Abstract
Template-free method is used to facilely synthesize TiO2 hollow microspheres via one-step hydrothermal method. Interestingly, the TiO2 hollow microspheres have porous shell with thickness of about 450 nm. The formation mechanism of the hollow@porous TiO2 microspheres involves the formation and aggregation of TiO2 nanoparticles followed by oriented growth, etching of HF, and then Ostwald ripening and transformation process. The prepared TiO2 microspheres show a reversible capacity of ~170 mAh g−1 after 150 cycles at a current density of 0.6 C and also exhibit a good rate capability of 40.8 mAh g−1 at a current density of 24 C due to the unique hollow@porous structure, which can offer more sites for the storage and insertion of Li ions, and accelerate electrolyte diffusion and Li ions transport. The results also indicate that the as-prepared TiO2 material possesses excellent electrochemical performances, which may be an ideal anode material for lithium ion battery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low-cost and convenient synthesis has been considered as a promising method; researchers are devoted themselves to explore how to reduce the work and make the cost lower in different fields. As we know, the hollow structure materials have raised interest in various fields such as photocatalysis, drug delivery, and others due to the advantages of high surface area, good adsorption, and low density. Researchers also have controllably synthesized hollow structure by diverse strategies. Among these methods, the hard and soft template methods are popularly used. The hard templates, such as carbon spheres, carbon nanotubes, silica, and some easy to be removed materials, are served as the sacrificial templates to prepare hollow structure by two-step methods, but they are high cost and troublesome. The soft template method, using ethylene glycol, polyvinylpyrrolidone, and sodium dodecyl benzene sulfonate as inducers, is a convenient method for synthesizing hollow structure, but the post-processing is difficult and the cost is increased [1, 2]. So it is obvious that a facile method with low cost to synthesize hollow structure materials massively is important and necessary.
Titanium dioxide (TiO2) is considered as a promising anode material because of the advantages of low cost, safety, non-toxic, structural stability, and high voltage plateau, and has attracted extensive attention. Some researchers fabricated graphene/TiO2 hybrid materials, carbon-tube/TiO2 composite, and TiO2@carbon to improve the electrochemical performance [3–5]. But many researchers still tend to study the hollow TiO2 materials because the hollow structures can make Li ions access the shells of the spheres from both sides, shorten the diffusion path and improve kinetics, leading to good cycle performance, high rate capacity, and excellent lithium storage. In addition, the hollow structure can buffer structural strain caused during Li ions insertion and expulsion, improving the structural stability and cycling life. Wang and Lou [6] prepared TiO2 nanocages by employing Cu2O polyhedra as the hard template to improve the reversible capacity and cycling performance. Xiao et al. [7] synthesized the uniform and monodispersed TiO2 hollow spheres by utilizing carboxyl-functionalized polystyrene spheres as hard templates, improving the discharge–charge capacity due to the facility and reversibility of lithium storage on surface. Zhang et al. [8] fabricated anatase TiO2 hollow spheres with high crystallinity by employing the carbon as template, which shows the excellent property of lithium storage. Ren et al. [9] obtained the uniform multishelled TiO2 hollow microspheres via sacrificing hard template to control the shell thickness and the number of internal shells. The above studies on fabricating hollow structure using template methods are troublesome and the cost is high. So to fabricate TiO2 hollow structure by the template-free method will be more convenient and lower cost, which is easy for the large-scale production. Therefore, the template-free method for the preparation of the hollow TiO2 is worthy to be explored.
Herein, we report a simple synthesis for hollow as well as porous TiO2 microspheres via one-step hydrothermal method without template. The formation and growth mechanism of these TiO2 microspheres have been explored by the intermediate products obtained at different reaction stages. Meanwhile, the hollow structure exhibits high reversible capacity, excellent rate capability, and cycling stability as the anode material and may have a good prospect for LIBs.
