Abstract
Graphene is a potential reinforcing material for polymeric materials due to high aspect ratio, surface area and electrical and mechanical properties. In this work, thermally reduced graphene oxide (TRGO)/acrylonitrile butadiene styrene (ABS) composites were developed using combined solution mixing and melt mixing techniques. The effect of wt% of pristine graphite and TRGO on the mechanical and thermal properties of composites was studied. Graphene oxide (GO) was prepared from graphite powder using improved Hummers’ method followed by thermal reduction to obtain TRGO. Characterization of GO, TRGO and as-developed ABS composites was performed using Fourier transmission infrared spectroscopy, scanning electron microscopy, atomic force microscopy, differential scanning calorimetry and thermogravimetric analysis. Tensile properties were determined by testing injection-molded dumbbell-shaped samples. The results showed that tensile properties of TRGO/ABS composites increased significantly at 0.2 wt% loading compared to corresponding graphite/ABS composites. However, increased content of both fillers decreased mechanical properties of the composites. TRGO, at 0.2 wt% loading, increased glass transition temperature of ABS by ca.7 °C. TRGO neither increased nor decreased thermal stability of ABS composites. This study showed that combined solution and melt mixing technique can significantly improve dispersion of TRGO in ABS matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Acrylonitrile butadiene styrene (ABS) is a widely utilized thermoplastic polymer due to its vital properties such as admirable mechanical properties, chemical resistance and recyclability. Being a terpolymer of three monomers, acrylonitrile, butadiene and styrene, it is very valuable among other polymeric materials. It can be readily processed using solution mixing and melt mixing methods [1]. The specific properties of ABS could be further enhanced by incorporation of fillers, and this could boost its demand [2]. These polymer composites are extensively employed in various industrial sectors such as automotive, aerospace and construction [3].
Carbon-based reinforcements, i.e., carbon black (CB), expanded graphite (EG), carbon nanotube (CNT), graphene, graphene oxide (GO) and thermally reduced graphene oxide (TRGO), are potential candidates for improving mechanical, electrical and thermal properties of the polymeric materials [4,5,6,7]. Nowadays, two-dimensional materials have attained much attention due to high aspect ratio and layered structure which give better dispersion in polymeric matrices.
Graphene is one of the two-dimensional materials with one-atom-thick planar sheet of sp2-bonded carbon atoms densely packed in a honeycomb crystal lattice [8]. Graphene, GO, TRGO or graphene nanoplatelets are considered to be superior fillers than other conventional carbon fillers (CNT, CB, EG, etc.) due to their inherent properties such as high surface area, good mechanical strength, excellent thermal conductivity, electrical conductivity, electromagnetic interference shielding and low coefficient of thermal expansion [9,10,11].
Few studies have been reported on graphene-reinforced ABS polymer composites. Pour et al. [12] developed graphene nanoplatelets (GNPs)-reinforced polycarbonate (PC)/ABS nanocomposites by melt blending using a twin-screw extruder. Commercial GNPs with a thickness of 5–10 nm and an average length of 15 µm were used to produce composites at loadings of 1, 3 and 5 wt%. The authors reported 30 and 54% increases in Young’s modulus and flexural modulus, respectively, at 3 wt% loading attributed to better dispersion of GNPs and some interaction between GNPs and matrix. However, they found a reduction in impact strength of nanocomposites. In another study, Wang et al. [13] investigated electrical conductivities and mechanical properties of ABS/ethylene propylene diene terpolymer (EPDM) composites filled with graphene filler developed using melt mixing and solution mixing methods. They reported better properties of melt-mixed composites up to 8% loading of graphene compared to dry and wet premixing composites. Waheed et al. [14] evaluated mechanical properties of few-layered graphene and multiwalled carbon nanotubes (MWCNTs) in ABS matrix produced by solution mixing. They reported a 43% increase in tensile strength of the composites produced with a few-layered graphene and a 22% increase for the composites produced with MWCNTs at similar loading. Panwar et al. [15] developed ABS matrix composites by incorporating GO, TRGO and chemically reduced graphene oxide (CRGO) using melt mixing route. They reported about 28, 20 and 80% increments in tensile strength at 2 wt% loading of GO, TRGO and CRGO, respectively, over neat ABS. All three fillers gave approximately 12% improvement in storage modulus. The authors also found about 8 to 11 °C increase in glass transition temperature (Tg) of ABS with the incorporation of graphene fillers.
