Abstract
A facile non-covalent surface treatment method is reported in this paper to modify graphene oxide (GO) sheets with the assistance of polyvinylpyrrolidone (PVP). The PVP-modified GO (PGO) was further adopted to fabricate PGO/styrene–butadiene rubber (SBR) nano-composites through the latex compounding method. The properties of PGO were carefully investigated and interaction between GO and PVP molecules was confirmed. The mechanical properties, dynamic mechanical properties, thermal stability, thermal conductivity as well as swelling properties of the PGO/SBR nano-composites were thoroughly studied. It was confirmed that PVP molecules could have strong interaction with GO via hydrogen bond; thus, the PGO significantly improved the strength of SBR matrix, e.g., 517 and 387 % increase in tensile strength and tear strength, respectively, with the presence of only 5 phr (parts per hundred rubber) PGO in the nano-composite. The presence of PGO had also greatly reduced the glass transition temperature (T g) and enhanced the storage modulus of SBR matrix in the nano-composites. Meanwhile, the maximum heat decomposition temperature (T max) was increased by 23.6 °C; equilibrium solvent uptake in toluene was reduced by 41 % and thermal conductivity was increased by 30 %. All the observations indicated that PVP modification of GO can achieve excellent exfoliation and dispersion of GO in the SBR matrix. These findings were further supported by X-ray diffraction and scanning electron microscopy measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mechanical control of nano-materials and nano-system is a hot topic in advanced nanoscience [1]. The fabricated materials would work as nice platforms for such hot mechanical nanotechnology [2]. Rubbers with excellent elasticity and mechanical strength have been extensively used in industry. However, some rubbers might not have balanced overall properties, e.g., the mechanical properties and thermal conductivity might not be sufficient for certain applications. To improve these properties, novel nano-fillers with high thermal conductivity and excellent reinforcing efficiency are commonly added into rubbers [3], which is always a hot topic in both academic and industrial studies [4].
Among a variety of fillers, graphene oxide (GO) is one of the ideal reinforcing and functional fillers for rubber nano-composites, due to its ultra-high mechanical strength (e.g., facture stress of 63 GPa), large surface area, good barrier property, high aspect ratio, water solubility, and versatile surface characteristics [5, 6]. GO has been extensively studied for the application in polar rubbers, where it has good compatibility with polar rubbers because of the presence of a large number of polar groups, such as hydroxyl, carboxyl, and epoxy groups on the surface [5, 7–10]. For example, Li et al. [7] observed that the GO sheets were dispersed homogeneously in the acrylonitrile–butadiene rubber (NBR) matrix, and both the coefficient of friction and specific wear rate of the GO/NBR nano-composites increased in proportion with GO content. Tang et al. [8] realized that butadiene–styrene–pyridine rubber (VPR) could form hydrogen bond with GO, which leads to the 200 % improvement in tensile strength of the GO/VPR nano-composite with the addition of 1.5 vol% of GO, compared with virgin VPR matrix. Wang et al. [5] reported that GO could greatly improve the mechanical properties and thermal conductivity of carboxylated acrylonitrile–butadiene rubber (xNBR). Kang et al. [11] further found that GO could effectively improve the gas barrier properties of xNBR through the hydrogen bond between GO and xNBR. Wei et al. [12] prepared GO/fluoroelastomer (GO/FKM) nano-composites by solution processing, and the thermal, mechanical, and liquid barrier properties of FKM with the addition of GO sheets were efficiently enhanced through excellent dispersion techniques and strong GO–matrix bond.
However, for non-polar rubber, the dispersion of GO is much more difficult because of the weaker interfacial interaction between polar GO and non-polar rubber matrix [13, 14]. Furthermore, the weak interfacial interaction may cause a poor stress transfer in rubber matrix and low reinforcing efficiency of GO as a result [15]. Therefore, improvement of interfacial interaction between GO and non-polar rubber is still a critical and challenging issue remaining to be solved in order to obtain good dispersion of GO in the non-polar rubber matrix and achieve the desired performance.
