Abstract
Tb3+-doped β-Ca2P2O7 phosphors were successfully prepared via a simple surfactant-free molten salt method for the first time and characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (SEM), and photoluminescence. The results of XRD and SEM suggest that the products belong to pure β-Ca2P2O7 nanoparticles with average particle size of about 88.6 nm. Under the UV light excitation, the as-prepared β-Ca2P2O7:Tb3+ phosphors exhibit green emission, which corresponds to the characteristic emissions of Tb3+ ion. The optimal doping concentration of Tb3+ ions in β-Ca2P2O7 phosphors was confirmed to be abound 10 mol% and the exchange interaction is responsible for energy transfer between Tb3+ ions in β-Ca2P2O7 phosphors. The fluorescent lifetime of 5D4 level of Tb3+ in β-Ca2P2O7 phosphors decreases with the increase of Tb3+ ions concentration because of self-generated quenching process, which was confirmed by Auzel’s model. The thermal quenching behaviors of β-Ca2P2O7:Tb3+ nanophosphors were also studied and the activation energy was deduced to be 0.265 eV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth (RE) ions doped luminescent materials have attracted considerable attention owing to their unique optical properties originating from the special electron configurations of 4f shell of the RE ions and have been widely applying in the fields of lighting, displays, lasers, etc. [1–4]. As one member of RE ions, it is well known that Tb3+ is frequently used as an activator of green-emitting materials due to its predominant 5D4 → 7F5 transition [5]. To achieve excellent luminescent performance of Tb3+ ion, it is necessary to select proper host materials. Among various host materials, the phosphate is a promising host material due to its remarkable thermal, structural diversity and a rather short wavelength of optical absorption edge, for example, Ca2P2O7 [6–9]. It is well known that Ca2P2O7 exists in three different crystal structures including γ-, β-, and α-Ca2P2O7, which depend on the temperature of firing [10]. RE-doped Ca2P2O7 phosphors provide a novel flexible method for designing new luminescent materials. Currently, studies on Ca2P2O7 phosphors have mainly been focused on Eu2+-doped materials and materials co-doped with Eu2+ and another metal ion, which can emit blue or white light [7–9]. Moreover, these Ca2P2O7 phosphors materials are usually synthesized by the solid-state reaction method, in which relatively high temperature and prolonged heating time are required. As a result, the size and morphology of the as-obtained Ca2P2O7 phosphors are not uniform and regular. To the best of our knowledge, however, the report on luminescent properties of other RE ion-doped Ca2P2O7 phosphor is rare. Especially, nanosized Ca2P2O7 phosphors prepared via molten salt method has not been reported now.
The molten salt method has been proved to be one of the most convenient, effective, and environmental friendly approaches for achieving single-phased nano- or micro-crystals at relatively lower temperature and shorter reaction time compared with the conventional solid-state reactions [11]. Therefore, this method has been extensively used for the preparation of electron ceramic powders and some other inorganic functional materials [12]. At the certain temperature, inorganic mixed salts, such as NaNO3 and KNO3, can melt into ionic liquids, which can be used as an effective chemical reaction medium to produce a high-temperature liquid environment for crystal growth. The fundamental basis of molten salt reactions is the use of different types of inorganic molten salts as the reaction medium. Moreover, the inorganic molten salts usually have several favorable physicochemical properties including a higher oxidizing potential, high mass transfer, high thermal conductivity, as well as relatively lower viscosities and densities, as compared with conventional solvents [13]. Therefore, the molten salt method is considered as one of the simplest, most versatile, and cost-effective approaches to obtain crystalline, chemically purified, single-phase powders at lower temperatures and often in overall shorter reaction times with little residual impurities as compared with conventional solid-state reactions. In recent years, many inorganic compounds have been synthesized through molten salt method, such as NaLuF4 [11], ZnWO4 [12], Gd2MO6 (M = W, Mo) [14], etc.