It is important to find that whether the impact of GO on the properties of polymers is better than that of graphite or not. Before GO, graphite was incorporated in polymers due to its low cost, thermal stability and good electrical and thermal conductivities. It was also incorporated in ABS polymer to improve its properties. For example, Uhl et al. [16] reported that the incorporation of graphite nanofiller in ABS and polystyrene composites slightly enhanced tensile strength (7–10%) of ABS/graphite composites. In another study, Pandey et al. [17] reported a very contradictory result as they mentioned that tensile strength of the ABS was damaged with the addition of graphite flakes; however, they found an improvement in the thermal stability of the ABS. Comparison of properties of graphite-filled ABS and graphene-filled ABS composites reported in the literature is presented in Table 1. It can be concluded on the basis of Table 1 that graphene could be better filler for ABS than graphite.
Composite production method is a key for better properties of composites as it would be helpful in achieving a uniform dispersion and distribution of filler in the polymer matrix. Further, development of better interfacial bonding between the filler and polymer matrix not only depends on effectiveness of coupling agent but also depends on good dispersion and distribution of the filler, which themselves depend on the processing techniques. An industrially important technique for the dispersion of fillers in thermoplastics is melt blending or melt mixing. In the melt mixing, polymer is melted and combined with the desired amount of the filler materials by using twin-screw extruders. After mixing of fillers, the filler/thermoplastic blends can be injection molded to fabricate a desired product. On the other hand, an important technique for academic studies is a solution mixing which is based on a solvent system in which the polymer or pre-polymer is soluble and the nanofillers may swell (for layered nanofillers). Solvent selection is critical for solution mixing technique. A solvent selected should not only be able to dissolve polymer but it should also be suitable for acquiring good dispersion of the filler. When both polymer and fillers are dispersed effectively in the solvent, the resulting dispersion can be casted to make membranes or films after evaporation of solvent. The resulting composite would have very well-dispersed fillers in the polymer, which can be further processed by melt mixing technique to produce desired products. Solution mixing method besides offering improved dispersion of filler in the polymer matrix creates environmental issues due to solvent evaporation, recovery and toxicity of solvent. Contrary to solution mixing, melt mixing is environmentally friendly technique but the dispersion of filler by melt mixing is somewhat compromised and largely dependent upon the sophistication and advancement of the extruders [19,20,21]. ABS-based composites are developed mainly by melt mixing technique due to thermoplastic nature of ABS [22]. Melt mixing route is feasible for large-scale production and therefore is of great interest for the industry. Incorporation of nanofillers in polymers via melt mixing route is very difficult due to high viscosity of molten polymers, low mixing speeds of commonly available extruders and longer mixing times required which could degrade polymers. All these factors are major hindrances in obtaining well-dispersed fillers in polymer matrices [23].
Direct mixing of graphene in ABS matrix by an extruder is not possible as few-layered graphene sheets are obtained by ultrasonication in solvents, and these solvent-dispersed GO fillers cannot be directly added into the extruder [14, 24]. For this purpose, solvent has to be removed first, but during this process graphene fillers tend to agglomerate or form GO paper and utilizing of GO like this cannot give a true benefit in polymer.
Therefore, this study aims to develop ABS/TRGO composites by a technique in which combined solution mixing and melt mixing approach was used. Briefly, TRGO was dispersed in acetone and ABS was separately dissolved in acetone. These two solutions were first mixed together to disperse TRGO in ABS by solution mixing process. The TRGO/ABS composite sheet was then obtained by removing solvents. The TRGO/ABS sheet was cut into flakes which was melt mixed using an extruder to draw composites in the form of wires. Pristine graphite composites were also produced following the same route to explore the true potential of TRGO in improving the mechanical properties of ABS.
2 Experimental
2.1 Materials and Reagents
Powder graphite, a product of Asbury Graphite Mills, USA, having a particle size of 10 μm was used for GO production. All chemicals, including sulfuric acid (H2SO4), phosphoric acid (H3PO4), potassium permanganate (KMnO4), hydrochloric acid (HCl) and acetone, used were of analytical grade and purchased from Sigma-Aldrich, USA. ABS used for this study was obtained from Nexeo Plastics (USA).
