Abstract
The Fe2SSe particles dispersed in the pores of carbon (Fe2SSe/PC) were prepared using a simple one-pot solid-state method, which were then characterized by XRD, SEM, TEM, XPS, and Raman spectrum techniques. As the anode material for lithium-ion batteries, Fe2SSe/PC displays an initial discharge capacity as high as 699.5 mAh/g at 0.1 C, and 327.9 mAh/g can be maintained after 200 cycles, much enhanced than those of pure Fe2SSe. The small particles of Fe2SSe wrapped in carbon can effectively buffer the volume expansion during charging/discharging to improve the electrochemical performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) are playing a more and more significant role in energy storage along with the rapid development and requirement of high-power current-consuming equipments [1–8]. As an important component of LIBs, the anode material makes great contribution to its capacity and influences the whole electrochemical performance of LIBs. The commercial graphite has been widely used as anode materials for LIBs because of its excellent cycling stability [9–11]. However, its low theoretical capacity (372 mAh/g) cannot meet the requirement of LIBs with high energy density and power density. So, it is continuously meaningful to explore novel anode materials.
As anode material for LIBs, ferrous sulfide (Fe S) has attracted much attention to be extensively studied [12–18] because of its high theoretical capacity (609 mAh/g) and the low cost of iron. However, the cycling stability of FeS is poor due to the large volume expansion during charge and discharge. Relatively, ferrous selenide (FeSe, theoretical capacity:397 mAh/g) has a better cycling stability and higher conductivity than FeS [19–22]. So, a double-anions compound, Fe2SSe, was designed and prepared. Fe2SSe combines the advantages of FeS and FeSe, and it has a higher theoretical capacity (480 mAh/g) and operating voltage (ca. 1.4 V vs. Li+/Li) than that of graphite (below 0.2 V vs. Li+/Li). J. B. Liu et al. prepared Fe2SSe using ball-milling method followed by sintering, and the as-prepared Fe2SSe could deliver an initial discharge capacity of 471 and 397.2 mAh/g could be remained after 100 cycles at 0.1 C [23]. So far, this is the only study of Fe2SSe as anode material for LIBs. Differently, one-pot solid-state method was employed to prepare Fe2SSe/PC composite here, which can be simply described as the synthesis of Fe2SSe and PAN carbonization finished at the same time in one reactive system. This method should be introduced to prepare other types of metal chalcogenides/carbon composites. It is necessary to investigate Fe2SSe much more to get better understanding and try to enhance its electrochemical performance.
In our work, the Fe2SSe particles wrapped in carbon using a simple one-pot solid-state method, namely, Fe2SSe/PC, exhibit a first discharge capacity of 699.5 mAh/g at 0.1 C and 327.9 mAh/g can be left after 200 cycles.
Experimental
Preparations and material characterizations
The Fe2SSe/PC was prepared by heating Fe (99 %, aladdin), Se (99.9 %, aladdin), S (99.95 %, aladdin), and PAN (M w 150000, Sigma-Aldrich) mixed with the molar ratios of 3:2 for (Fe, Se, S):PAN and 2:1:1 for Fe:Se:S in an evacuated quartz tube. The quartz tube was firstly heated from room temperature to 400 °C with the speed of 0.5 °C/min and homogenized at 400 °C for 5 h. Then the temperature was heated at 900 °C with the same heating rate and maintained for 10 h. Finally, the black sample was obtained through natural cooling to room temperature. In addition, pure Fe2SSe and carbonized PAN were prepared using the same method.
Powder X-ray diffraction (PXRD, Bruker D8 Advance) analysis was performed at 40 kV and 100 mA for Cu-Kα radiation (λ = 1.5406 Å) with a scan speed of 5 °/min at room temperature. Energy-dispersive X-ray spectroscopy (EDS, Bruker, Quantax) was used to analysis the element content. Energy-dispersive X-ray spectroscopy element mapping was employed to show the distributions of Fe, S, Se, and C in Fe2SSe/PC. Scanning electron microscopy (SEM, Supra 55 Sapphire) and transmission electron microscopy (TEM, Philips Tecnai12) were used to observe the surface morphology and size. Raman spectrum (Renishaw in via) and X-ray photoelectron spectroscopy (XPS, Thermofisher Scientific, ESCALAB250Xi) were used to analyze the chemical structures and chemical composition, respectively.
