii) PPy nanoparticles are formed by in situ vapor-phase polymerization of pyrrole monomers supplied as vapor, giving the NCG/PPy-1 composite alcogel. (iii) Repeating the second step two or three times gives NCG/PPy-2 and NCG/PPy-3 composite alcogels, respectively. (iv) Drying with supercritical CO2 gives NCG/PPy composite aerogel. (b) Schematic representation of pyrrole polymerization with FeCl3. Macroscopic views of NCG/PPy-3 composite hydrogels under (c) bending, (d) rolling (internal diameter of 10 mm), and (e) torsional loading, and (f) NCG/PPy-3 composite aerogel. Reproduced from [24] with permission from Shi et al. (2014)
It should be pointed out that although the efforts have been made to exploit new oxidants, such as ammonium persulfate(APS), BC/PPy·FeCl3 composites seem to present higher interaction of functional groups than BC/PPy·APS composites so that the former provides higher electrical and mechanical properties [30]. Instead, by doping with stretchable polymer or carbon materials, the flexibility and conductivity have been successfully raised. A highly conductive membrane based on BC/PPy/multi-walled carbon nanotubes (MWCNTs) with a high mass loading in the range of 7–12 mg/cm2 and efficient performance was reported. The value of its capacitance (2.43 F/cm2 at a discharge current of 2 mA/cm2) was higher than that of PPy-coated papers (1.5 F/cm2) [31].
Polyaniline (PANI), owing to its low cost, ease of preparation, environmental stability, and reversible acid/base doping characteristics, is now widely used in conducting polymer composites [32, 33]. Various strategies, including template synthesis, self-assembly, electrospinning, electrochemical methods, rapid mixing polymerization, and interfacial polymerization, have been developed for the synthesis of PANI nanofibers and nanotubes [34]. With BC material as the template, PANI/BC conductive nanocomposites have been successfully fabricated by oxidative polymerization of aniline using ammonium persulfate as the oxidant. The process is shown in Fig. 4. It was found that the PANI particles could uniformly deposit on the surface of BC to form a continuous nanosheath by taking along the BC fibers, which greatly increased the thermal stability of BC [35]. The electrical conductivity was enhanced from 10−8 to 10−2 S/cm by controlling the reaction time and the amount of PANI used [36–38]. In addition, the composites showed amazing combination of flexibility and conductivity, which could be bent by 180° without breaking. The work opens a new field to prepare flexible conducting films with BC materials [39]. Wang et al. developed a highly conductive BC/PANI nanocomposite with conductivity hitting 5.1 S/cm. They dissolved BC in a mixed solvent of DMF and distilled water before reaction steps. Figure 5 shows that with the PANI coating, the morphology of the 3D network of nanocellulose changes into a flake structure. From Fig. 5a, b, c, d of homogeneous BC/PANI fibers, DMF may play an important role in diffusion of aniline monomer in the mixture, resulting in concentrating aniline on the surface of BC nanofibers and inner 3D networks for polymerization. In addition, it was found that BC/PANI electrode retained about 94.3 % (234.7 F/g) of initial capacitance after 1000 cycles. This good stability may be ascribed to the well-ordered BC/PANI composites and strong interaction between the BC core and the PANI shell [40]. Furthermore, PANI/BC composites could also be constructed as an excellent three-dimensional template for hydrothermal growth of MoS2 nanosheets after nitrogen-doped carbonization [41]. The hierarchical nanostructures and coarse surface of N-doped carbon nanofibers were beneficial to the diffusion of MoS2 precursors into the inner space. These synergistic effects ensure the excellent electrocatalytic activity and stability of the composites, which provide a new strategy for design and application of bacterial cellulose and MoS2-based nanocomposites in energy conversion and storage areas.
Photographs of a BC hydrogel and b BC–PAni hydrogel and c schematic diagram showing the experiment process, the chemical structure and synthesis of PANi in the BC hydrogel. Reproduced from [35] with permission from Shi et al. (2012)
FE-SEM images of BC/PANI nanocomposites with 86 wt% PANI prepared from a pure H2O, b DMF/H2O (1:2, v/v), c DMF/H2O (2:2, v/v), and d DMF/H2O (3:2, v/v). Reproduced from [40] with permission from Wang et al. (2012)
Another electrode material with attractive morphology was fabricated via in situ synthesis of PANI on regenerated cellulose microspheres (CM) using phytic acid (PA) [42]. PA with phosphorus oxygen groups was believed to form strong hydrogen bonding with polyaniline serving like a bridge between PANI and CM. From the SEM images in Fig. 6, the CM microspheres exhibited homogeneous porous structure with pore size ranging from about 100 to 200 nm, and the loose structure induced the formation and rapid aggregation of coralline PANI on the surface of the CM. Due to this homogeneous micro- and nanoporous architecture, the PANI/PA/CM exhibited excellent cycling stability (over 12,000 cycles), high rate capability, and good conductivity as an electrode material. They open a new gate with large scale and cost-efficient strategy to construct highly efficient electrode materials in energy-storage devices.