In this paper, Tb3+-doped β-Ca2P2O7 phosphors were prepared via molten salt method using NaNO3–KNO3 as the medium. X-ray diffraction (XRD) and scanning electron microscopy (SEM) results suggest that the as-prepared samples are pure phase of β-Ca2P2O7. The optimal doping concentration of Tb3+ ions in Ca2P2O7 phosphors was confirmed to be around 10 mol%. In addition, the energy transfer and thermal quenching behavior of Tb3+-doped β-Ca2P2O7 phosphors were studied in detail.
Experimental
Sample synthesis
Tb3+-doped β-Ca2P2O7 phosphors were prepared via molten salt process for the first time. In a typical procedure, the certain amounts of calcium nitrate, ammonia phosphate, terbium nitrate hexahydrate, sodium nitrate, and potassium nitrate (the molar ratio: (1−x):1:x:50:25, where x = 0.5–12 mol%) were mixed and ground thoroughly in an agate mortar for about 30 min. After being ground, the as-obtained mixture was transferred into an alumina crucible with a lip and heated at 350 °C for 3 h. After cooling to room temperature naturally, the as-prepared sample was washed with distilled water and collected via centrifugation several times. Finally, the product was dried at 80 °C in air for 10 h.
Characterization
X-ray powder diffraction (XRD) of the as-prepared samples was performed on a Shimadzu XRD-6000 diffractometer with Cu K α1 radiation (λ = 0.15406 nm). The XRD data were collected using a scanning mode in 2θ ranging from 10° to 60° with a step size of 0.02° and a rate of 4.0°/min. The morphology of the samples was observed by field emission scanning electron microscopy (FE-SEM, Hitachi S-4800). The excitation & emission spectra and fluorescent decays were recorded with a Hitachi F-4600 spectrophotometer equipped with a 150 W xenon lamp as excitation source. A homemade temperature control system was used to measure temperature-dependent emission spectra from 25 to 250 °C. The measuring and controlling accuracy of the temperature is about ±0.5 °C.
Results and discussion
Crystal structure of as-prepared samples
The typical XRD patterns for 0.5 and 10 mol% Tb3+-doped β-Ca2P2O7 phosphors are shown in Fig. 1. It can be found that all the diffraction peak positions from the 0.5 and 10 mol% Tb3+-doped β-Ca2P2O7 phosphors are well consistent with that of the pure tetragonal phase of Ca2P2O7 with space group P41(76), which was reported in JCPDS card with No. 71-2123. No extra diffraction peaks corresponding to any impurities are observed even in the sample with highest concentration of Tb3+. This fact indicates that the tetragonal phase of Ca2P2O7 polycrystals can be formed directly via simple molten salt process, and that the crystal structure of the products is influenced slightly by the introduction of Tb3+ ions. Herein, Tb3+ ions should substitute the sites of Ca2+ ions in β-Ca2P2O7 host because of the similar ionic radius and Na+ are K+ ions may be used as charge compensators during the synthesis process. In addition, we can also see that the XRD peaks of the prepared samples are intense and sharp, suggesting that the products are crystallized well. This is in the favor of high efficient luminescence of RE ions as well.
SEM observations
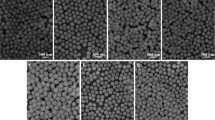
Figure 2a and b shows the SEM images of 0.5 and 10 mol% Tb3+-doped β-Ca2P2O7 phosphors prepared using NaNO3 and KNO3 as molten salt. It can be seen that both of them present the similar morphology and size distribution. However, the size and morphology of the phosphors are not relatively uniform and regular. Meanwhile, some particles are agglomerated into bulk and the size range of particles is from 30 to 140 nm. By counting 100 identifiable particles not including the agglomerated bulk, the average particle size was estimated from the size distribution to be around 88.6 nm. Although the aggregation of particles is still observed though the molten salt method, the temperature is much lower than that of solid-state reaction and the reaction period is much shorter. Therefore, this can save a lot of energy resource.