Electrochemical measurements
The electrodes were composed of 80 wt% active material (Fe2SSe/PC or Fe2SSe), 10 wt% carbon black, and 10 wt% polyvinylidene fluoride (PVDF), respectively. The slurry was prepared by stirring the mixture in a certain amount of N-methyl-2-pyrrolidinone (NMP), then coated the slurry onto a copper foil. After dried at 120 °C for 12 h in a vacuum oven, the foil was cut into disks with diameter of 1.6 cm.
The CR-2032-type coin cells were assembled using a Li foil as the counter electrode and a Celgard 2325 film as the separator in a glovebox (VAC-Omni 102283) filled with argon, where oxygen and water contents were less than 1 ppm. The electrolyte was 1 M LiPF6 in 1:1 DEC/EC. Cyclic voltammetry (C–V) measurements were carried out on an electrochemical workstation (CHI660D) in 1.0–3.5 V with a scan rate of 0.1 mV/s. Electrochemical impedance spectroscopies (EIS) were measured over a frequency range of 0.01 Hz–100 kHz. The galvanostatic charge/discharge tests were carried out at 0.1 C (48 mAh/g) in the voltage range of 1.0–3.5 V using NEWARE CT-3008 battery charge–discharge system. The charge–discharge data were recorded after the first discharge to 1.0 V.
Results and discussion
Structure and morphology characterization
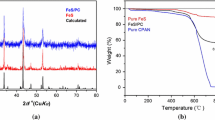
The content of Fe2SSe in Fe2SSe/PC was determined by heating the sample in the air and calculating the weight of Fe2O3, which is about 88 %.
As seen in Fig. 1, the main PXRD peaks of Fe2SSe/PC and pure Fe2SSe were located at 29.8°, 33.04°, 42.6°, 51.9°, 55.56°, 62.92°, and 69.52°, corresponding to the calculated ones, indicating the obtained samples were pure Fe2SSe/PC and Fe2SSe. Energy-dispersive X-ray spectroscopy elemental analysis of Fe2SSe/PC shows that C, Fe, S, and Se exist and the ratio of Fe, Se, and S is around 2:1:1 (Fig. 2a). The EDS element mapping analysis (Fig. 2b) of Fe2SSe/PC indicates that Fe, S, Se, and N are evenly distributed on the surface of carbon [24–27].
Figure 3 shows the Raman spectrum of Fe2SSe/PC. Two peaks appear at 1348.79 and 1589.62 cm−1, which are caused by the effects of defects (D) and graphitization (G), respectively [27]. Furthermore, the intensity of D band is much higher than that of G band, indicating that there are some defects and interspaces on the surface of the carbon.
Figure 4 shows the X-ray photoelectron spectroscopy (XPS) spectrum which is used to analyze the chemical composition and valence of Fe2SSe/PC. The smooth C 1s spectrum (Fig. 4a) shows four peaks at 284.78, 284.80, 285.88, and 287.28 eV, indicating that there are a large number of C=C, C–C, C–N, and C=N bonds, respectively [28]. The Fe 2p (Fig. 4b) spectrum has two strong peaks at 710.78 and 724.98 eV, which are consistent with those of Fe2+. Furthermore, the characteristic peaks of S2− appear at 161.78 and 163.18 eV from the S 2p spectrum (Fig. 4c). The Se 3d (Fig. 4d) spectrum shows two peaks at 54.08 and 55.88 eV, which approve that Se is in form of negative bivalent in the composite [21, 29]. All these XPS peaks indicate that the as-prepared sample is really Fe2SSe and carbon with abundant single and double bonds, corresponding to the results of PXRD, EDS, and Raman spectrum.