a, c SEM image of a cellulose microsphere (CM); b high magnification SEM image of the surface; d, f SEM image of the PANI/PA/CM; e a high magnification SEM image of the surface; g electrochemical performance for a two-electrode device with PANI/PA/CM films as the electrodes: normalized CV curves under a sweep rate from 2 to 100 mV/s; h cycling stability and coulombic efficiency for 12,000 cycles under 2 mA. Reproduced from [42] with permission from Xu et al. (2015)
Recently, BC conducting composites incorporating with poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) (PEDOT:PSS) have attracted considerable interest, because of their strong chemical interactions, ionic nature, as well as high conductivity [43, 44]. The composites were usually prepared by ex situ incorporation PEDOT: PSS into BC pellicles so that the three-dimensional structure of BC could be preserved. The electrical conductivity could raise up to 12.17 S/cm for only 31.24 wt% PEDOT:PSS loading, which confirmed PEDOT: PSS was more efficient than PANi or PPy [43]. Chen et al. [45, 46] made efforts to explore both biocompatible and electrical performance of 3D BC/PEDOT/PSS nanofibers. Human mesenchymal stem cells (hMSCs) presented good attachment and proliferation on the scaffolds. Moreover, PC12 cells responded with the conformational changes of the proteins, when they received the electrical stimuli of charge injection. They confirmed these 3D BC/PEDOT nanofibers could also meet the needs of bio-sensing devices, smart drug delivery systems, and implantable electrodes for tissue engineering. What is more, Wang et al. [47] reported a soft biomolecule actuator based on carboxylated bacterial cellulose (CBC), ionic liquid (IL), and PEDOT:PSS electrodes. Carboxylic acid groups in TEMPO oxidized bacterial cellulose could greatly contribute to the absorption of ionic liquid so that enhancing ion transport properties. The soft CBC–IL actuator exhibited better tip displacement in both the step and harmonic response cases due to the higher ionic exchange capacity, ionic conductivity, and an exceptionally superior elongation. This high performance electroactive actuator shows great potential in soft wearable electronics and flexible bio-medical devices.
Combination with graphene
Produced in lab in 2003, graphene (GE) and graphene oxide (GO) have emerged as novel versatile electronic carbon materials brought into sharp focus, which form unique two-dimensional structures with unexpected physical properties including high conductivity, accessible surface area, and high mechanical strength [48–50]. Due to enough functional groups including hydroxyl, carbonyl, and epoxy groups on their basal planes and edges, they have served as polymer matrixes reinforcement [51, 52].
To the authors’ best knowledge, just two systematic attempts to obtain the combination of GO with BC have been reported so far (as shown Fig. 7) [53–55]. For one thing, fabricated BC as flexible substrates are covalently bonded with GO nanosheets via mechanical mixing method [54]. Feng et al. [32] prepared free-standing disk-like BC/GO films. Compared with pristine BC, the modulus, strength, and electrical properties were obviously enhanced, but the elongation at break was decreased due to the brittle nature of the GO sheets. However, this method has the drawback that the conductivity is in pace with the GO content while the higher conductive composites could be extremely fragile. On the base of this, Liu et al. [53] reported parallel-aligning GO covalently reinforced with BC via one-pot esterification. They formed an excellent conductive material with the electrical conductivity of 171 S/m, which was increased by 4 orders of magnitude than that in the literature [32]. What is more, compared with the preparation by physical mixing (BC/GOPM) without dicyclohexylcarbodiimide (DCC), as shown in Fig. 8, we can see obviously greater aggregation of GO sheets in Fig. 8a, b than those in 8c, d, e, f, so it can be readily ascertained that DCC plays an important role in strongly crosslinking carboxyl groups of GO and hydroxyl groups of BC to form the interconnected structure in esterification. Moreover, the unique 3D composites exhibit outstanding capacitance retention about 90.3 % after 2000 charge/discharge cycles, shown in Fig. 8g, which have the potential for robust supercapacitor application.
a Schematic illustration of BC/GO composites synthesized through mechanical mixing strategy. Reproduced from [54] with permission from Luo, et al. (2014). b Schematic illustration of BC/GO composites synthesized through in situ fermentation strategy. Reproduced from [55] with permission from Yun et al. (2014)
a, b TEM images of BC/GOPM composites showing GO-rich domains. c Top-view SEM image showing GO sheets covalently functionalized with BC and the bonding of the parallel GO sheets with the BC network; d corresponding TEM image for (c). e Zoom-in SEM image showing the covalent bonding of parallel-aligned GO sheets with the BC network; f corresponding TEM image for (e). g Recycling performance of BC/GO at 0.4 A/g. Reproduced from [55] with permission from Yun et al. (2014)
Luo et al. [54] added GE suspension into BC culture medium, the hybrid nanostructures can ensure an even distribution of graphene nanosheets in the BC matrix and retain the unique network structure of BC in the meanwhile. After adding graphene, the crystallinity of hybrid BC was decreased while the structure of graphene sheets was still unchanged. However, GO has been reported to have remarkable antimicrobial property which may have great effect on bacteria to produce cellulose in the fermentation [56, 57]. Therefore, BC hybridized with antibacterial agents has yet to be well resolved. The highest electrical conductivity (1320 S/m) and the largest volumetric capacitance (278 F/cm3) ever shown by intercalating graphene sheets with BC nanofibers were reported by Liu et al. [58]. With wrapping PPy onto chemically bonded BC/GO hybrid, well-defined 3D electrical conduction path was constructed and the negative effect of oxygen-containing groups on GO surface was eliminated. This PPy/BC/GO composite also revealed high specific capacitance and remarkable recyclability (95.2 % capacitive retention for asymmetric after 5000 recycles), which was first reported on such chemically bonded hybrid composites as working electrodes for supercapacitors.