Covalent and non-covalent surface modification of GO are two effective methods to improve the interfacial interaction between GO and non-polar rubbers and achieve good dispersion of GO in the matrix. Covalent modification approach includes the reaction of coupling agents [15, 16] and in situ polymerization [17]. This method has good surface modification effects; however, the process is complicated and even causes undesired permanent structure changes [18]. Such covalent modification processes may usually be conducted in organic solvents, which would not be environmental friendly [15, 19]. Therefore, a simple non-covalent modification method is always preferred, which typically takes advantage of van der Waals forces [20], electrostatic interaction [13, 21], hydrogen bond [22], etc., without altering the intrinsic properties of GO. Lian et al. [20] adopted cetyltrimethylammonium bromide (CTAB) to modify the surface of GO and fabricated the modified GO/butyl rubber (MGO/IIR) nano-composites by solution processing. The Young’s modulus of IIR nano-composite was greatly improved; however, the tensile strength of the MGO/IIR nano-composites was only about 0.8 MPa, which was too low to qualify for the requirement of inner tyre (about 8.4 MPa [23]) in particular applications. Mao et al. [21] applied VPR to modify GO, and the tear strength of the nano-composite, with the presence of only 2.0 vol% of modified GO, was increased by up to 208 % over that of virgin styrene–butadiene rubber (SBR). However, the elastic response of virgin SBR toward deformation was strongly influenced by the presence of modified GO sheets. Liu et al. [13] modified GO with alkylamine via electrostatic interaction, and the tensile strength of the modified GO/SBR nano-composite with 3 wt% of modified GO increased by 78 % compared to that of the unmodified GO/SBR nano-composite. However, the plasticizing effect of alkylamine molecules on the rubber network made it difficult to further improve tensile strength of the modified GO/SBR nano-composites. Therefore, it is still challenging to achieve balanced overall performances through proper modification of GO as the filler in the rubber matrix.
Polyvinylpyrrolidone (PVP), an amphipathic polymer, has been widely used in biological and chemical species [24]. It has been reported that hydrophilic GO can form hydrogen bond with PVP [22], which provided an effective way to change the surface properties of GO. This work opened up a new pathway to fabricate rubber nano-composites with well-dispersed GO in non-polar rubber through latex compounding. To the best of our knowledge, it has not been reported yet in the literature the fabrication of PVP-modified GO (PGO)/rubber nano-composites through latex compounding.
In this article, we report a novel, facile, and low-cost approach to prepare PGO to strengthen the SBR matrix, taking advantage of the hydrogen bond between GO and PVP. The PGO was incorporated into SBR latex by a latex compounding method. The mechanical properties, dynamic mechanical properties, thermal stability, thermal conductivity as well as swelling properties of the PGO/SBR nano-composites were studied and the results will be discussed in the following sections.
Experimental
Materials
SBR1712 latex (solid content: 23 wt%) was received from YPC-GPRO (Nanjing) Rubber Co. Ltd. (YGC). Natural graphite powder (average particle size ≤30 μm, purity ≥99.85 %), PVP (K30, \( \mathop {M_{\text{n}}}\limits^{\_\_} \) = 40,000), H2O2 (30 %), H2SO4 (98 %), HCl (37 %), NaCl, NaNO3, and KMnO4 with analytical grade were purchased from the Sinopharm Chemical Reagent Co. Ltd., China. Curing agents including stearic acid (SA), zinc oxide (ZnO), sulfur (S), and N-cyclohexyl-2-benzothiazole sulfenamide (CZ) with industry grade were provided by Nanjing Jinsanli Rubber Plastic Co. Ltd., China.
Preparation of PGO
GO aqueous suspension with a concentration of 1 mg mL−1 was prepared through 1-h ultrasonic treatment of graphite oxide according to Hummers’ method [25]. Then, PVP was added to the GO suspension with the ratio of 3:1. The PGO aqueous suspension was finally obtained after 2-h ultrasonic treatment. To facilitate the following characterization and tests, the PGO solid was separated from suspension through centrifugation at 10,000 rpm for 30 min, followed by washing with deionized water several times. After that, the PGO powder was obtained by drying in a vacuum oven at 60 °C.
Preparation of PGO/SBR nano-composites
The PGO powder was dispersed in water by ultrasonic treatment, and then a certain amount of PGO suspension was dropped into SBR latex with stirring for 30 min, followed by coagulation with adding 5 wt% NaCl solution. The solid formed by coagulation was isolated by filtration and washed with deionized water several times. It was further dried under vacuum at 60 °C until its weight remained constant. All the samples have the same curing agents, the addition of the curing agents was carried out in an LN-120 open two-roll mill (LINA machinery Industrial Co. Ltd., China) at room temperature according to Table 1. As control experiments, GO/SBR compounds were prepared by the same process in the absence of PVP. All the samples were cured at 160 °C up to their optimum cure time with 15 MPa.