2.2 Production of GO and TRGO
Graphite oxide was synthesized by improved Hummers’ method from graphite powder without using NaNO3 [25]. Details of the procedure can be found in our previous papers [26, 27]. Briefly, graphite powder was treated in a mixture of H3PO4/H2SO4 (1:9) containing oxidizing agent KMnO4 under continuous mechanical stirring. Then, the treated product was filtered and washed with HCl followed by washing with deionized (DI) water several times to achieve neutral pH. The residue left is called graphite oxide which was used to produce TRGO.
Graphite oxide produced via improved Hummers’ method was first thermally exfoliated in a microwave oven [28] and then sonicated in ethanol to produce TRGO. When the brownish product, graphite oxide, of improved Hummers’ process was exfoliated in a microwave oven, it turned into a blackish product, and the reaction was completed with a spark in the microwave oven. The process not only exfoliated graphite oxide but also reduced it to some extent. The thermally exfoliated graphite oxide (TRGO) powder was dispersed in acetone to obtain isolated sheets.
2.3 Development of Composites
Polymeric composites for this study were developed by combined solution and melt mixing, the details of which are given in the following subsections.
2.3.1 Solution Mixing
First of all, a homogeneous suspension of TRGO in acetone was made by ultrasonication in an ultrasonic bath (Elmasonic-E30H with ultrasonic power of 240 W) until no residual material was seen. Similarly, ABS was dissolved in the acetone at 50 °C with the aid of ultrasonication. Afterward, TRGO and ABS solutions in acetone were mixed by continuous mechanical stirring for 4 h at 30 °C. Further, solution mixing of the ABS/TRGO was carried out in a ball mill for 6 h using zirconia balls (diameters = 1, 2 and 3 mm having a ratio of 60:20:20, respectively). As-obtained homogeneous dispersion of ABS/TRGO was dried in a vacuum oven at 45 °C for 24 h to completely remove acetone. The product obtained was in the form of sheet.
2.3.2 Melt Mixing
To achieve better dispersion, dried product, ABS/TRGO, in the form of sheets was cut into smaller pieces and passed from the custom-made extruder at 190 °C and speed of 200 rev/min. The product was passed three times through an extruder to achieve better dispersion and to remove any air trapped. Similar procedures were adopted for ABS/graphite composites and neat ABS for comparison. The compositions of the developed composites in this study are presented in Table 2.
2.4 Characterization
The thickness of GO and TRGO sheets was measured by using a semi-contact mode atomic force microscope (AFM) (Nano-Solver, NT-MDT). The presence of functional groups on the surface of GO and TRGO was analyzed using Fourier transform infrared spectroscopy (FTIR) using ATR mode (Agilent Cary 630). Morphology and dispersion of graphite and TRGO in ABS matrix were investigated by analyzing the freeze-fractured samples using a scanning electron microscope (SEM) (FEI, Inspect S50). Au–Pd alloy sputter-coated samples were analyzed by SEM. Thermal stability was studied using thermogravimetric analysis (TGA) (SDT_Q600, TA Instruments). TGA was conducted on 10 mg of cuboidal samples of composites and neat ABS in the temperature range of 30 to 600 °C at the rate of 10 °C/min under the flow of nitrogen gas at 10 ml/min. Differential scanning calorimetry (DSC) (Q 200, TA Instruments) analysis was conducted to investigate the effect of filler on the glass transition temperature. For this purpose, a 10-mg sample was heated in the temperature range of 30 to 600 °C at the rate of 10 °C/min under the flow of nitrogen gas at 10 ml/min at constant pressure. For mechanical testing, dumbbell-shaped samples were prepared by injecting various ABS composites melt at 150 °C into a steel die using an injection molding machine. The tensile samples were made according to BS 2782-3 (overall length 75 mm, gauge length 20 mm, width within gauge length 4 mm and thickness 2 mm). Before tensile testing, cast samples were annealed at 100 °C for 8 h in the oven to reduce temperature effect that occurred during injection molding. Tensile properties (fracture strength, ultimate tensile strength (UTS), percentage elongation, modulus and toughness) reported were averaged by testing at least three specimens of each composition using a tensometer (Monsanto Tensometer, UK) with a crosshead speed of ca. 2 mm/min.