Combination with carbon nanotubes
In recent years, carbon nanotubes(CNTs) have inspired a great deal of interest for flexible electrochemical capacitor applications [59], owing to their high specific surface area, high electrical conductivity, excellent electrochemical properties, and controllable regular pore structure [60]. Meanwhile, BC inherent characteristics as higher strength, good network structure as well as high purity and crystallinity attract more researchers to incorporate CNTs into BC hydrogel as a smart material used in sensors, actuators, and electrodes. This type of composites provides numerous advantages such as the improvement of elastic modulus, mechanical properties, and optic transition. The combination methods are also manifold which involves adding CNTs in the media culture, complexing CNTs with regenerated BC or modified BC, compressing the composites by vacuum filtration.
Since Park et al. confirmed that the biocompatibility of MWCNT to bacteria G.xylium [61], MWCNTs, and BC composites could be produced simply by adding acid-treated MWCNTs into a static culture medium. The acid-treated MWCNTs with –OH groups were dispersed uniformly in the medium, so that BC ribbons with the MWCNTs were able to form a 3D network [62, 63]. Furthermore, to obtain a homogeneous MWCNTs solution, Yoon et al. introduced cationic cetyltrimethyl ammonium bromide (CTAB) as surfactant and found that the MWCNTs were effectively embedded in cellulose pellicle, which gave an electrical conductivity approximate to 0.14 S/cm. It indicated that the incorporation process was a useful method not only for dispersing MWCNTs in an ultrafine fibrous network structure but also for enhancing the electrical conductivity of the polymeric membranes [64]. The conductivity of BC/MWCTs was much higher than BC/DWCNTs, in the range from 0.12 to 1.6 S/cm instead of 0.034 to 0.39 S/cm. What is more, MWCNT-treated cellulose showed higher sensitivity than that of DWCNT [65].
BC/CNTs composites were also gradually developed in the application of supercapacitors [66–68]. Kang et al. developed a flexible supercapacitor with high physical flexibility, desirable electrochemical properties, and excellent mechanical integrity (Fig. 9), which was realized by rationally exploiting unique properties of BC, carbon nanotubes, and ionic liquid-based polymer gel electrolytes. The supercapacitors owned a specific capacitance, energy, and power of 20.2 mF/cm2, 15.5 mWh/g, and 1.5 W/g, respectively. Moreover, they showed excellent cyclic stability with <0.5 % change in Csp over 5000 charge/discharge cycles. Owing to the excellent interfacial quality between the three different layers, the performance of the supercapacitors was well retained over the bending cycles [69].
a Cross-sectional view of a CNT/BNC paper. b Photograph of a light-emitting diode (LED) turned on by the flexible supercapacitors. c CV curves measured before and after 200 bending cycles. d Capacitance retention over 5000 cycles of charge/discharge at a current density of 10 A/g. Reproduced from [69] with permission from Kang et al. (2012)
Vacuum filtration was usually used in the preparation of this compound, due to its simplicity and preserving good properties [70]. Another BC/MWCNTs composites prepared simply by vacuum filtering were used as BC-CNT-GO composite electrodes with immobilizing GO on the sample surface. In this work, this electrode showed a pair of well-defined redox peaks and indicated that BC-CNT electrodes were efficient for direct electron transfer by GO on the electrodes [71]. What is more, the electrical conductivity seemed to be much lower using regenerated bacterial cellulose and wet-spinning technique compared with other methods, which may result from the change of BC structure after dissolution. However, there was still an approximate 300–430 % increase in the modulus of the regenerated BC/MWCNTs composite fibers, and the thermal stability was dramatically improved as well [72, 73].
Carbonized as carbon nanofibers
Recently, carbon nanofibers (CNF) derived from BC have been widely investigated in electrochemical energy storage because of their outstanding multifunctional properties [74–76]. These low-cost carbon nanomaterials can be easily fabricated on a large scale via a new simple way reported by Yu [77], CNF can serve as a stable platform by polymerization not only on the cellulose surface but also within the network to afford supporting substrates. Besides, the electrical conductivity of CNF aerogel is highly sensitive to the compressive strain, thereby it could be used for pressure sensors and other potential applications including 3D electrode materials for lithium-ion batteries and catalyst supports [78–80].