Characterizations
Atomic force microscope (AFM) images were taken by a Nanoscope III D Multimode scanning probe microscope (Bruker Corporation, Switzerland) in a tapping mode. The AFM tip used in this work was a silicon tip (RTESP) with a resonant frequency of 300 kHz. The PGO dispersion was spin-coated onto a freshly exfoliated mica substrate at 2000 rpm and dried at room temperature. Fourier transform infrared (FTIR) spectroscopy was conducted in a transmission mode in KBr pellets at a scanning resolution of 4 cm−1 with a Shimazdu FTIR-8400S spectrometer. UV–Vis absorption spectra were recorded on a UV-6100S spectrophotometer (Shanghai Mapada Co. Ltd., China) with the scanning range from 200 to 400 nm at room temperature. Solid-state nuclear magnetic resonance (NMR) spectroscopy was performed with a Bruker Avance III NMR spectrometer (Bruker Corporation, Switzerland) operating at 400 and 100 MHz for 1H and 13C, respectively. The magic angle spin rate of the sample tube for the NMR measurement was 10 kHz. The spectra were recorded from a single-pulse experiment using high-power decoupling. The π/2 pulse width for 13C was 6 μs with a 3-μs width decoupling pulse for 1H. The number of scans for acquisition of spectra was 30 k, and the frequency resolution of NMR is not more than 0.005 Hz. Raman spectroscopy measurement was carried out at ambient temperature with an argon-ion laser Raman spectrometer at an excitation wavelength of 514.5 nm and a resolution of 1 cm−1 (Renishaw Invia-H31894). X-ray diffraction (XRD) patterns were recorded by a D8 Advanced X-ray diffractometer (Bruker Corporation, Switzerland) with Cu Kα radiation (λ = 0.154 nm) under a voltage of 40 kV and a current of 40 mA. Scanning was performed at a speed of 3° min−1, from 5° to 40°. The morphologies of the freeze-fractured surfaces of the rubber sample were observed using a JSM-6380LV scanning electron microscope (SEM) (JEOL Ltd., Japan). The tensile and tear tests were measured on a universal testing machine (Shenzhen SANS Co. Ltd., China) at a cross-head speed of 500 mm min−1 according to ASTM D-412 and ASTM D-624, respectively. The results were averaged based on five measurements. The storage modulus (E′) and dynamic loss factor (tanδ) were measured by dynamic mechanical analysis (DMA) Q800 (TA Co. Ltd., USA) under a nitrogen atmosphere at a heating rate of 3 °C min−1 from −60 to 40 °C and a tensile mode at 1 Hz. The results were averaged based on three measurements. Thermogravimetric analysis (TGA) studies were carried out in a Shimadzu differential thermogravimetric (DTG)-60 under a nitrogen atmosphere at a heating rate of 10 °C min−1 from 25 to 600 °C. A Netzsch laser flash analysis LFA447 was used to measure thermal diffusivity (α) and specific heat (C p) of the samples according to ASTM/E1461-2011. The thermal conductivity (λ) of the samples was further calculated as follows:
where ρ is the density of the sample. The results of thermal conductivity were averaged based on five measurements. The swelling properties of the samples were tested in toluene at room temperature according to ASTM D 471-06. The equilibrium solvent absorption constant (Q e) was calculated as follows [26]:
where w 1 is the mass of rubber nano-composites before swollen and w e is the mass of rubber nano-composites at swollen equilibrium with stable mass in analytical balance. M s is the molar mass of toluene (92.14 g mol−1).
Results and discussion
Characterizations of PGO
AFM was adopted to get information about the morphology of the produced PGO. Figure 1 shows a tapping-mode AFM image of PGO and its corresponding height profile. The results show that the thickness of PGO sheets is 3 nm, which is larger than that of GO (1.27 nm) [5]. It is attributed to the adsorption of PVP molecules on the surface of GO.
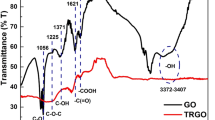
To shed light on the interaction between GO and PVP, FTIR was used to detect the molecular bonds’ details of the PVP, GO, and PGO, which is shown in Fig. 2. In the spectrum of GO, the broad peak at 3418 cm−1 results from the vibration of hydroxyl groups, while sharp peaks at 1719, 1628, 1393, 1225, and 1055 cm−1 can be attributed to the stretching vibration of C=O, stretching vibration of C=C, O–H deformation from C–OH, and stretching vibration of C–O from the C–OH and C–O–C, respectively [27]. For PVP, a band at about 3545 cm−1 is assigned to the O–H stretching, and two obvious peaks at 1670 and 1283 cm−1 are related to C=O and C–N, respectively [28]. In the spectrum of PGO, the curve is a combination of the curves of GO and PVP, indicating that PVP interacts with GO and successfully bounded on GO surface. This close interaction between PVP and GO may be due to two reasons. Firstly, PVP may interact with the structurally intact part of GO (graphene) through hydrophobic force [29]. Secondly, PVP may interact with the structural defects through hydrogen bonding [22], and by the following experiments (UV–Vis and NMR, etc.).