3 Results and Discussion
FTIR analysis of GO and TRGO is shown in Fig. 1. FTIR spectra of GO have clear peaks at 3372 cm−1, 1726 cm−1 and 1621 cm−1 which correspond to –OH, –COOH and –C(=O) oxide functional groups [29, 30]. These functional groups are reduced or decomposed by thermal reduction of GO as shown by FTIR spectrum of TRGO. FTIR analysis (Fig. 1) showed that GO was successfully reduced by thermal reduction. A reduction of functional groups resulted in higher thermal stability of TRGO as validated by TGA analysis (Fig. 3).
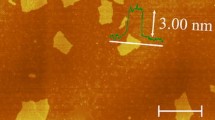
Figure 2 shows a semi-contact mode AFM images of GO and TRGO with height profiles. It is clear from height profile that the average thickness of GO sheets is 1 nm with 300-nm lateral dimensions, while the AFM image of TRGO shows single layer and agglomerated sheets. The height profile of agglomerated sheets of TRGO shows the average thickness of 2 nm with lateral dimensions of 250 nm. AFM image of TRGO clearly shows the successful exfoliation of GO.
TGA curves of TRGO and GO presented in Fig. 3 show higher thermal stability of TRGO than GO up to 600 °C. TRGO shows 23% residual weight at 600 °C, whereas GO almost completely decomposed at this temperature. Higher mass loss of GO than TRGO is attributed to decomposition of functional groups which were excessively available on GO. Similar trend was previously observed in the case of GO and TRGO by Shen et al. [28]. Because of this fact, TRGO was used to produce composites as it will be more hydrophobic in nature due to lack of functional groups [31], and this character would be useful to achieve good dispersion of TRGO in ABS.
TGA/DTG curves of neat ABS, graphite/ABS and TRGO/ABS composites are shown in Fig. 4a, and TGA/DTG data are presented in Table 3. TGA curves show that a significant weight loss of neat ABS took place in the range of 380–440 °C due to breakdown of the ABS chains. A similar behavior was reported for pure ABS by Panwar et al. [15].
The addition of fillers in the ABS matrix has no significant effect on the decomposition temperature. Graphite/ABS composites showed some improvement in the decomposition temperature of ABS in agreement with Pandey et al. [17]. The thermal stability, high thermal conductivity and no functional groups presence could be the reasons for better thermal stability of graphite/ABS composites. On the other hand, TRGO/ABS composites at loadings of 0.2 and 1 wt% showed a slight reduction in the degradation temperature of ABS. The main cause for this reduced thermal stability of ABS in the presence of TRGO was the presence of few remaining functional groups on the surface of TRGO which starts to decompose at a relatively lower temperature [12]. DTG showed that there is a slight change in point of inflection of neat ABS and ABS composites at various loadings. It showed that thermal stability of composites is not much affected by the addition of nanofillers.
DSC curves of neat ABS, graphite/ABS and TRGO/ABS composites are shown in Fig. 4b, and the values of glass transition temperature (Tg) are presented in Table 3. TRGO/ABS composites at loading of 0.2 wt% enhanced the glass transition temperature of neat ABS by ~ 7 °C, which means that the good dispersion of graphene sheets in the ABS matrix restricted movement of the segments of polymeric chains [32]. On the other hand, the higher content (1 wt%) of TRGO showed a very small effect on ‘Tg’ which could be due to some agglomerates as shown in SEM (Fig. 7d). In the case of graphite/ABS composites, both compositions have improved ‘Tg’ of the neat ABS.