Photoluminescent properties
The excitation spectra of the as-prepared β-Ca2P2O7:x mol% Tb3+ (x = 0.5–12) phosphors by monitoring 545 nm emission are shown in Fig. 3. It can be found that the excitation spectra have the similar profiles except for the intensity and are composed of two parts: One is a broad band ranging from 200 to 275 nm, which corresponds to the 4f → 5d of Tb3+ transitions and the other is a set of some weak lines, which can be attributed to the f–f transitions within the 4f8 configuration of Tb3+ ion [15]. Under the 375 nm excitation, the as-prepared β-Ca2P2O7:x mol%Tb3+ (x = 0.5–12) phosphors exhibit green emission and the typical emission spectra are shown in Fig. 4. The emission peaks located at 490, 545, 585, and 623 nm can be observed, which correspond to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions of Tb3+, respectively. The green emission of 5D4 → 7F5 transition is dominant in the emission spectra. Therefore, the samples exhibit green color under the UV light excitation. The emissions from the 5D3 level cannot be observed even in the samples with lower Tb3+ concentration (0.5 mol%), which is due to the relative high phonon energy of host and unnegligible cross-relaxation effect, i.e., 5D3 +7F6 → 5D4 +7F0 [15].
The doping concentration of luminescent activators is an important factor that can influence the phosphor performance greatly. Therefore, it is necessary to confirm the optimal doping concentration for obtaining strongest emission intensity. The inset in Fig. 4 shows the dependence of the integrated emission intensity of 5D4 → 7F5 transition on Tb3+ concentration in β-Ca2P2O7 phosphors. It can be seen that the integrated emission intensity of 5D4 → 7F5 transition increases firstly with the increase of Tb3+ doping concentration and then reaches its maximum at around 10 mol% Tb3+. When the doping concentration of Tb3+ ions is more than 10 mol%, the emission intensity decreases. Namely concentration quenching occurs. Therefore, the optimal concentration of Tb3+ in β-Ca2P2O7 phosphors can be confirmed to be about 10 mol%.
Energy transfer and self-generated quenching of Tb3+ in β-Ca2P2O7
Van Uitert has developed a phenomenological model to explain the relationship between the luminescent intensity and the concentration of luminescent center, which can be written by [16, 17]
where C is doping concentration of Tb3+ ions in the present case; K and β are constants for a certain system; Q represents the interaction type between luminescence center and quenching center, here Q = 3, 6, 8, or 10, indicating, respectively, the exchange interaction, electric dipole–dipole (D–D), electric dipole-quadrupole (D–Q), and electric quadrupole–quadrupole (Q–Q) interactions. In order to understand the energy transfer mechanism between Tb3+ ions in β-Ca2P2O7 phosphors, the Eq. (1) was used to fit the experimental data in inset in Fig. 4. It can be found that Eq. (1) can fit well with the experimental data, and the Q value was deduced from the fitting process to be 3.15, which suggests the exchange interaction is responsible for energy transfer of the 5D4 level of Tb3+ in β-Ca2P2O7 phosphors. Considering that luminescence quenching is caused by the energy transfer within the same RE ions, the critical distance (R C) can be estimated in terms of the equation developed by Blasse [18]
where V is the volume of the unit cell, and X c is the critical concentration, which is the concentration of Tb3+ ions at the half of strongest emission intensity. Herein, X c is about 12 mol%. N is the number of available crystallographic sites occupied by the activator ions in the unit cell. The values of V and N for the tetragonal Ca2P2O7 are 1078.65 Å3 and 8, respectively (JCPDS 71-2123). Therefore, the R C for Tb3+ in β-Ca2P2O7 phosphor is about 2.88 Å.