UV–Vis absorption spectra of PVP, GO, and PGO dispersions are illustrated in Fig. 3. For PVP, only a peak at 217 nm is shown, which corresponds to the π → π*(C=O) transitions [30]. For GO, characteristic peaks at 230 and 300 nm can be attributed to the π → π*(C–C) and n → π*(C=O) transitions, respectively [31]. The absorption curve of PGO and its multi-peaks Lorentz fitting results are shown in the inset of Fig. 3. It is shown that the characteristic peak of PVP molecules shifts from 217 to 208 nm, while the characteristic peak at 230 nm of GO shifts to 244 nm. It indicates that the electronic structure of GO changed which might be due to the formation of hydrogen bond between hydroxyl groups on the surface of GO and carbonyl group of PVP [32].
Solid-state 13C NMR spectroscopy has been applied to detect the interactions between two materials, according to the changes of chemical shifts of 13C NMR spectra. The 13C NMR spectra of PVP, GO, and PGO are presented in Fig. 4. In the 13C NMR spectrum of PVP, four peaks at 173.7, 40.0, 29.4, and 16.4 ppm are observed, corresponding to C*=O, C*–N, C*–C (O), and C*–C, respectively [33]. As for GO, peaks at 131.9, 69.4, and 59.3 ppm can be attributed to sp 2 carbons, C*–OH, and C*–O–C, respectively [34]. In the case of PGO, there is a broad peak at 115.4 ppm and four sharp peaks at 174.3, 39.5, 28.5, and 15.1 ppm. Compared with pure PVP, the peak of C=O in PGO spectrum shifts to low field for 0.6 ppm. It indicates the hydrogen bond between PVP and GO, which will lead to a lower field shift of PVP carbon atoms in 13C spectrum due to the homogenization of electron density [35]. However, the other signals at 40.0, 29.4, and 16.4 ppm shift to 39.5, 28.5, and 15.1 ppm, respectively. It may be due to the shielding effect of magnetic field induced by π electron circulation of graphitic structure in GO [36]. It also suggests that the PVP molecules have been adsorbed on the basal plane of GO instead of the sheet edge.
Raman spectra were used to investigate of interaction between PVP molecules and GO, and are shown in Fig. 5. The Lorentzian function is used to fit the curve of each Raman spectrum in order to improve the accuracy of the determination of Raman spectroscopic parameters. The detailed Raman spectroscopic parameters are summarized in Table 2. It can be seen that both the spectra of GO and PGO show two main bands at around 1600 (G-band) and 1350 cm−1 (D-band). The G-band corresponds to the C–C vibration of delocalized π electrons in graphitic structures on the surface of GO, and D-band corresponds to the carbon of sp 3 arising from the disorder and defects in GO surface [13]. It is worthy to point out that the position of the D-band of PGO shifts to lower wavenumber by 9 cm−1 (from 1357 to 1348 cm−1), while the G-band of PGO almost remains unchanged. The wavenumber shift also points to the possible strong hydrogen bond between the PVP molecules and GO [37].
It is interesting to observe that the intensity (peak area) of the G-band of PGO spectrum decreases, while the ratio of D-band and G-band intensity (I D/I G) increases. The intensity contributed from amorphous component in PGO spectrum decreases as well. These observations indicate the increase of interstitial disorder in graphitic structure, which can be determined by L a:
As expected, the PGO shows the lower microcrystalline planar size, as indicated by a lower L a value, compared with that of GO. This suggests that the adsorption of PVP on the GO graphitic crystals through hydrogen bond can separate the graphitic crystals, which causes the decrease of L a. A sketch of the hydrogen bond between GO and PVP is shown in Fig. 6.
Dispersion of PGO in SBR matrix
It is known that the exfoliation and/or dispersion of the GO in polymer matrix and the interfacial interaction between GO and polymer have great impacts on the properties of GO/polymer nano-composites [15]. Thus, the exfoliation of GO in SBR is detected by XRD. XRD patterns of SBR, GO5, and PGO5 are chosen as examples to study the GO exfoliation and dispersion (Fig. 7a). Because of the amorphous phase of SBR, all SBR nano-composites show a broad diffraction peak, except that there is a small diffraction peak at 2θ = 26.7° in the XRD pattern of GO5. It corresponds to a 0.35 nm interlayer (002) space of GO, which indicates the existence of ordered GO structure in SBR matrix. However, no diffraction peaks are observed at 2θ = 26.7° in PGO5, which clearly indicates no ordered structure in the PGO/SBR nano-composite. It is safe to conclude that the GO is fully exfoliated into single-sheet or few-sheet stacks in SBR matrix once the GO is treated by PVP.