The representative tensile stress–strain curves of various composites are presented in Fig. 5. The tensile properties of neat ABS, graphite/ABS and TRGO/ABS composites are presented in Table 4, and comparison is shown in Fig. 6. Both graphite/ABS and TRGO/ABS composites showed a noticeable improvement in tensile strength and elastic modulus. However, TRGO demonstrated much improvement in tensile properties than graphite. TRGO at loading of 0.2 wt% increased tensile strength and elastic modulus of ABS by 106 and 61%, respectively. On the other hand, at similar loading of graphite the tensile strength and elastic modulus of ABS increased by 83 and 32%, respectively. At 1 wt% loading of both TRGO and graphite, an increase in tensile properties of ABS was lower compared to that obtained at 0.2 wt% loading. The tensile strength and modulus of 1 wt% TRGO/ABS composites were found to be 77 and 32%, respectively, higher than those of neat ABS. At similar loading, graphite composites (1 wt% graphite/ABS) showed 62 and 44% increases in tensile strength and modulus, respectively, over neat ABS. For both types of composites, percentage elongation was not significantly changed compared to neat ABS.
Previous research groups [12, 14, 15], as presented in Table 1, used either melt mixing or solution mixing for development of graphene/ABS composites. Unlike findings of Pour et al. [12], we found a 50% increase in tensile strength at about 15 times lower loading of TRGO. The higher tensile strength of our composites achieved at significantly lower loading of filler is attributed to much thinner and well-dispersed TRGO. Similarly, our 0.2 wt% TRGO/ABS composites demonstrated 78% higher tensile strength than corresponding composites reported by Panwar et al. [15] who dispersed graphene in ABS by melt blending. This suggests that melt mixing was not able to provide good degree of dispersion of graphene in ABS. On the other hand, our findings are in good agreement with Waheed et al.’s [14] findings who determined a 43% increase in the tensile strength of ABS composite at 0.2 wt% loading of few-layered graphene dispersed by solution mixing, which is in agreement with our findings, confirming the fact that solution mixing plays an important role in achieving better dispersion of graphene in thermoplastic matrix.
The tensile strength and modulus of composites produced with TRGO and graphite showed a decreasing trend with increased loading. The incorporation of 0.2 wt% TRGO in ABS matrix increased the tensile strength of ABS by 2 × which is significantly higher than previously reported by Pour et al. [9] and Panwar et al. [15] for graphene/ABS composites produced at higher filler loadings (3 wt% and 2 wt% loadings, respectively). The possible reason for lower tensile strength at higher loading is non-homogenous dispersion of the filler, resulting in stress concentration sites due to agglomeration [33]. Due to the combined effect of the solution and melt mixing, the strength of graphite/ABS and TRGO/ABS composites at higher loading was still higher than that of neat ABS. Furthermore, lower tensile strength of graphite/ABS composites might also be due to the presence of agglomerate as shown in Fig. 7b.
Figure 6b presents the elastic modulus of neat ABS, graphite/ABS and TRGO/ABS composites. Composites (graphite/ABS and TRGO/ABS) showed increased elastic modulus than the neat ABS polymer. Similar to tensile strength of TRGO/ABS composites, modulus of elasticity of composite produced at low loading of 0.2 wt% was higher than that of the composite produced at higher loading of 1 wt%. Again, this behavior could be attributed to agglomeration which reduced the stiffness of the composites. TRGO having higher surface area could easily agglomerate at higher loading content [2].
Conversely, graphite/ABS composites showed an increase in modulus with increased loading. This finding contradicts with that of Pandey et al. [17] who reported that modulus of graphite/ABS composite decreased with an increase of wt % of graphite. Further, for both types of composites (graphite/ABS and TRGO/ABS) small increment in the elongation (Fig. 6c) suggests that the carbon reinforcement has not hindered movement of the polymeric chains. The results of mechanical and thermal analysis of composites suggest that lower concentration of the TRGO is favorable in improving the mechanical properties of ABS.
SEM images of composites and TRGO sheets are presented in Fig. 7. It can be observed in the case of graphite/ABS composites that agglomerates increased with an increase in graphite loading (Fig. 7a, b). Further, the fractured surface of graphite/ABS composites showed brittle fracture of composites which could be due to weak interfacial bonding between the pristine graphite and ABS matrix [17]. On the other hand, TRGO/ABS composites have uniform dispersion of graphene sheets with fewer agglomerates as shown in Fig. 7c, d. Nevertheless, dimple-like fracture was seen for almost all samples, which is an evidence of the ductile fracture [12]. Furthermore, the composites showed no pores which were removed due to multiple passes through a single-screw extruder. So the use of combined solution and melt mixing leads to better dispersion of nanofiller and no pores in composites. This might also be the reason for better mechanical properties of ABS polymeric composites. Figure 7e, f shows SEM images of TRGO. SEM micrographs show crumples and sheet-like structure of TRGO which is due to thermal exfoliation.