Figure 5 shows the SEM images of Fe2SSe/PC, pure CPAN (carbon form the carbonization of PAN), and pure Fe2SSe. Compared with pure Fe2SSe, the Fe2SSe particles in Fe2SSe/PC (Fig. 5c) have much smaller sizes with around hundreds of nanometers, which are partially inserted to the pores on the surface of CPAN. It can be observed that the Fe2SSe particles in Fe2SSe/PC have smaller sizes than those of pure ones, so the specific surface area of Fe2SSe in Fe2SSe/PC is larger than that of pure Fe2SSe, which can expand the area of effective interaction between the active materials and electrolyte. What is more, smaller size is beneficial to shorten the Li+ diffusion route and enhances the kinetics of charge carrier transport [14]. The TEM image (Fig. 5d) further shows that the Fe2SSe particles are wrapped in the carbon, which is well consistent with the results of the SEM images.
Electrochemical performance
The redox reaction occurred on the electrode was studied using cyclic voltammetry (C–V) measurement. Figure 6a, b show the test results for pure Fe2SSe and Fe2SSe/PC for the first three cycles, respectively. Both of the C–V curves are similar. For pure Fe2SSe (Fig. 6a), a reduction peak is located at 1.36 V on the first loop, which could be described as follows [23]:
The broad oxidation peak appearing at 1.93 V indicates that Fe2SSe phase formed again. After the first cycle, the location of the reduction peak was at 1.43 V and stable. The oxidation peaks were located at 1.93 V all the time, which can be explained using the following reaction [23, 30].
Electrochemical impedance spectroscopies of pure Fe2SSe and Fe2SSe/PC were investigated to know more about the interface performance of electrode materials in Fig. 7. Both of the impedance curves consist of a semicircle set in high frequency and a straight line of low-frequency region, which reflect the charge transfer resistance at the interface of the electrode and the electrolyte, and ion diffusion resistance in the electrolyte, respectively. It is obvious that the radius of the semicircle for the Fe2SSe/PC is much smaller than that of pure Fe2SSe in the high frequency, indicating a lower resistance. This as well proves that CPAN plays an important role for the enhancement of Fe2SSe/PC’s electrical conductivity.
The cyclic performance of the electrode material for pure Fe2SSe (Fig. 8a) and Fe2SSe/PC (Fig. 8b) was evaluated between 1.0 and 3.5 V at 0.1 C. Obviously, the initial discharge capacity of pure Fe2SSe was only 395.3 mAh/g. On the contrary, Fe2SSe/PC electrode prepared at the same conditions could deliver about two times initial capacity of 699.5 mAh/g than that of pure Fe2SSe. The capacity enhancement is most probably ascribed to stabilized PAN matrix, which can effectively prevent active material loss and bear the volume changes during Li+ insertion/extraction process [31]. Besides, Fe2SSe/PC could still remain the capacity of 327.9 mAh/g after 200 cycles, which is much higher than the capacity of 102.3 mAh/g for the pure Fe2SSe electrode (Fig. 8c). Furthermore, Fe2SSe/PC displays a good Coulomb efficiency, which is maintained above 99 % during the charge and discharge for 200 cycles.
The rate capabilities of Fe2SSe/PC and pure Fe2SSe were also performed (Fig. 8d). Obviously, the specific capacities were decreased along with the increase of the discharge/charge rates from 0.1 to 2 C. The third cycle discharge capacities of Fe2SSe/PC reach around 643.8, 560.5, 466.9, 360.6, and 277.6 mAh/g at 0.1, 0.2, 0.5, 1 and 2 C, respectively. The discharge capacity of 423.7 mAh/g can be still left when the current density comes back to 0.1 C, indicating that the Fe2SSe/PC has a better reversibility and rate capacity than that of pure Fe2SSe. These results reveal that the incorporation of CPAN into pure Fe2SSe can effectively enhance its electrochemical performance.
Conclusions
The Fe2SSe/PC was successfully prepared using a simple one-pot solid-state method. It exhibits an initial discharge capacity of 699.5 mAh/g at 0.1 C and 327.9 mAh/g can be still maintained after 200 cycles when it is used as the anode material for LIBs. In our opinions, this method can be also used to prepare other M 2SSe@PC (M: transition metal) composites.