The dispersion of fillers in rubber matrix is further characterized by SEM. The SEM images of freeze-fractured surfaces of SBR, GO5, and PGO5 are shown in Fig. 7b–d. In contrast with the smooth texture of the fractured surfaces of virgin SBR (Fig. 7b), the fractured surface of GO5 shows a wrinkled and folded texture (Fig. 7c white ellipse), suggesting the aggregation of GO in SBR matrix and extraction of GO from SBR matrix due to poor adhesion between the filler and matrix. In the case of PGO5 (Fig. 7d), however, the fracture surface is coarser than that of GO5, and there is no stacked texture on the surface, which agrees well with the conclusion based on XRD study. In addition, the interface between PGO and SBR is ambiguous, and there is no obvious pulled out of GO in fractured surface because GO sheets are deeply embedded in the SBR matrix. It is due to the good compatibility between the PGO sheets and SBR matrix. All the results indicate that PVP modification of GO can achieve excellent exfoliation and dispersion of GO in PGO5.
Mechanical properties
The stress–strain curves of all samples are shown in Fig. 8. The effects of PGO content on the tensile modulus at 100 % elongation (M 100), tensile modulus at 300 % elongation (M 300), and elongation at break of SBR nano-composites are shown in Fig. 9. It is worthy to mention that both GO and PGO are able to improve the strength of SBR matrix. Compared with GO, PGO exhibits more pronounced reinforcing effect for SBR matrix. For GO5, compared with those of virgin SBR, the M 100 and M 300 are increased by 82 and 308 %, respectively. After modification, the M 100 and M 300 of PGO5 are increased by 221 and 386 %, respectively. However, the changes in elongation at break of PGO/SBR and GO/SBR nano-composites with the filler content are almost similar.
The tensile strength and tear strength of SBR, GO/SBR, and PGO/SBR nano-composites are shown in Fig. 10. The tensile strength of GO5 is increased by 332 % compared with that of virgin SBR. It is due to the efficient distribution of external force through the orientation of SBR chains on the surface of GO with a slippage [8]. However, after modification, the PGO5 shows a greater reinforcement on SBR with a significant increase of tensile strength by 517 %. The improved reinforcement of PGO may be due to the greater uniform distribution of PGO in SBR and better interfacial interaction between PGO and SBR, in which energy dissipation and load transfer between PGO and SBR matrix are more effective. It is clear that tear strength exhibits a trend similar to that of tensile strength. Both the PGO/SBR and GO/SBR nano-composites demonstrate higher tear strength than that of virgin SBR, and PGO provides a higher tear strength than that of the GO system.
Generally, filler with surface modification will improve the properties of polymer more effectively [38]. However, different kinds of modification lead to different impacts on the final properties of polymer. In Mao’s research [16], GO was covalently modified by bis(triethoxysilylpropyl)tetrasulfide (Si69), 3-aminopropyltrimethoxysilane (KH550), and the combination of Si69 and KH550, respectively. The modified GO/SBR nano-composites were prepared by a complicated method, which combined latex compounding and spray drying. The tensile strength of three SBR nano-composites increased by only 7.6, 15, and 39 % with adding 5 phr modified GO, respectively, with respect to the unmodified GO/SBR nano-composite. However, in the present study, the GO interacts with PVP via hydrogen bond, and the tensile strength of PGO5 fabricated through a simple method increases 43 %, compared with that of GO5.
Dynamic mechanical properties
The dynamic mechanical properties of GO5 and PGO5 are shown in Fig. 11. As shown in Fig. 11a, the storage modulus (E′) value of GO5, which measures the stiffness and load-bearing capability of the nano-composites, shows a significant improvement in the rubbery region compared with virgin SBR. It can be ascribed to the enormous difference between the modulus of the SBR matrix and the reinforcing phase to generate a significant reinforcing effect [5]. The E′ value of PGO5 shows a further increase in the rubbery region compared to unmodified GO5, owing to the strong interfacial interaction between SBR molecules and PGO sheets [39].
Glass transition temperature (T g) of GO5 and PGO5 is determined from the peak of the tan δ–temperature curve in Fig. 11b. In comparison with virgin SBR, the T g of GO5 is slightly shifted to lower temperature, because of the decrease in the fraction of polymer in the composite [40]. The T g of PGO5 has almost no change compared to that of GO5. It should be noticed that the peak intensity of PGO5 decreases compared with GO5, which reflects that PGO has immobilized SBR chains near the polymer–particle interface effectively due to the stronger interfacial interaction [13].