Figure 5 shows the fluorescent decay curves of the 5D4 level of Tb3+ ions in β-Ca2P2O7:x mol% Tb3+ (x = 0.5–12) phosphors under the 375 nm excitation. It can be seen that all these decays follow single-exponential function, indicating that the energy transfer between Tb3+ ions is an energy migration process [19, 20]. In this case, the excitation energy quickly transfers between Tb3+ ions, and finally is captured by the quenchers, which may be the lattice defects or unintended dopants. The decay time constants were derived via single-exponential fitting and are also listed in inset in Fig. 5. The dotted circles in inset in Fig. 5 show the dependence of average fluorescent lifetime on the doping concentration of Tb3+. It can be found that with the increase of Tb3+ concentration the lifetime of the 5D4 level of Tb3+ decreases. It is well known that there is no energy transfer quenching 5D4 level of Tb3+. Therefore, the self-generated quenching process should be a dominant mechanism for the lifetime variation. The self-generated quenching process occurs, usually, in the instance that the average distance between activators is short enough, and the energy transfer from the studied level does not exist, which has been proved above [20]. Auzel has developed a physical model to describe the dependence of fluorescent lifetime on the doping concentration for the self-generated quenching system, where their relationship can be mathematically expressed as [21]
where τ(c) is fluorescent lifetime at concentration c, τ 0 is radiative transition lifetime, c 0 is a constant with the same dimension as the concentration c, and N is the number of phonon required for quenching the studied level via cascade multiphonon process. The fluorescent lifetime data were fitted by Eq. (3) and the solid line in inset in Fig. 5 shows the fitting curve. As seen in Fig. 5, the experimental data can be fitted well by Eq. (3). In the fitting process, the N value is confirmed to be 12.5, indicating that the 5D4 level can be quenched via nonradiative relaxation by generating about 13 phonons. It is known that the energy difference between 5D4 and 7F0 is around 14784 cm−1, which is around 14 times as the highest phonon energy (1051 cm−1) of the host [22]. Therefore, it requires 14 phonons at least to bridge these two levels, which is close to fitting value of N. This fact means that Auzel’s model can well explain the self-generated quenching process of Tb3+ in β-Ca2P2O7 phosphors. Additionally, the intrinsic radiative transition lifetime τ 0 of 5D4 level of Tb3+ ions in β-Ca2P2O7 phosphors was also obtained to be 3.81 ms via the fitting process. The internal quantum efficiency can be written by
where τ s is the measured fluorescence lifetime. Therefore, the maximally external quantum efficiency is 96.59 % for the 0.5 mol% Tb3+-doped sample. The external quantum efficiency decreases to 44.88 % when doping concentration increases to 12 mol% because of the increase of the nonradiative transition rate.
Thermal quenching behavior
Usually, the spectral properties of luminescence materials are temperature dependent and thermal effect of luminescence materials is also an important aspect of spectroscopic investigations. Comprehensively understanding the thermal deterioration and fluorescence temperature quenching of the phosphors is beneficial to improve phosphors’ performance via material design and synthesis. To examine the thermal performance of all the as-prepared β-Ca2P2O7 phosphors, their emission spectra were measured at temperatures ranging from 298 K to 523 K upon 375 nm excitation. As an example, the emission spectra for β-Ca2P2O7:10 mol%Tb3+ phosphor excited at different temperature are shown in Fig. 6. It can be found that the emissions from the 5D4 level decrease with the increase of temperature. Namely thermal quenching occurs. It is known that the luminescent ion can go back to the ground state via thermal quenching. In other words, it can reach the ground state when the excited state and the ground state energy curves cross at an energy which is thermally accessible from the relaxed excited state. It is assumed that the nonradiative rate k nr can be expressed as [23]
where A is a constant, k is Boltzmann’s constant, and ΔE is the activation energy for the thermal quenching process. Thus, the emission intensity decreases due to the increase of probability of nonradiative transition with temperature. According to Eq. (5), a lower value of ΔE means a more rapid nonradiative rate at a given temperature. The Arrhenius equation can be used to describe the temperature dependence of the luminescent intensity [23–25].
where I 0 is the initial luminescent intensity, I(T) is the luminescent intensity at given temperature T, C is a constant, k is Boltzmann’s constant, and ΔE is the activation energy for the thermal quenching process. The inset in Fig. 6 shows the dependence of integrated emission intensity of the 5D4 level on temperature. Equation (6) is used to nonlinearly fit the experimental data of thermal quenching; the solid line shows the fitting curve. It can be found that the experimental data are fitted well with Eq. (6). From the fitting process, the activation energy was confirmed to be 0.265 eV.