4 Conclusions
-
TRGO increased tensile strength of ABS by 106% at 0.2 wt% loading. Higher loading of TRGO (1 wt%) reduced tensile strength of ABS; perhaps, at this loading TRGO tends to agglomerate as observed by SEM analysis.
-
TRGO at 0.2 wt% loading produced ~ 61% increment in elastic modulus compared to neat ABS. Graphite at similar loading increased elastic modulus of ABS by 30%. The higher stiffness of TRGO-based composite is attributed to good dispersion of TRGO in ABS and better interaction with ABS matrix due to higher surface area.
-
Solution mixing and sonication prior to melt mixing enabled improved dispersion of TRGO in ABS matrix, which resulted in improved mechanical properties of composites.
-
TRGO neither improved nor damaged the thermal stability of ABS appreciably at 0.2 wt% loading. However, TRGO composite at 1 wt% loading showed slightly lower thermal stability than ABS due to decomposition of remnant functional groups on TRGO.
-
This study shows that TRGO can improve mechanical properties of ABS, but only at very low loading (0.2 wt%).
References
Brydson, J.A.: Plastics Materials. Butterworth-Heinemann, Oxford (1999)
Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S.: Graphene-based composite materials. Nature 442(7100), 282–286 (2006)
Kar, K.K.; Rana, S.; Pandey, J.: Handbook of Polymer Nanocomposites Processing, Performance and Application. Springer, New York (2015)
Bouhfid, R.; Arrakhiz, F.; Qaiss, A.: Effect of graphene nanosheets on the mechanical, electrical, and rheological properties of polyamide 6/acrylonitrile–butadiene–styrene blends. Polym. Compos. 37(4), 998–1006 (2016)
Jayanth, N.; Senthil, P.: Application of 3D printed ABS based conductive carbon black composite sensor in void fraction measurement. Compos. B Eng. 159, 224–230 (2019)
Mura, A.; Adamo, F.; Wang, H.; Leong, W.S.; Ji, X.; Kong, J.: Investigation about tribological behavior of ABS and PC-ABS polymers coated with graphene. Tribol. Int. 134, 335–340 (2019)
Raza, M.A.; Mujadid, M.; Hussain, M.; Ali, H.Q.; Rehman, Z.U.; Inam, A.: Mechanical properties of graphene oxide coated-glass fiber reinforced unsaturated polyester composites. Mater. Res. Express 6(11), 115303 (2019)
Allen, M.J.; Tung, V.C.; Kaner, R.B.: Honeycomb carbon: a review of graphene. Chem. Rev. 110(1), 132–145 (2009)
Bunch, J.S.; Van Der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L.: Electromechanical resonators from graphene sheets. Science 315(5811), 490–493 (2007)
Eda, G.; Chhowalla, M.: Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv. Mater. 22(22), 2392–2415 (2010)
Raza, M.A.; Westwood, A.; Brown, A.; Hondow, N.; Stirling, C.: Characterisation of graphite nanoplatelets and the physical properties of graphite nanoplatelet/silicone composites for thermal interface applications. Carbon 49(13), 4269–4279 (2011)
Pour, R.H.; Hassan, A.; Soheilmoghaddam, M.; Bidsorkhi, H.C.: Mechanical, thermal, and morphological properties of graphene reinforced polycarbonate/acrylonitrile butadiene styrene nanocomposites. Polym. Compos. 37(6), 1633–1640 (2016)
Wang, F.; Zhang, Y.; Zhang, B.; Hong, R.; Kumar, M.; Xie, C.: Enhanced electrical conductivity and mechanical properties of ABS/EPDM composites filled with graphene. Compos. B Eng. 83, 66–74 (2015)
Waheed, Q.; Khan, A.N.; Jan, R.: Investigating the reinforcement effect of few layer graphene and multi-walled carbon nanotubes in acrylonitrile-butadiene-styrene. Polymer 97, 496–503 (2016)
Panwar, V.; Pal, K.: An optimal reduction technique for rGO/ABS composites having high-end dynamic properties based on Cole–Cole plot, degree of entanglement and C-factor. Compos. B Eng. 114, 46–57 (2017)