Experimental
Materials
Titanium tetrachloride (TiCl4, 99.0 %) and ammonium hexafluorotitanate (IV) ((NH4)2TiF6, 98.0 %) were purchased from Aladdin industrial Corporation (P. R. China). All reagents were of analytical grade and were employed without further purification. Milli-Q water (Millipore Corp., with resistivity of 18.2 MΩ cm) was used for the experiments.
Synthesis of hollow@porous TiO2 microspheres
Hollow@porous TiO2 microspheres were synthesized via one-step hydrothermal method. Firstly, (NH4)2TiF6 solution (0.1 mol L−1, 10 mL) was poured into the TiCl4 solution (0.1 mol L−1, 10 mL) under magnetic stirring for 5 min. Then, the mixture was transferred into a Teflon-lined stainless autoclave and heated at 150 °C for 10 h. After cooling to room temperature, the product was collected by centrifugation, rinsed with Milli-Q water for several times, and dried at 80 °C for 10 h. The weight of obtained product is 0.1120 g.
The time-dependent experiments for hydrothermal time of 10 min, 1, and 6 h were also performed under the same conditions.
Characterization
The X-ray diffraction (XRD) patterns were performed on DX-2700 (Dandong Haoyuan instrument Co., Ltd., P. R. China) using Cu-Kα (λ = 1.5406 Å) radiation, and the accelerating voltage and applied current were 35 kV and 25 mA. Field emission scanning electron microscopy (FESEM) images were taken with a Hitachi S4800 scanning electron microscope. Transmission electron microscopy (TEM) measurements were carried out on JEM model 100SX electron microscopes (Japan Electron Co., Ltd.) operated at an accelerating voltage of 200 kV. Energy dispersive spectra were analyzed on INCA x-act energy dispersive spectrometer with a voltage 15 kV (Oxford Instruments).
Electrochemical measurement
The working electrodes were composed of active material, acetylene black, and polyvinylidene fluoride (PVDF) in a weight ratio of 70:20:10. The mass loading of active material for each circular electrode (d = 14 mm) is about 2.2 mg (1.43 mg cm−2). Copper foil, metallic lithium, and polyethylene film (Celgard, 2400) were employed as the collector, counter electrode, and separator, respectively. LiPF6 (1 M) in the mixed solution of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1:1 in volume ratio) was used as the electrolyte. The coin cells (CR2032) were assembled in an argon-filled glove box. The galvanostatic charge and discharge were performed on a battery testing system (CT-3008W-5V 10 mA, Neware Technology Co., Ltd., P. R. China) at various current densities between 1.0 and 3.0 V (vs. Li+/Li). Cyclic voltammetry (CV) measurement was carried out on an electrochemical workstation (CHI660D, Shanghai CH instruments Co., Ltd. P. R. China) between 1.0 and 3.0 V (vs. Li+/Li) at a scanning rate of 0.1 mV s−1. The voltage selection of above experiments was according to the references [8, 10–12]. Electrochemical impedance spectroscopy (EIS) was measured on the electrochemical workstation with the frequency from 100 kHz to 0.01 Hz.
Results and discussion
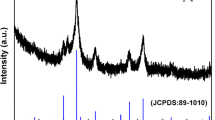
Figure 1a represents the XRD patterns of the TiO2 synthesized at hydrothermal reaction time of 10 h. The nine diffraction peaks at 2θ of 25.28°, 37.80°, 48.05°, 53.89°, 55.06°, 62.69°, 68.76°, 70.31°, and 75.03° can be indexed as the anatase TiO2 for the lattice plane of (101), (004), (200), (105), (211), (204), (116), (220), and (215), which is consistent with the standard card (JCPDS NO. 21-1272). The sharp diffraction peaks indicate the good crystallization of the as-prepared TiO2. The TiO2 sample is also confirmed as a high purity because there is no diffraction peaks of other impurity phase as shown in Fig. 1a. Meanwhile, we can find that only the peaks of titanium and oxygen elements are detected in the EDS spectrum hollow@porous TiO2 microspheres (Fig. S1). The atomic ratio of the two elements is 1: 2.11, which is closed to the composition of TiO2. The result further proofs that the sample is pure. Figure 1b, c exhibits SEM images of TiO2 sample obtained at hydrothermal time of 10 h. The TiO2 microspheres with porous surfaces composed of nanosheets are clearly discovered. From the broken part of the sphere in Fig. 1b, we find that the TiO2 microspheres are hollow, which is confirmed by the TEM images (Fig. 1d) of TiO2 microspheres. The contrast of uneven darker edges and pale centers further prove the TiO2 microspheres are with hollow structure and porous surface. The shell thickness of hollow@porous TiO2 microspheres is about 450 nm.