Thermogravimetric analysis
The TGA and DTG curves of SBR, GO/SBR, and PGO/SBR nano-composites are shown in Fig. 12. The results of TGA are summarized in Table 3. Because of the low decomposition temperature of GO [41], T 5 of GO/SBR nano-composites decreases with the increase of GO, while T 50 and T max increase. As for PGO/SBR nano-composites, T 5 decreases due to the decomposition of the organic molecules on the surface of GO [20]; however, the increase of T 50 and T max is profound. TGA and DTG curves of SBR, GO5, and PGO5 are chosen as examples. Compared with virgin SBR, the increase in T 50 and T max of GO5 is 7.9 and 14.9 °C, respectively. It is due to the physical barrier effect of GO, which can form a jammed network of layers that slows down the escape of decomposition products and subsequently delays further degradation [42]. For PGO5, T 50 and T max increase by 13.4 and 23.6 °C with respect to virgin SBR, respectively. In the previous study [20], CTAB-modified GO (5 phr) can improve the T max of IIR by only 6 °C. It may due to the improved dispersion of PGO, which leads to the increased decomposition temperature.
Thermal conductivity
The results of the thermal conductivity for all nano-composites are shown in Fig. 13. It can be found that the thermal conductivity of PGO/SBR nano-composites is far superior to that of the corresponding GO/SBR nano-composites. For example, compared with that of virgin SBR, the thermal conductivity of PGO5 is increased by 30 %; however, for GO5, there is only a 17 % increment. Generally, the phonon scattering or acoustic impedance mismatch at the interface between thermally conductive particles and polymeric matrix should be reduced, in order to have good thermal transport properties in thermally conductive polymers [43, 44]. PGO can be effectively exfoliated into a few layers and forms a great interfacial adhesion between rubber chains, thus reducing the acoustic impedance mismatch [43]. As a result, the thermal conductivity of PGO/SBR nano-composites is improved compared to that of GO/SBR nano-composites.
Swelling properties
Solvent sorption curves of SBR, GO/SBR, and PGO/SBR nano-composites are shown in Fig. 14. It can be seen that the addition of both GO and PGO can suppress swelling. However, when the filler content is increased further, Q e of GO/SBR nano-composites tends to increase. This may be attributed to the aggregation of GO at high filler loadings. It is worthy to note that the Q e of PGO/SBR nano-composites is decreased more obviously compared to that of GO/SBR nano-composites, and maintains a downward trend at high filler loadings. For example, the Q e of PGO5 is decreased by 41 and 30 % compared to those of virgin SBR and GO5, respectively. A perfect dispersion of PGO in SBR matrix may be responsible for this phenomenon [18].
Conclusions
In summary, this study has shown that non-covalent treatment of GO with PVP can be successfully performed through a simplified polymeric adsorption method. PVP molecules can interact with GO via strong hydrogen bond. Modified GO with PVP introduced a polarity matching for strong SBR–filler interaction and a better compatibility between the filler and matrix. PGO was able to enhance the dispersion of GO in SBR matrix due to the additional rubber–filler interaction. Therefore, it successfully acted as a reinforcement agent and an elasticity improver. When the loading of PGO was 5 phr, tensile strength and tear strength of PGO/SBR nano-composite were significantly enhanced by 517 and 387 %, respectively, compared with those of virgin SBR. Enhanced platelet dispersion of PGO provided tortuous path that enhanced blend resistance toward solvent penetration and improved the thermal conductivity. The thermal conductivity of PGO/SBR nano-composite with adding 5 phr PGO showed a 30 % increment and the equilibrium solvent uptake decreased by 41 % as well, compared with those of virgin SBR. In addition, the PGO had significantly enhanced T max of SBR matrix.