To date, some research works have been devoted to using BC as a precursor material to explore the development of applications in the different fields, such as supercapacitors (shown in Table 1) [55], adsorbing toxic organic dyes [81], and medical fields [6]. Tong et al. reported a network structured CNF/Ru composites, which derived from pyrolyzed BC for flexible Li-O2 battery [82]. Our group [83] prepared a CNF-Pt anode catalytic material, in which, Pt was evenly dispersed on the surfaces of the CNF with narrow size distribution. The volatile components such as carbon monoxide, carbon dioxide, alcohols provided more holes, and effectively shortened the diffusion pathway for ion transport [84]. Based on this 3D-interconnected nanostructure, we also successfully fabricated a high sulfur loading (81 wt%) CNF composite, the procedure is shown in Fig. 10. During the production, the CNF interlayer could also act as an additional collector for sulfur to alleviate the over-aggregation of insulated sulfur on the cathode surface [85]. What is more, Wang et al. [86] prepared CNF with the SBET up to 670 m2/g, which made it efficient in electrons or ions transportation and offered more electrochemical active sites. Wan et al. prepared a flexible nano-Fe3O4-anchored CNFs as electrocatalyst for lithium-ion batteries, showing high reversible capacity and excellent rate capability [87].
On the left The procedure for preparing S/CBC cathode and CBC interlayer, and their specific location in the Li–S battery configuration. On the right SEM images of a CBC and b S/CBC composites. Reproduced from [85] with permission from Huang et al. (2015)
To obtain advanced performance, one widely recognized approach is to dope with heteroatoms, such as nitrogen (N), phosphorus (P) to tailor the electronic structure of the matrix. These heteroatoms could influence the valence orbital energy levels of the adjacent C atoms in carbon fibers to induce a synergistically enhanced reactivity [88, 89]. Accordingly, N-doping carbonized bacterial cellulose has attracted a great deal of research interest. The general strategy for fabricating free-standing heteroatom-doped carbon nanofibers is illustrated in Fig. 11.
a Schematic illustration of the process to fabricate the free-standing heteroatom-doped carbon nanofibers. b Specific capacitor values of different N,P-CNF-based supercapacitors at the current density of 1.0 A/g, where N,P-CNF-1, N,P-CNF-2, N,P-CNF, and N,P-CNF-3 were prepared with a NH4H2PO4 aqueous solution molarity of 0.02, 0.05, 0.1, and 0.2 mol/L, respectively. c Specific capacitor values of different carbon nanofiber-based supercapacitors at the current density of 1.0 A/g. Reproduced from [97] with permission from Chen et al. (2013)
Chen et al. [90] reported a 3D N-doped activated CNF derived from pyrolyzed BC. The as-prepared pyrolyzed BC (p-BC)/N could offer more electrochemically active sites for improving the capacitive properties of the materials(173.32 F/g), which was much higher than that of p-BC (only 77.70 F/g) [25]. Previous studies have demonstrated that doping-N with ammonia gas for hours exhibited higher electrochemical stability when compared with undoped sample [91]. Similarly, Liang et al. [92] developed a highly active N-CNF aerogel catalyst, which possessed high N-doping contents about 5.8 at.% and superb porous performance (SBET, 916 m2/g; pore volume, 0.71 cm3/g). That is to say, NH3 treatment is an efficient manner, which can increase the porosity significantly for N-CNF aerogels. However, post-treatment of N-doped CNF like treating with NH3 [93–95] or pyrolyzing of N-enriched precursor [96] always involves awkward working conditions or time-consuming synthetic procedures [97]. Hence, Chen et al., examined N-modified activated pyrolyzed bacterial cellulose (A-p-BC–N) as the electrode material via employing an ammonia solution during hydrothermal synthesis which can overcome these drawbacks [90]. The as-obtained A-p-BC–N manifested good supercapacitive performance of up to 390.53 kW/kg power density and a superior cycling durability with 95.9 % specific capacitance retained after 5000 continuous cycles. More strikingly, highly mechanical flexible and recoverable properties prevent the supercapacitor from deformation under extreme bending conditions.
Conclusions and future perspectives
In this review, we showed a natural and biocompatible material, bacterial cellulose, which has dramatically gained attention in various application fields especially in fabricating electroconductive composites. It can not only act as a matrix to represent the unique 3D nanostructure, significant water-holding capacity as well as purity, the carbon nanofibers carbonized from it also grant the pursuit of applications in conducting applications, which can be ascribed to its innate excellent tensile strength and Young’s modulus. The low thermal expansion of BC combined with high conducting material or inorganic nanoparticles make them a potential material for fuel cells, flexible supercapacitors, and electronic papers. Even though the challenges like poor durability in most environments are still the obstacle to overcome for further industrial scale production, and the application in energy-storage device remains to be profoundly explored, and these natural polymers open up the important and rapidly expanding fields of personal care, medicine, and life sciences.