References
Liu X, Zhang K, Lei KX, Li FJ, Tao ZL, Chen J (2016) Facile synthesis and electrochemical sodium storage of CoS2 micro/nano-structures. Nano Res 9:198–206
Douglas A, Carter R, Oakes L, Share K, Cohn AP, Pint CL (2015) Ultrafine iron pyrite (FeS2) nanocrystals improve sodium-sulfur and lithium–sulfur conversion reactions for efficient batteries. ACS Nano 9:11156–11165
Zeng ZY, Zhang XW, Bustillo K, Niu KY, Gammer C, Xu J, Zheng HM (2015) In situ study of lithiation and delithiation of MoS2 nanosheets using electrochemical liquid cell transmission electron microscopy. Nano Lett 15:5214–5220
Xiong FY, Cai ZY, Qu LB, Zhang PF, Yuan ZF, Asare OK, Xu WW, Lin C, Mai LQ (2015) Three-dimensional crumpled reduced graphene oxide/MoS2 nanoflowers: a stable anode for lithium-ion batteries. ACS Appl Mater 7:12625–12630
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Jiang H, Ge YQ, Fu K, Lu Y, Chen C, Zhu JD, Dirican M, Zhang XW (2015) Centrifugally-spun tin-containing carbon nanofibers as anode material for lithium-ion batteries. J Mater Sci 50:1094–1102. doi:10.1007/s10853-014-8666-5
Xia L, Qiu KH, Gao YY, He X, Zhou FD (2015) High potential performance of Cerium-doped LiNi0. 5Co0. 2Mn0. 3O2 cathode material for Li-ion battery. J Mater Sci 50:2914–2920. doi:10.1007/s10853-015-8856-9
Zhang PG, Zhang CY, Xie AJ, Li C, Song JM, Shen YH (2016) Novel template-free synthesis of hollow@porous TiO2 superior anode materials for lithium ion battery. J Mater Sci 51:3448–3453. doi:10.1007/s10853-015-9662-0.pdf
Deng TS, Zhou XP (2016) Porous graphite prepared by molybdenum oxide catalyzed gasification as anode material for lithium ion batteries. Mater Lett 176:151–154
Wu GH, Li RY, Liu JK, Gu ZG, Wang GL (2015) N-doped graphene/graphite composite as a conductive agent-free anode material for lithium ion batteries with greatly enhanced electrochemical performance. Electrochim Acta 171:156–164
Ji JY, Liu JL, Lai LF, Zhao X, Zhen YD, Lin JY, Zhu YW, Ji HX, Zhang LL, Ruoff RS (2015) In situ activation of nitrogen-doped graphene anchored on graphite foam for a high-capacity anode. ACS Nano 9:8609–8616
Zhao L, Yu XQ, Yu JZ, Zhou YG, Ehrlich SN, Hu YS, Su D, Li H, Yang XQ, Chen LQ (2014) Remarkably improved electrode performance of bulk MnS by forming a solid solution with FeS-understanding the Li storage mechanism. Adv Funct Mater 24:5557–5566
Guo SP, Li CX, Chi Y, Ma Z, Xue HG (2016) Novel 3-D network SeS x /NCPAN composites prepared by one-pot in situ solid-state method and its electrochemical performance as cathode material for lithium-ion battery. J Alloys Compd 664:92–98
Xu C, Zeng Y, Rui XH, Xiao N, Zhu JX, Zhang WY, Chen J, Liu WL, Tan HT, Hng HH, Yan QY (2012) Controlled soft-template synthesis of ultrathin C@FeS nanosheets with high-Li-storage performance. ACS Nano 6:4713–4721
Fei L, Williams BP, Yoo SH, Carlin JM, Joo YL (2016) A general approach to fabricate free-standing metal sulfide@carbon nanofiber networks as lithium ion battery anodes. Chem Commun 52:1501–1504
Fei L, Lin QL, Yuan B, Chen G, Xie P, Li YL, Xu Y, Deng SD, Smirnov S, Luo HM (2013) Reduced graphene oxide wrapped fes nanocomposite for lithium-ion battery anode with improved performance. ACS Appl Mater 5:5330–5335