Uhl, F.M.; Yao, Q.; Wilkie, C.A.: Formation of nanocomposites of styrene and its copolymers using graphite as the nanomaterial. Polym. Adv. Technol. 16(7), 533–540 (2005)
Pandey, A.K.; Kumar, R.; Kachhavah, V.S.; Kar, K.K.: Mechanical and thermal behaviours of graphite flake-reinforced acrylonitrile–butadiene–styrene composites and their correlation with entanglement density, adhesion, reinforcement and C factor. RSC Adv. 6(56), 50559–50571 (2016)
Difallah, B.B.; Kharrat, M.; Dammak, M.; Monteil, G.: Mechanical and tribological response of ABS polymer matrix filled with graphite powder. Mater. Des. 34, 782–787 (2012)
Moniruzzaman, M.; Winey, K.I.: Polymer nanocomposites containing carbon nanotubes. Macromolecules 39(16), 5194–5205 (2006)
Dennis, H.; Hunter, D.; Chang, D.; Kim, S.; White, J.; Cho, J.; Paul, D.R.: Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 42(23), 9513–9522 (2001)
Ercan, N.; Durmus, A.; Kaşgöz, A.: Comparing of melt blending and solution mixing methods on the physical properties of thermoplastic polyurethane/organoclay nanocomposite films. J. Thermoplast. Compos. Mater. 30(7), 950–970 (2017)
Caradonna, A.; Colucci, G.; Giorcelli, M.; Frache, A.; Badini, C.: Thermal behavior of thermoplastic polymer nanocomposites containing graphene nanoplatelets. J. Appl. Polym. Sci. 134, 20 (2017)
Burk, L.; Gliem, M.; Lais, F.; Nutz, F.; Retsch, M.; Mülhaupt, R.: Mechanochemically carboxylated multilayer graphene for carbon/ABS composites with improved thermal conductivity. Polymers 10(10), 1088 (2018)
Dul, S.; Fambri, L.; Pegoretti, A.: Fused deposition modelling with ABS–graphene nanocomposites. Compos. A Appl. Sci. Manuf. 85, 181–191 (2016)
Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M.: Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010)
Raza, M.A.; Rehman, Z.U.; Ghauri, F.A.; Ahmad, A.; Ahmad, R.; Raffi, M.: Corrosion study of electrophoretically deposited graphene oxide coatings on copper metal. Thin Solid Films 620, 150–159 (2016)
Ghauri, F.A.; Raza, M.A.; Baig, M.S.; Ibrahim, S.: Corrosion study of the graphene oxide and reduced graphene oxide-based epoxy coatings. Mater. Res. Express 4, 125601 (2017)
Shen, J.; Li, T.; Long, Y.; Shi, M.; Li, N.; Ye, M.: One-step solid state preparation of reduced graphene oxide. Carbon 50(6), 2134–2140 (2012)
Nekahi, A.; Marashi, P.; Haghshenas, D.: Transparent conductive thin film of ultra large reduced graphene oxide monolayers. Appl. Surf. Sci. 295, 59–65 (2014)
Maqsood, M.F.; Raza, M.A.; Ghauri, F.A.; et al.: Corrosion study of graphene oxide coatings on AZ31B magnesium alloy. J. Coat. Technol. Res. (2020). https://doi.org/10.1007/s11998-020-00350-3
Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S.: The chemistry of graphene oxide. Chem. Soc. Rev. 39(1), 228–240 (2010)
Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q.: Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 69, 467–480 (2014)
Ghaleb, Z.; Mariatti, M.; Ariff, Z.: Graphene nanoparticle dispersion in epoxy thin film composites for electronic applications: effect on tensile, electrical and thermal properties. J. Mater. Sci. Mater. Electron. 28(1), 808–817 (2017)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raza, M.A., Maqsood, M.F., Rehman, Z.U. et al. Thermally Reduced Graphene Oxide-Reinforced Acrylonitrile Butadiene Styrene Composites Developed by Combined Solution and Melt Mixing Method. Arab J Sci Eng 45, 9559–9568 (2020). https://doi.org/10.1007/s13369-020-04845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04845-4