References
Gatenholm P, Klemm D (2010) Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull 35(03):208–213
Brown AJ (1886) XLIII.—On an acetic ferment which forms cellulose. J Chem Soc Trans 49:432–439
Yoshinaga F, Tonouchi N, Watanabe K (1997) Research progress in production of bacterial cellulose by aeration and agitation culture and its application as a new industrial material. Biosci Biotechnol Biochem 61(2):219–224
Thiruvengadam V, Vitta S (2013) Ni–bacterial cellulose nanocomposite; a magnetically active inorganic–organic hybrid gel. RSC Adv 3(31):12765–12773
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci 35(2):261–270. doi:10.1023/A:1004775229149
Huang Y, Zhu C, Yang J, Nie Y, Chen C, Sun D (2014) Recent advances in bacterial cellulose. Cellulose 21(1):1–30
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26(9):1561–1603
Luo C, Stoyanov SD, Stride E, Pelan E, Edirisinghe M (2012) Electrospinning versus fibre production methods: from specifics to technological convergence. Chem Soc Rev 41(13):4708–4735
Jradi K, Bideau B, Chabot B, Daneault C (2012) Characterization of conductive composite films based on TEMPO-oxidized cellulose nanofibers and polypyrrole. J Mater Sci 47(8):3752–3762. doi:10.1007/s10853-011-6226-9
Yang J, Sun D, Li J, Yang X, Yu J, Hao Q, Liu W, Liu J, Zou Z, Gu J (2009) In situ deposition of platinum nanoparticles on bacterial cellulose membranes and evaluation of PEM fuel cell performance. Electrochim Acta 54(26):6300–6305
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44(22):3358–3393
Olsson RT, Samir MA, Salazar-Alvarez G, Belova L, Ström V, Berglund LA, Ikkala O, Nogues J, Gedde UW (2010) Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat Nanotechnol 5(8):584–588
Legnani C, Vilani C, Calil V, Barud H, Quirino W, Achete C, Ribeiro S, Cremona M (2008) Bacterial cellulose membrane as flexible substrate for organic light emitting devices. Thin Solid Films 517(3):1016–1020
Zakirov AS, Yuldashev SU, Wang HJ, Lee JC, Kang TW, Mamadalimov AT (2010) Study on electrical transport and photoconductivity in iodine-doped cellulose fibers. J Mater Sci 46(4):896–901. doi:10.1007/s10853-010-4832-6
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50(24):5438–5466
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40(7):3941–3994
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196(1):1–12
Ramya R, Sivasubramanian R, Sangaranarayanan M (2013) Conducting polymers-based electrochemical supercapacitors—progress and prospects. Electrochim Acta 101:109–129
Malhotra BD, Chaubey A, Singh S (2006) Prospects of conducting polymers in biosensors. Anal Chim Acta 578(1):59–74
Bhadra S, Singha NK, Khastgir D (2008) Effect of aromatic substitution in aniline on the properties of polyaniline. Eur Polym J 44(6):1763–1770
Mihranyan A, Esmaeili M, Razaq A, Alexeichik D, Lindström T (2012) Influence of the nanocellulose raw material characteristics on the electrochemical and mechanical properties of conductive paper electrodes. J Mater Sci 47(10):4463–4472. doi:10.1007/s10853-012-6305-6
Zhang X, Zhang J, Song W, Liu Z (2006) Controllable synthesis of conducting polypyrrole nanostructures. J Phys Chem B 110(3):1158–1165
Müller D, Rambo C, Recouvreux D, Porto L, Barra G (2011) Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synth Met 161(1):106–111
Shi Z, Gao H, Feng J, Ding B, Cao X, Kuga S, Wang Y, Zhang L, Cai J (2014) In situ synthesis of robust conductive cellulose/polypyrrole composite aerogels and their potential application in nerve regeneration. Angew Chem Int Ed 53(21):5380–5384
Wang H, Bian L, Zhou P, Tang J, Tang W (2013) Core–sheath structured bacterial cellulose/polypyrrole nanocomposites with excellent conductivity as supercapacitors. J Mater Chem A 1(3):578–584
Xu J, Zhu L, Bai Z, Liang G, Liu L, Fang D, Xu W (2013) Conductive polypyrrole–bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org Electron 14(12):3331–3338
Li S, Huang D, Yang J, Zhang B, Zhang X, Yang G, Wang M, Shen Y (2014) Freestanding bacterial cellulose–polypyrrole nanofibres paper electrodes for advanced energy storage devices. Nano Energy 9:309–317
Su F, Poh CK, Chen JS, Xu G, Wang D, Li Q, Lin J, Lou XW (2011) Nitrogen-containing microporous carbon nanospheres with improved capacitive properties. Energy Environ Sci 4(3):717–724
Olsson H, Carlsson DO, Nyström G, Sjödin M, Nyholm L, Strømme M (2012) Influence of the cellulose substrate on the electrochemical properties of paper-based polypyrrole electrode materials. J Mater Sci 47(13):5317–5325. doi:10.1007/s10853-012-6418-y