Conclusion
In conclusion, Tb3+-doped β-Ca2P2O7 phosphors with average size of 88.6 nm have been successfully prepared via simple and environmental friendly molten salts method using NaNO3 and KNO3 as the medium. The luminescent properties of Tb3+-doped β-Ca2P2O7 phosphors have been studied as well. The results suggested that the optimal doping concentration of Tb3+ ions in β-Ca2P2O7 phosphors is about 10 mol% and a critical distance of 2.88 Å was obtained for energy transfer between Tb3+ ions. In addition, it was found that the self-generated quenching process is responsible for concentration quenching of Tb3+ ions in β-Ca2P2O7 phosphors according to the Auzel’s model and the intrinsic radiative transition lifetime τ 0 of 5D4 level was deduced to be 3.81 ms. The luminescent intensities of Tb3+-doped β-Ca2P2O7 phosphors decrease with the increase of ambient temperature because of temperature quenching and the activation energy was confirmed to be 0.265 eV.
References
Tian Y, Qi XH, Wu XW, Hua RN, Chen BJ (2009) Luminescent properties of Y2(MoO4)3:Eu3+ red phosphors with flowerlike shape prepared via coprecipitation method. J Phys Chem C 113:10767–10772
Lakshminarayana G, Qiu JR (2009) Photoluminescence of Eu3+, Tb3+ and Tm3+ doped transparent SiO2-Al2O3-LiF-GdF3 glass ceramics. J. Alloys Compd 476:720–727
Lakshminarayana G, Buddhudu S (2007) Spectral analysis of Eu3+ and Tb3+: B2O3-ZnO-PbO glasses. Mater Chem Phys 102:181–186
Lakshminarayana G, Qiu JR (2009) Photoluminescence of Pr3+, Sm3+ and Dy3+-doped SiO2-Al2O3-BaF2-GdF3 glasses. J. Alloys Compd 476:470–476
Han LL, Wang YH, Wang YZ, Zhang J, Tao Y (2013) Observation of efficient energy transfer from host to rare-earth ions in KBaY(BO3)2:Tb3+ phosphor for plasma display panel. J. Alloys Compd 551:485–489
Yonesaki Y (2013) Sensitized red luminescence from Mn2+-doped olgite-type phosphate. J Solid State Chem 197:166–171
Hao ZD, Zhang JH, Zhang X, Lu SZ, Luo YS, Ren XG, Wang XJ (2008) Phase dependent photoluminescence and energy transfer in Ca2P2O7:Eu2+, Mn2+ phosphors for white LEDs. J Lumin 128:941–944
Pang R, Li CY, Zhang S, Su Q (2009) Luminescent properties of a new blue long-lasting phosphor Ca2P2O7:Eu2+, Y3+. Mater Chem Phys 113:215–218
Hao ZD, Zhang JH, Zhang X, Sun XY, Luo YS, Lu SZ, Wang XJ (2007) White light emitting diode by using α-Ca2P2O7:Eu2+, Mn2+ phosphor. Appl. Phys. Lett. 90:261113-1–261113-3
Bian JJ, Kim DW, Hong KS (2004) Phase transformation and sintering behavior of Ca2P2O7. Mater Lett 58:347–351
Niu N, Yang PP, He F, Zhang X, Gai SL, Li CX, Lin J (2012) Tunable multicolor and bright white emission of one-dimensional NaLuF4:Yb3+, Ln3+ (Ln = Er, Tm, Er/Tm, Tm/Ho) microstructures. J Mater Chem C 22:10889–10899
Yan B, Lei F (2010) Molten salt synthesis, characterization and luminescence of ZnWO4:Eu3+ phosphors. J Alloys Compd 507:460–464
Volkov SV (1990) Chemical reactions in molten salts and their classification. Chem Soc Rev 19:21–28