References
Ariga K, Mori T, Hill JP (2012) Mechanical control of nanomaterials and nanosystems. Adv Mater 24:158–176
Ariga K, Li J, Fei J, Ji Q, Hill JP (2016) Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv Mater 28:1251–1286
Wang J, Jia H, Zhang J, Ding L, Huang Y, Sun D, Gong X (2014) Bacterial cellulose whisker as a reinforcing filler for carboxylated acrylonitrile-butadiene rubber. J Mater Sci 49:6093–6101. doi:10.1007/s10853-014-8336-7
Rahmat M, Hubert P (2011) Carbon nanotube–polymer interactions in nanocomposites: a review. Compos Sci Technol 72:72–84
Wang J, Jia H, Tang Y, Ji D, Sun Y, Gong X, Ding L (2013) Enhancements of the mechanical properties and thermal conductivity of carboxylated acrylonitrile butadiene rubber with the addition of graphene oxide. J Mater Sci 48:1571–1577. doi:10.1007/s10853-012-6913-1
Potts JR, Shankar O, Murali S, Du L, Ruoff RS (2013) Latex and two-roll mill processing of thermally-exfoliated graphite oxide/natural rubber nanocomposites. Compos Sci Technol 74:166–172
Li Y, Wang Q, Wang T, Pan G (2012) Preparation and tribological properties of graphene oxide/nitrile rubber nanocomposites. J Mater Sci 47:730–738. doi:10.1007/s10853-011-5846-4
Tang Z, Wu X, Guo B, Zhang L, Jia D (2012) Preparation of butadiene–styrene–vinyl pyridine rubber–graphene oxide hybrids through co-coagulation process and in situ interface tailoring. J Mater Chem 22:7492–7501
Chouhan DK, Rath SK, Kumar A, Alegaonkar P, Kumar S, Harikrishnan G, Patro TU (2015) Structure-reinforcement correlation and chain dynamics in graphene oxide and laponite-filled epoxy nanocomposites. J Mater Sci 50:7458–7472. doi:10.1007/s10853-015-9305-5
Silva LC, Silva GG, Ajayan PM, Soares BG (2015) Long-term behavior of epoxy/graphene-based composites determined by dynamic mechanical analysis. J Mater Sci 50:6407–6419. doi:10.1007/s10853-015-9193-8
Kang H, Zuo K, Wang Z, Zhang L, Liu L, Guo B (2014) Using a green method to develop graphene oxide/elastomers nanocomposites with combination of high barrier and mechanical performance. Compos Sci Technol 92:1–8
Wei J, Jacob S, Qiu J (2014) Graphene oxide-integrated high-temperature durable fluoroelastomer for petroleum oil sealing. Compos Sci Technol 92:126–133
Liu X, Kuang W, Guo B (2015) Preparation of rubber/graphene oxide composites with in situ interfacial design. Polymer 56:553–562
Kim H, Kobayashi S, AbdurRahim MA, Zhang MJ, Khusainova A, Hillmyer MA, Abdala AA, Macosko CW (2011) Graphene/polyethylene nanocomposites: effect of polyethylene functionalization and blending methods. Polymer 52:1837–1846
Wu J, Huang G, Li H, Wu S, Liu Y, Zheng J (2013) Enhanced mechanical and gas barrier properties of rubber nanocomposites with surface functionalized graphene oxide at low content. Polymer 54:1930–1937
Mao Y, Zhang S, Zhang D, Chan TW, Liu L (2014) Enhancing graphene oxide reinforcing potential in composites by combined latex compounding and spray drying. Mater Res Express 1:025009
Zhao XW, Zang CG, Wen YQ, Jiao QJ (2015) Thermal and mechanical properties of liquid silicone rubber composites filled with functionalized graphene oxide. J Appl Polym Sci 132:42582
Razak JA, Ahmad SH, Ratnam CT, Mahamood MA, Mohamad N (2015) Effects of poly (ethyleneimine) adsorption on graphene nanoplatelets to the properties of NR/EPDM rubber blend nanocomposites. J Mater Sci 50:6365–6381. doi:10.1007/s10853-015-9188-5
Yang H, Li F, Shan C, Han D, Zhang Q, Niu L, Ivaska A (2009) Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J Mater Chem 19:4632–4638
Lian H, Li S, Liu K, Xu L, Wang K, Guo W (2011) Study on modified graphene/butyl rubber nanocomposites. I. Preparation and characterization. Polym Eng Sci 51:2254–2260
Mao Y, Wen S, Chen Y, Zhang F, Panine P, Chan TW, Zhang L, Liang Y, Liu L (2013) High performance graphene oxide based rubber composites. Sci Rep 3:2508
Chang X, Wang Z, Quan S, Xu Y, Jiang Z, Shao L (2014) Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly (vinylylidenefluoride)(PVDF) ultrafiltration membrane performance. Appl Surf Sci 316:537–548
Zhang X, Zhang J (2005) Studies on the fundamental properties of butyl rubber. Petrochem Ind Technol 12:1–4
Lin J, Chen D, Dong J, Chen G (2015) Preparation of polyvinylpyrrolidone-decorated hydrophilic graphene via in situ ball milling. J Mater Sci 50:8057–8063. doi:10.1007/s10853-015-9373-6