Muller D, Rambo CR, Porto LM, Schreiner WH, Barra GM (2013) Structure and properties of polypyrrole/bacterial cellulose nanocomposites. Carbohydr Polym 94(1):655–662. doi:10.1016/j.carbpol.2013.01.041
Yuan L, Yao B, Hu B, Huo K, Chen W, Zhou J (2013) Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ Sci 6(2):470–476
Feng Y, Zhang X, Shen Y, Yoshino K, Feng W (2012) A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr Polym 87(1):644–649. doi:10.1016/j.carbpol.2011.08.039
Casado U, Quintanilla R, Aranguren M, Marcovich N (2012) Composite films based on shape memory polyurethanes and nanostructured polyaniline or cellulose–polyaniline particles. Synth Met 162(17):1654–1664
Kebiche H, Debarnot D, Merzouki A, Poncin-Epaillard F, Haddaoui N (2012) Relationship between ammonia sensing properties of polyaniline nanostructures and their deposition and synthesis methods. Anal Chim Acta 737:64–71
Shi Z, Zang S, Jiang F, Huang L, Lu D, Ma Y, Yang G (2012) In situ nano-assembly of bacterial cellulose–polyaniline composites. Rsc Adv 2(3):1040–1046
Hu W, Chen S, Yang Z, Liu L, Wang H (2011) Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. J Phys Chem B 115(26):8453–8457
Park M, Cheng J, Choi J, Kim J, Hyun J (2013) Electromagnetic nanocomposite of bacterial cellulose using magnetite nanoclusters and polyaniline. Colloids Surf B 102:238–242
Lin Z, Guan Z, Huang Z (2013) New bacterial cellulose/polyaniline nanocomposite film with one conductive side through constrained interfacial polymerization. Ind Eng Chem Res 52(8):2869–2874
Liu D, Sui G, Bhattacharyya D (2014) Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Compos Sci Technol 99:31–36
Wang H, Zhu E, Yang J, Zhou P, Sun D, Tang W (2012) Bacterial cellulose nanofiber-supported polyaniline nanocomposites with flake-shaped morphology as supercapacitor electrodes. J Phys Chem C 116(24):13013–13019
Lai F, Miao Y-E, Huang Y, Zhang Y, Liu T (2015) Nitrogen-doped carbon nanofiber/molybdenum disulfide nanocomposites derived from bacterial cellulose for high-efficiency electrocatalytic hydrogen evolution reaction. ACS Appl Mater Interfaces. doi:10.1021/acsami.5b06274
Xu D, Xiao X, Cai J, Zhou J, Zhang L (2015) Highly rate and cycling stable electrode materials constructed from polyaniline/cellulose nanoporous microspheres. J Mater Chem A 3(32):16424–16429
Khan S, Ul-Islam M, Khattak WA, Ullah MW, Park JK (2015) Bacterial cellulose–poly (3, 4-ethylenedioxythiophene)–poly (styrenesulfonate) composites for optoelectronic applications. Carbohydr Polym 127:86–93
Huang L, Chen K, Peng C, Gerhardt RA (2011) Highly conductive paper fabricated with multiwalled carbon nanotubes and poly (3,4-ethylenedioxythiophene)-poly (styrenesulfonate) by unidirectional drying. J Mater Sci 46(20):6648–6655. doi:10.1007/s10853-011-5617-2
Chen C, Yu Y, Li K, Zhao M, Liu L, Yang J, Liu J, Sun D (2015) Facile approach to the fabrication of 3D electroconductive nanofibers with controlled size and conductivity templated by bacterial cellulose. Cellulose 22(6):3929–3939
Chen C, Zhang T, Zhang Q, Feng Z, Zhu C, Yu Y, Li K, Zhao M, Yang J, Liu J (2015) Three-dimensional BC/PEDOT composite nanofibers with high performance for electrode-cell interface. ACS Appl Mater Interfaces. doi:10.1021/acsami.5b07273
Wang F, Jeon J-H, Park S, Kee C-D, Kim S-J, Oh I-K (2016) A soft biomolecule actuator based on a highly functionalized bacterial cellulose nano-fiber network with carboxylic acid groups. Soft Matter. doi:10.1039/c5sm00707k
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6(3):183–191
Castro Neto AH, Guinea F, Peres NMR, Novoselov KS, Geim AK (2009) The electronic properties of graphene. Rev Mod Phys 81(1):109–162. doi:10.1103/RevModPhys.81.109
Geim AK (2009) Graphene: status and prospects. Science 324(5934):1530–1534
Li K, Feng L, Shen J, Zhang Q, Liu Z, Lee ST, Liu J (2014) Patterned substrates of nano-graphene oxide mediating highly localized and efficient gene delivery. ACS Appl Mater Interfaces 6(8):5900–5907. doi:10.1021/am5008134
Valentini L, Bittolo Bon S, Fortunati E, Kenny JM (2013) Preparation of transparent and conductive cellulose nanocrystals/graphene nanoplatelets films. J Mater Sci 49(3):1009–1013. doi:10.1007/s10853-013-7776-9
Shao W, Liu H, Liu X, Wang S, Zhang R (2015) Anti-bacterial performances and biocompatibility of bacterial cellulose/graphene oxide composites. RSC Adv 5(7):4795–4803
Luo H, Xiong G, Yang Z, Raman SR, Si H, Wan Y (2014) A novel three-dimensional graphene/bacterial cellulose nanocomposite prepared by in situ biosynthesis. Rsc Adv 4(28):14369–14372