Figure 2 shows time-dependent SEM images of TiO2 samples. The TiO2 microspheres with rough surfaces are assembled by many TiO2 nanoparticles with average diameter of 140 nm, which can be detected in Fig. 2a at hydrothermal time of 10 min. When the reaction time reaches 1 h, the nanoparticles changed to nanoplates with a thickness of about 10 nm, as shown in Fig. 2b, c. As the reaction time prolonged to 6 h, many holes are observed on the nanoplates and make the surface of the TiO2 microspheres very rough (Fig. 2d, e). When the hydrothermal time is 10 h, the nanoplates with holes disappear, and then the rough surface of TiO2 microspheres with pores is seen in Fig. 1b, c. We can also detect that the peak intensity at 2θ of 25.28° in Fig. 2f and Fig. 1a increases clearly with the time extension, indicating the oriented growth of (101) crystal plane.
The probable formation mechanism of hollow TiO2 microspheres with porous shell is illustrated in Fig. 3. When TiCl4 is added into water, Ti-OH groups are generated due to the hydrolysis of TiCl4 and accompanied by the generation of a large amount of hydrochloric acid (HCl). In the initial of the hydrothermal reaction, Ti-F groups of (NH4)TiF6 can easily combine with Ti-OH groups to form Ti–O–Ti oxygen bridge bonds by releasing hydrofluoric acid (HF) [13], resulting in the formation of TiO2 nanoparticles followed by their aggregation to form TiO2 microspheres (Fig. 2a). With the reaction time extended, the surface energy of the (001) crystal plane can be reduced to a lower level than that of (101) crystal plane due to the adsorption of F− on the (001) crystal plane, resulting in the oriented growth of (101) crystal plane and the exposure of (001) crystal plane [14, 15], then the nanoplates will appear (Fig. 2b, c). As the reaction continued, HF generated at the initial stage of hydrothermal reaction etches the (001) crystal plane and the etching started from the central area of the TiO2 nanoplates due to the higher density of planar defects [14], resulting in the formation of TiO2 microspheres with rough surface (Fig. 2d, e). Ostwald ripening can be employed to explain the formation of hollow microspheres with porous shell [15, 16]. The interiors of TiO2 microspheres with relative low crystallinity are gradually dissolved by HF shown in Eq. (1), then transferred to the external surface, and recrystallized on the shell of the TiO2 microspheres indicated by Eq. (2) [17].
Figure 4a shows the cyclic voltammogram (CV) of hollow@porous TiO2 microsphere electrode at a scan rate of 0.1 mV s−1. A pair of oxidation and reduction peaks can be noticed at 2.066 V (anodic sweep) and 1.699 V (cathodic sweep), corresponding to extraction and insertion of lithium ion in TiO2. The initial three charge–discharge curves of TiO2 hollow microsphere electrode at a current density of 0.6 C are exhibited in Fig. 4b. Two voltage plateaus appear at ~1.7 and ~2.0 V during the discharge and charge processes, which are consistent with the above CV analysis as well as the previous reports [9, 18]. The first discharge capacity is 196.6 mAh g−1 and the following charge capacity is 168.4 mAh g−1, leading to a capacity loss of 14.4 %. The irreversible capacity loss is attributed to the trapping of lithium ion in TiO2 nanostructure. Furthermore, the subsequent cycles are almost the same, which is also shown in Fig. 4c. The discharge and charge capacities of hollow@porous TiO2 microspheres are 167.4 and 167.9 mAh g−1 at a current density of 0.6 C after 150 cycles, respectively. The coulombic efficiency is nearly 100 % at the cycles. The rate capability of the hollow@porous TiO2 microsphere electrode is estimated by the discharge and charge at various current densities from 0.6 to 24 C as shown in Fig. 4d. When the current density is 0.1 A g−1, the capacity is 172.2 mAh g−1, and the capacity is still retained in 40.8 mAh g−1 at high current density of 24 C. After the discharge–charge cycles at high current densities, the capacity of 170.6 mAh g−1 can be regained when the current density is reduced to 0.6 C, indicating the good rate capability and the stable structure of TiO2 hollow microspheres.