Lei F, Yan B, Chen HH, Zhao JT (2009) Molten salt synthesis, characterization, and luminescence properties of Gd2MO6:Eu3+ (M = W, Mo) phosphors. J Am Ceram Soc 92:1262–1267
Tian Y, Chen BJ, Tian BN, Sun JS, Li XP, Zhang JS, Cheng LH, Zhong HY, Zhong H, Meng QY, Hua RN (2012) Ionic liquid-assisted hydrothermal synthesis of dendrite-like NaY(MoO4)2:Tb3+ phosphor. Phys B 407:2556–2559
Van Uitert LG (1967) Characterization of energy transfer interactions between rare earth ions. J Electrochem Soc 114:1048–1053
Tian Y, Chen BJ, Tian BN, Hua RN, Sun JS, Cheng LH, Zhong HY, Li XP, Zhang JS, Zheng YF, Yu TT, Huang LB, Meng QY (2011) Concentration-dependent luminescence and energy transfer of flower-like Y2(MoO4)3:Dy3+ phosphor. J Alloy Compd 509:6096–6101
Blasse G (1986) Energy transfer between inequivalent Eu3+ ions. J Solid State Chem 62:207
van der Ziel JP, Kopf L, Van Uitert LG (1972) Quenching of Tb3+ luminescence by direct transfer and migration in aluminum garnets. Phys Rev B 6:615–623
Tian Y, Chen BJ, Hua RN, Sun JS, Cheng LH, Zhong HY, Li XP, Zhang JS, Zheng YF, Yu TT, Huang LB, Yu HQ (2011) Opitical transition, electron-phonon coupling and fluorescent quenching of La2(MoO4)3:Eu3+ phosphor. J Appl Phys 109:053511-1–053511-6
Auzel F (2002) A fundamental self-generated quenching center for lanthanide-doped high purity solids. J Lumin 100:125–130
Cornilsen BC, Condrate RA Sr (1979) The vibrational spectra of β-Ca2P2O7 and γ-Ca2P2O7. J Inorg Nucl Chem 41:602–605
Tian BN, Chen BJ, Tian Y, Sun JS, Li XP, Zhang JS, Zhong HY, Cheng LH, Hua RN (2012) Concentration and temperature quenching mechanisms of Dy3+ luminescence in BaGd2ZnO5 phosphors. J Phys Chem Solid 73:1314–1319
Tian BN, Chen BJ, Tian Y, Li XP, Zhang JS, Sun JS, Zhong HY, Cheng LH, Fu SB, Zhong H, Wang YZ, Zhang XQ, Xia HP, Hua RN (2013) Excitation pathway and temperature dependent luminescence in color tunable Ba5Gd8Zn4O21 phosphors. J Mater Chem C 1:2338–2344
Tian Y, Chen BJ, Hua RN, Yu NS, Liu BQ, Sun JS, Cheng LH, Zhong HY, Li XP, Zhang JS, Tian BN, Zhong H (2012) Self-assembled 3D flower-shaped NaY(WO4)2:Eu3+ microarchitectures: microwave-assisted hydrothermal synthesis, growth mechanism and luminescent properties. CrystEngComm 14:1760–1769
Acknowledgements
This work was partially supported by National Natural Science Foundation of China (NSFC, Grant Nos. 11374044 and 51302182), The National High Technology Research and Development Program (“863” Program) of China (2015AA016901), The Qualified Personnel Foundation of Taiyuan University of Technology (QPFT) (No: tyut-rc201361a), and The Program for the outstanding Innovative Teams of Higher Learning Institutions of Shanxi.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tian, Y., Fang, Y., Tian, B. et al. Molten salt synthesis, energy transfer, and temperature quenching fluorescence of green-emitting β-Ca2P2O7:Tb3+ phosphors. J Mater Sci 50, 6060–6065 (2015). https://doi.org/10.1007/s10853-015-9155-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9155-1