Liu Y, Zhou J, Zhu E, Tang J, Liu X, Tang W (2015) Facile synthesis of bacterial cellulose fibres covalently intercalated with graphene oxide by one-step cross-linking for robust supercapacitors. J Mater Chem C 3(5):1011–1017. doi:10.1039/C4TC01822B
Wang J, Feng M, Zhan H (2014) Preparation, characterization, and nonlinear optical properties of graphene oxide-carboxymethyl cellulose composite films. Opt Laser Technol 57:84–89
Muller D, Silva JP, Rambo C, Barra G, Dourado F, Gama F (2013) Neuronal cells’ behavior on polypyrrole coated bacterial nanocellulose three-dimensional (3D) scaffolds. J Biomater Sci Polym Ed 24(11):1368–1377
Liu Y, Zhou J, Tang J, Tang W (2015) Three-dimensional, chemically bonded polypyrrole/bacterial cellulose/graphene composites for high-performance supercapacitors. Chem Mater 27(20):7034–7041
Lota G, Fic K, Frackowiak E (2011) Carbon nanotubes and their composites in electrochemical applications. Energy Environ Sci 4(5):1592–1605
Kim B, Chung H, Kim W (2012) High-performance supercapacitors based on vertically aligned carbon nanotubes and nonaqueous electrolytes. Nanotechnology 23(15):155401
Park W-I, Kim H-S, Kwon S-M, Hong Y-H, Jin H-J (2009) Synthesis of bacterial celluloses in multiwalled carbon nanotube-dispersed medium. Carbohydr Polym 77(3):457–463
Yan Z, Chen S, Wang H, Wang B, Wang C, Jiang J (2008) Cellulose synthesized by Acetobacter xylinum in the presence of multi-walled carbon nanotubes. Carbohydr Res 343(1):73–80
Yan Z, Chen S, Wang H, Wang B, Jiang J (2008) Biosynthesis of bacterial cellulose/multi-walled carbon nanotubes in agitated culture. Carbohydr Polym 74(3):659–665
Yoon SH, Jin H-J, Kook M-C, Pyun YR (2006) Electrically conductive bacterial cellulose by incorporation of carbon nanotubes. Biomacromolecules 7(4):1280–1284
Farjana S, Toomadj F, Lundgren P, Sanz-Velasco A, Naboka O, Enoksson P (2013) Conductivity-dependent strain response of carbon nanotube treated bacterial nanocellulose. J Sens
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett 10(10):4025–4031
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Sci Mag 321(5889):651–652
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7(11):845–854
Kang YJ, Chun S-J, Lee S-S, Kim B-Y, Kim JH, Chung H, Lee S-Y, Kim W (2012) All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano 6(7):6400–6406
O-Rak K, Ummartyotin S, Sain M, Manuspiya H (2013) Covalently grafted carbon nanotube on bacterial cellulose composite for flexible touch screen application. Mater Lett 107:247–250
Kim YH, Park S, Won K, Kim HJ, Lee SH (2013) Bacterial cellulose–carbon nanotube composite as a biocompatible electrode for the direct electron transfer of glucose oxidase. J Chem Technol Biotechnol 88(6):1067–1070
Chen P, Kim H-S, Kwon S-M, Yun YS, Jin H-J (2009) Regenerated bacterial cellulose/multi-walled carbon nanotubes composite fibers prepared by wet-spinning. Curr Appl Phys 9(2):e96–e99
Chen P, Yun YS, Bak H, Cho SY, Jin H-J (2010) Multiwalled carbon nanotubes-embedded electrospun bacterial cellulose nanofibers. Mol Cryst Liq Cryst 519(1):169–178
Huang HX, Chen SX, Ce Yuan (2008) Platinum nanoparticles supported on activated carbon fiber as catalyst for methanol oxidation. J Power Sources 175(1):166–174
Yuan D, Huang X, Yan J, Yu W, Meng H, Rong J (2013) Porous carbon nanofibers derived from bacterial cellulose for sustainable energy storage. Sci Adv Mater 5(11):1694–1700
Liu Y, Qin W, Wang Q, Liu R, Liu H (2014) Glassy carbon nanofibers from electrospun cellulose nanofiber. J Mater Sci 50(2):563–569. doi:10.1007/s10853-014-8612-6
Wu Z-Y, Li C, Liang H-W, Zhang Y-N, Wang X, Chen J-F, Yu S-H (2014) Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci Rep. doi:10.1038/srep04079
Wu ZY, Li C, Liang HW, Chen JF, Yu SH (2013) Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew Chem 125(10):2997–3001
Dumanlı AG, Windle AH (2012) Carbon fibres from cellulosic precursors: a review. J Mater Sci 47(10):4236–4250. doi:10.1007/s10853-011-6081-8
Lee K-Y, Qian H, Tay FH, Blaker JJ, Kazarian SG, Bismarck A (2012) Bacterial cellulose as source for activated nanosized carbon for electric double layer capacitors. J Mater Sci 48(1):367–376. doi:10.1007/s10853-012-6754-y
Wu Z-Y, Liang H-W, Li C, Hu B-C, Xu X-X, Wang Q, Chen J-F, Yu S-H (2014) Dyeing bacterial cellulose pellicles for energetic heteroatom doped carbon nanofiber aerogels. Nano Res 7(12):1861–1872