The good electrochemical performance of hollow@porous TiO2 microspheres as anode materials for LIBs can be considered from two viewpoints. First, the porous shell of TiO2 hollow microspheres makes the electrolyte more easily immersed into the interiors of TiO2 microspheres. The electrolyte is closely contact with the active materials, which shortens the diffusion distance of lithium ion in the discharge–charge processes, promoting the reversible and fast lithium extraction and insertion. Second, the hollow structure can prevent the aggregation of active materials, which maintains the integrity of electrode and prolongs cycle numbers.
Electrochemical impedance spectroscopy (EIS) is further utilized to analyze electrochemical impedance of TiO2 hollow microsphere electrode. The Nyquist plots of the hollow TiO2 microsphere electrode before and after cycles are shown in Fig. 5, which display a semicircle in the high-frequency region and an inclined line in the low-frequency range, respectively, corresponding to the charge transfer resistance on the electrode and electrolyte interface and the solid-state diffusion of lithium ion in the electrode materials. The intercept at the real axis in high frequency is related to the electrolyte resistance [19]. The charge transfer is reduced as evidenced by smaller diameter of the semicircle in Nyquist plot of EIS after 150 cycles. The possible reason is likely related to better wetting of the electrolyte to hollow@porous TiO2 active materials, making more sufficient contact between the active materials and the electrolyte, and then lithium ions and electron can be transferred effectively in the interface of active materials and electrolyte [20, 21]. This results in the enhanced hollow@porous TiO2 microsphere electrode reaction kinetics and the better cycling performance. In theory, the angle of the inclined line is absolutely 45°, but it is rare in the real cases. Here, the deviation of the 45° line is likely caused by convolution between Warburg diffusion and the capacitance between the two current collectors [22].
Conclusion
The hollow@porous TiO2 microspheres are successfully synthesized by a facile hydrothermal method without template. The hollow@porous TiO2 microsphere electrode keeps a reversible capacity of ~170 mAh g−1 at a current density of 0.6 C for 150 cycles. Meanwhile, the electrode exhibits a good rate capability, which is evaluated by the discharge–charge cycles at various current densities. The hollow@porous structure of TiO2 microspheres is beneficial to enhance the electrochemical performances. These hollow@porous TiO2 microspheres will be a promising material applied in LIBs or other areas.