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Maria HJ, Lyczko N, Nzihou A, Mathew C, George SC, Joseph K, Thomas S (2013) Transport of organic solvents through natural rubber/nitrile rubber/organically modified montmorillonite nanocomposites. J Mater Sci 48:5373–5386. doi:10.1007/s10853-013-7332-7
Barkauskas J, Dakševič J, Juškėnas R, Mažeikienė R, Niaura G, Račiukaitis G, Selskis A, Stankevičienė I, Trusovas R (2012) Nanocomposite films and coatings produced by interaction between graphite oxide and Congo red. J Mater Sci 47:5852–5860. doi:10.1007/s10853-012-6485-0
Saroj A, Singh R, Chandra S (2013) Studies on polymer electrolyte poly (vinyl) pyrrolidone (PVP) complexed with ionic liquid: effect of complexation on thermal stability, conductivity and relaxation behaviour. Mater Sci Eng, B 178:231–238
Bourlinos AB, Georgakilas V, Zboril R, Steriotis TA, Stubos AK, Trapalis C (2009) Aqueous-phase exfoliation of graphite in the presence of polyvinylpyrrolidone for the production of water-soluble graphenes. Solid State Commun 149:2172–2176
Abdelghany A, Mekhail MS, Abdelrazek E, Aboud M (2015) Combined DFT/FTIR structural studies of monodispersed PVP/Gold and silver nano particles. J Alloys Compd 646:326–332
Xing W, Tang M, Wu J, Huang G, Li H, Lei Z, Fu X, Li H (2014) Multifunctional properties of graphene/rubber nanocomposites fabricated by a modified latex compounding method. Compos Sci Technol 99:67–74
Mehrdad A, Niknam Z (2015) Spectroscopic and viscometric studies on the interaction of ionic liquid, 1-butyl-3-methylimidazolium bromide with polyvinyl pyrrolidone. Fluid Phase Equilib 391:72–77
Zhao C, Xu X, Chen J, Yang F (2014) Optimization of preparation conditions of poly (vinylidene fluoride)/graphene oxide microfiltration membranes by the Taguchi experimental design. Desalination 334:17–22
Fan X, Peng W, Li Y, Li X, Wang S, Zhang G, Zhang F (2008) Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv Mater 20:4490–4493
Oh T-J, Nam J-H, Jung YM (2009) Molecular miscible blend of poly (2-cyano-1, 4-phenyleneterephthalamide) and polyvinylpyrrolidone characterized by two-dimensional correlation FTIR and solid state 13 C NMR spectroscopy. Vib Spectrosc 51:15–21
Liu X, Pan X, Shen W, Ren P, Han X, Bao X (2012) NMR study of preferential endohedral adsorption of methanol in multiwalled carbon nanotubes. J Phys Chem C 116:7803–7809
Fini A, Cavallari C, Ospitali F (2008) Raman and thermal analysis of indomethacin/PVP solid dispersion enteric microparticles. Eur J Pharm Biopharm 70:409–420
Li X, Deng H, Li Z, Xiu H, Qi X, Zhang Q, Wang K, Chen F, Fu Q (2015) Graphene/thermoplastic polyurethane nanocomposites: surface modification of graphene through oxidation, polyvinyl pyrrolidone coating and reduction. Compos Part A 68:264–275
Das A, Kasaliwal GR, Jurk R, Boldt R, Fischer D, Stöckelhuber KW, Heinrich G (2012) Rubber composites based on graphene nanoplatelets, expanded graphite, carbon nanotubes and their combination: a comparative study. Compos Sci Technol 72:1961–1967
Matos CF, Galembeck F, Zarbin AJ (2014) Multifunctional and environmentally friendly nanocomposites between natural rubber and graphene or graphene oxide. Carbon 78:469–479
Zheng D, Tang G, Zhang H-B, Yu Z-Z, Yavari F, Koratkar N, Lim S-H, Lee M-W (2012) In situ thermal reduction of graphene oxide for high electrical conductivity and low percolation threshold in polyamide 6 nanocomposites. Compos Sci Technol 72:284–289
Potts JR, Dreyer DR, Bielawski CW, Ruoff RS (2011) Graphene-based polymer nanocomposites. Polymer 52:5–25
Hu Y, Shen J, Li N, Ma H, Shi M, Yan B, Huang W, Wang W, Ye M (2010) Comparison of the thermal properties between composites reinforced by raw and amino-functionalized carbon materials. Compos Sci Technol 70:2176–2182
Kim H, Abdala AA, Macosko CW (2010) Graphene/polymer nanocomposites. Macromolecules 43:6515–6530
Acknowledgements
This work was financially supported by Jiangsu Province Key Project (BE2015158), Priority Academic Program Development of Jiangsu Higher Education Institutions and Scientific Foundation of Nanjing Institute of Technology (Project No. YKJ201508).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, B., Wang, J., Jia, H. et al. Enhanced mechanical properties and thermal conductivity of styrene–butadiene rubber reinforced with polyvinylpyrrolidone-modified graphene oxide. J Mater Sci 51, 5724–5737 (2016). https://doi.org/10.1007/s10853-016-9874-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-9874-y