Tong S, Zheng M, Lu Y, Lin Z, Zhang X, He P, Zhou H (2015) Binder-free carbonized bacterial cellulose-supported ruthenium nanoparticles for Li–O2 batteries. Chem Commun 51(34):7302–7304
Huang Y, Wang T, Ji M, Yang J, Zhu C, Sun D (2014) Simple preparation of carbonized bacterial cellulose–Pt composite as a high performance electrocatalyst for direct methanol fuel cells (DMFC). Mater Lett 128:93–96
Wang S, Liu K, Liu J, Yu ZT, Xu X, Zhao L, Lee T, Lee EK, Reiss J, Lee YK, Chung LW, Huang J, Rettig M, Seligson D, Duraiswamy KN, Shen CK, Tseng HR (2011) Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew Chem Int Ed Engl 50(13):3084–3088. doi:10.1002/anie.201005853
Huang Y, Zheng M, Lin Z, Zhao B, Zhang S, Yang J, Zhu C, Zhang H, Sun D, Shi Y (2015) Flexible cathodes and multifunctional interlayers based on carbonized bacterial cellulose for high-performance lithium–sulfur batteries. J Mater Chem A 3(20):10910–10918
Wang L, Schütz C, Salazar-Alvarez G, Titirici M-M (2014) Carbon aerogels from bacterial nanocellulose as anodes for lithium ion batteries. RSC Adv 4(34):17549–17554
Wan Y, Yang Z, Xiong G, Guo R, Liu Z, Luo H (2015) Anchoring Fe3O4 nanoparticles on three-dimensional carbon nanofibers toward flexible high-performance anodes for lithium-ion batteries. J Power Sources 294:414–419
Cruz-Silva E, Lopez-Urias F, Munoz-Sandoval E, Sumpter BG, Terrones H, Charlier J-C, Meunier V, Terrones M (2009) Electronic transport and mechanical properties of phosphorus-and phosphorus—nitrogen-doped carbon nanotubes. ACS Nano 3(7):1913–1921
Hu Z, Li S, Cheng P, Yu W, Li R, Shao X, Lin W, Yuan D (2015) N, P-co-doped carbon nanowires prepared from bacterial cellulose for supercapacitor. J Mater Sci 51(5):2627–2633. doi:10.1007/s10853-015-9576-x
Chen LF, Huang ZH, Liang HW, Guan QF, Yu SH (2013) Bacterial-cellulose-derived carbon nanofiber@ MnO2 and nitrogen-doped carbon nanofiber electrode materials: an asymmetric supercapacitor with high energy and power density. Adv Mater 25(34):4746–4752
Meng F, Li L, Wu Z, Zhong H, Li J, Yan J (2014) Facile preparation of N-doped carbon nanofiber aerogels from bacterial cellulose as an efficient oxygen reduction reaction electrocatalyst. Chin J Catal 35(6):877–883
Liang H-W, Wu Z-Y, Chen L-F, Li C, Yu S-H (2015) Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: an efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 11:366–376
Chen L-F, Zhang X-D, Liang H-W, Kong M, Guan Q-F, Chen P, Wu Z-Y, Yu S-H (2012) Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 6(8):7092–7102
Hulicova-Jurcakova D, Kodama M, Shiraishi S, Hatori H, Zhu ZH, Lu GQ (2009) Nitrogen-enriched nonporous carbon electrodes with extraordinary supercapacitance. Adv Funct Mater 19(11):1800–1809
Cao B, Zhang B, Jiang X, Zhang Y, Pan C (2011) Direct synthesis of high concentration N-doped coiled carbon nanofibers from amine flames and its electrochemical properties. J Power Sources 196(18):7868–7873
Lota G, Lota K, Frackowiak E (2007) Nanotubes based composites rich in nitrogen for supercapacitor application. Electrochem Commun 9(7):1828–1832
Chen L-F, Huang Z-H, Liang H-W, Yao W-T, Yu Z-Y, Yu S-H (2013) Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ Sci 6(11):3331–3338
Yu W, Lin W, Shao X, Hu Z, Li R, Yuan D (2014) High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J Power Sources 272:137–143. doi:10.1016/j.jpowsour.2014.08.064
Chen LF, Huang ZH, Liang HW, Gao HL, Yu SH (2014) Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv Funct Mater 24(32):5104–5111. doi:10.1002/adfm.201400590
Li S, Huang D, Zhang B, Xu X, Wang M, Yang G, Shen Y (2014) Flexible supercapacitors based on bacterial cellulose paper electrodes. Adv Energy Mater 4(10):1301655–1301661
Acknowledgements
This work was supported by “The Fundamental Research Funds for the Central Universities (No. 30920130121001),” “National Natural Science Foundation of China (No. 51272106) (No. 21206076),” “Natural Science Foundation of Jiangsu Province (No. BK2012401),” and “A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, China).” The authors are grateful to “Synergetic Research Center for Advanced Micro-Nano-Materials and Technology of Jiangsu Province.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao Chen and Fanshu Yuan have contributed equally.
Rights and permissions
About this article
Cite this article
Chen, X., Yuan, F., Zhang, H. et al. Recent approaches and future prospects of bacterial cellulose-based electroconductive materials. J Mater Sci 51, 5573–5588 (2016). https://doi.org/10.1007/s10853-016-9899-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-9899-2