References
Wang HE, Jin J, Cai Y et al (2014) Facile and fast synthesis of porous TiO2 spheres for use in lithium ion batteries. J Colloid Interface Sci 417:144–151
Zhang P, Zhang J, Xie A, Li S, Song J, Shen Y (2015) Hierarchical flower-like Bi2WO6 hollow microspheres: facile synthesis and excellent catalytic performance. RSC Adv 5:23080–23085
Yan Z, Liu L, Guo H et al (2014) One-pot synthesis of FCNTs-wired TiO2 nanocomposites as anode materials for high-rate lithium ion batteries. Electrochim Acta 123:551–559
Trang NT, Ali Z, Kang DJ (2015) Mesoporous TiO2 spheres interconnected by multiwalled carbon nanotubes as an anode for high-performance lithium ion batteries. ACS Appl Mater Interfaces 7:3676–3683
Zhao C, Liu L, Zhang Q, Rogers J, Zhao H, Li Y (2015) Synthesis of carbon-TiO2 nanocomposites with enhanced reversible capacity and cyclic performance as anodes for lithium-ion batteries. Electrochim Acta 155:288–296
Wang Z, Lou XW (2012) TiO(2) nanocages: fast synthesis, interior functionalization and improved lithium storage properties. Adv Mater 24:4124–4129
Xiao L, Cao M, Mei D et al (2013) Preparation and electrochemical lithium storage features of TiO2 hollow spheres. J Power Sources 238:197–202
Zhang G, Wu HB, Song T, Paik U, Lou XW (2014) TiO2 hollow spheres composed of highly crystalline nanocrystals exhibit superior lithium storage properties. Angew Chem 53:12590–12593
Ren H, Yu R, Wang J et al (2014) Multishelled TiO2 hollow microspheres as anodes with superior reversible capacity for lithium ion batteries. Nano Lett 14:6679–6684
Yi J, Lu D, Li X, Hu S, Li W, Lei J, Wang Y (2011) Preparation and performance of porous titania with a trimodal pore system as anode of lithium ion battery. J Solid State Electrochem 16:443–448
Xia T, Zhang W, Murowchick J, Liu G, Chen X (2013) Built-in electric field-assisted surface-amorphized nanocrystals for high-rate lithium-ion battery. Nano Lett 13:5289–5296
Madej E, Ventosa E, Klink S, Schuhmann W, La Mantia F (2014) Aging effects of anatase TiO2 nanoparticles in Li-ion batteries. Phys Chemistry Chem Phys 16:7939–7945
Yu J, Zhang J (2010) A simple template-free approach to TiO2 hollow spheres with enhanced photocatalytic activity. Dalton Trans 39:5860–5867
Shi Q, Li Y, Zhan E, Ta N, Shen W (2014) Anatase TiO2 hollow nanosheets: dual roles of F−, formation mechanism and thermal stability. CrystEngComm 16:3431–3437
Lv K, Cheng B, Yu J, Liu G (2012) Fluorine ions-mediated morphology control of anatase TiO2 with enhanced photocatalytic activity. Phys Chem Chem Phys 14:5349–5362
Yang HG, Zeng HC (2004) Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. J Phys Chem B 108:3492–3495
Yu J, Xiang Q, Ran J, Mann S (2010) One-step hydrothermal fabrication and photocatalytic activity of surface-fluorinated TiO2 hollow microspheres and tabular anatase single micro-crystals with high-energy facets. CrystEngComm 12:872–879
Wang X, Wang Y, Yang L, Wang K, Lou X, Cai B (2014) Template-free synthesis of homogeneous yolk–shell TiO2 hierarchical microspheres for high performance lithium ion batteries. J Power Sources 262:72–78
Wang S, Xing Y, Su H, Zhang S (2014) MnO nanoparticles interdispersed in 3D porous carbon framework for high performance lithium-ion batteries. ACS Appl Mater Interfaces 6:12713–12718
Jadhav HS, Kalubarme RS, Park CN, Kim J, Park CJ (2014) Facile and cost effective synthesis of mesoporous spinel NiCo2O4 as an anode for high lithium storage capacity. Nanoscale 6:10071–10076
Wu F, Huang R, Mu D, Wu B, Chen S (2014) New synthesis of a foamlike Fe3O4/C composite via a self-expanding process and its electrochemical performance as anode material for lithium-ion batteries. ACS Appl Mater Interfaces 6:19254–19264
Wen CJ, Ho C, Boukamp BA, Raistrick ID, Weppner W, Huggins RA (1981) Use of electrochemical methods to determine chemical-diffusion coefficients in alloys: application to ‘LiAl’. Int Metals Rev 5:253–268
Acknowledgements
This work was supported by the National Nature Science Foundation of China (21173001 and 21371003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, P., Zhang, C., Xie, A. et al. Novel template-free synthesis of hollow@porous TiO2 superior anode materials for lithium ion battery. J Mater Sci 51, 3448–3453 (2016). https://doi.org/10.1007/s10853-015-9662-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9662-0