Xing CC, Zhang D, Cao K, Zhao SM, Wang X, Qin HY, Liu JB, Jiang YZ, Meng L (2015) In situ growth of FeS microsheet networks with enhanced electrochemical performance for lithium-ion batteries. J Mater Chem A 3:8742–8749
Zhu CB, Wen YR, Yu Y (2015) High lithium storage performance of FeS nanodots in porous graphitic carbon nanowires. Adv Funct Mater 25:2335–2342
Chen GY, Sun Q, Yue JL, Shadike L, Yang Y, Ding F, Sang L, Fu ZW (2015) Conversion and displacement reaction types of transition metal compounds for sodium ion battery. J Power Sour 284:115–121
Zhang K, Hu Z, Liu X, Tao ZL, Chen J (2015) FeSe2 microspheres as a high-performance anode material for Na-ion batteries. Adv Mater 27:3305–3309
Huang SS, He QQ, Chen WL, Zai JT, Qiao QQ, Qian XF (2015) 3D hierarchical FeSe2 microspheres: controlled synthesis and applications in dye-sensitized solar cells. Nano Energy 15:205–215
Chen D, Wang XS, Chen JT, Ren Z, Xue MQ, Chen GF (2015) Rewriting the superconductivity in iron-based superconductors by lithium-ion insertion and extraction. Adv Mater 27:4224–4228
Liu JB, Yang Y, Liu XJ, Xue MZ, Liu JS, Cui YH (2016) Facile synthesis and electrochemical properties of Fe2SeS for lithium ion batteries. J Power Sour 306:317–321
Tang J, Liu J, Li CL, Li YQ, Tade MO, Dai S, Yamauchi Y (2015) Synthesis of nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles. Angew Chem Int Ed 54:588–593
Li L, Wang CL, Liao JY, Manthiram A (2015) Dual-template synthesis of N-doped macro/mesoporous carbon with an open-pore structure as a metal-free catalyst for dye-sensitized solar cells. J Power Sour 300:254–260
Liang HW, Wu ZY, Chen LF, Li C, Yu SH (2015) Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: an efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 11:366–376
Ren YP, Lv WM, Wen FS, Xiang JY, Liu ZY (2016) Microwave synthesis of SnS2 nanoflakes anchored graphene foam for flexible lithium-ion battery anodes with long cycling life. Mater Lett 174:24–27
Liu YC, Zhang N, Yu CM, Jiao LF, Chen J (2016) MnFe2O4@C nanofibers as high-performance anode for sodium-ion batteries. Nano Lett 16:3321–3328
Kong DS, Wang HT, Lu ZY, Cui Y (2014) CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. J Am Chem Soc 136:4897–4900
Kitajou A, Yamaguchi J, Hara S, Okada S (2014) Discharge/charge reaction mechanism of a pyrite-type FeS2 cathode for sodium secondary batteries. J Power Sour 247:391–395
Liu J, Wen Y, Wang Y, van Aken PA, Maier J, Yu Y (2014) Carbon-encapsulated pyrite as stable and earth-abundant high energy cathode material for rechargeable lithium batteries. Adv Mater 26:6025–6030
Acknowledgements
We gratefully acknowledge the financial support by the Higher Education Science Foundation of Jiangsu Province (No. 15KJB150031), State Key Laboratory of Structural Chemistry Fund (No. 20150009), the Qing Lan project, and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We would also like to acknowledge the technical support received from the Testing Center of Yangzhou University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, JC., Ma, Z., Chi, Y. et al. The electrochemical properties of one-pot prepared Fe2SSe/porous carbon composite as anode material for lithium-ion batteries. J Mater Sci 52, 1573–1580 (2017). https://doi.org/10.1007/s10853-016-0451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0451-1