Abstract
The removal of hazardous dyes from process water has become one of the most important tasks for water clarification. In this work, a novel type of latex, prepared by the emulsion polymerization of butyl acrylate (BA) and acrylamide (AM) in the presence of hemicellulose, was developed. Under the optimal polymerization conditions, the conversion of monomers reached as high as 97.6 %. The resulting hemicellulose-containing latex (HCL) was assessed as an absorbent toward dyes, methylene blue (MB) in particular. Much effort was made to revealing the impact of adsorption conditions of the dye removal induced by HCL. The maximum removal of the dye by HCL was about 93.8 %, much higher than that of the control sample. The dye adsorption isotherm was determined to further understand the adsorption mechanism. The adsorption data were found to fit Langmuir model well (R 2=0.99904). The maximum adsorption capacity obtained at 25 °C was 42.7 mg g−1, suggesting that the HCL is promising as an excellent absorbent for the removal of dyes from effluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hemicelluloses are the second most abundant biopolymers in the cell wall of cellulose materials [1]. It is mainly made of low molecular weight monosaccharide, such as xylose, arabinose, glucose, galactose, and mannose. Recently much attention has been paid to exploring the utilization of hemicellulose for a variety of applications; and the market potential of resulting products from hemicellulose is significant [2–4]. One of the potential applications of hemicellulose is to act as an important composition of pulp to increase fiber swelling and flexible in papermaking industry. However, the high-value utilization of hemicellulose is still highly demanded.
Dyes have been extensively used in many industries, such as textile, papermaking, leather, food technology, and hair coloring [5, 6]. Discharging large amount of dyes in water accompanied with organics, bleaches, and salts can deteriorate the physical and chemical properties of freshwater. It is estimated that the annual dye production world-wide exceeds 7 × 105 tons and more than 100,000 commercially available dyes of different chemical and physical properties are being used [7–9]. In addition to their undesirable colors, some of these dyes may degrade to produce carcinogens and toxic products. Therefore, it is necessary to reduce dye concentration in the wastewater before their biological treatment processes. However, the removal of dyes from wastewater is still a challenge due to the complex properties of dyes. A number of methods have been attempted for the removal of dyes from wastewater, including both chemical and physical methods [10]. Among these methods, using absorbents, such as activated carbon [11, 12] and kaolinite, to absorb dyes is an efficient approach. However, not all these adsorbents are cost-effective and highly efficient. Therefore, the green-based and biodegradable adsorbents with low-cost and high efficiency recycled are highly demanded. A wide variety of materials such as jute fiber carbon [13], modified clays [14], activated sludge [15], wood chips [16], carbon nanotube [17], palm-fruit bunch particles [18], nanosize modified silica [19], and many others have been used as absorbents currently.
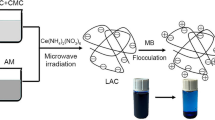
In this study, a kind of hemicellulose-containing latex (HCL) was prepared and applied to absorb methylene blue for its removal from waste water. The effect of influencing factors such as initial methylene blue concentration, mass of absorbent, pH, temperature, and contact time on absorptive capacity of the HCL was tested. The adsorption isotherm of the dye on HCL was also studied to further understand the adsorption mechanism of methylene blue onto the HCL. The incorporation of hemicellulose into the latex particles not only creates the stable latex with enhanced absorptive capacity, but also improved the biodegradability of the latex.
Materials and methods
Materials
Hemicellulose used in this work was isolated from the pre-hydrolysis liquor (PHL) of the Kraft-based dissolving pulp production process [20]. All chemicals and reagents used for experiments and analysis were analytical grade and purchased from Sigma–Aldrich Canada. The dye used in this work was methylene blue. The emulsifier was sodium dodecyl sulfate. The initiator was ammonium persulfate.
Preparation of hemicellulose-containing latex
Hemicellulose was dispersed in distilled water with magnetic stirring at 85 °C for 60 min before the solution was cooled to room temperature. Then, the hemicellulose dispersion liquid was mixed with an emulsifier for 1 h; and the mixture was heated to 65 °C; and then added with monomer (butyl acrylate and acrylamide) and initiator, while N2 bubbled through the flask. The polymerization in the presence of hemicellulose proceeded for 2 h under constant stirring with the rate of 500 rpm at 70 °C. The emulsion polymerization of butyl acrylate and acrylamide in the absence of hemicellulose was performed under the same reaction conditions to generate a control sample.
The particle sizes of the HCL were measured using BIC particle size analyzer (model: ZetaPlus, Brookhaven Instruments Corporation). The particle images were obtained from a transmission electron microscope (TEM) using a JEOL JEM-2011 Scanning Transmission Electron Microscope equipped with a Gatan GIF2001 Gatan Image Filter, a Gatan MSC Multiscan camera, a Gatan Dualvision 300W CCD camera, a Gatan Ultrascan 4000SP camera, and an EDAX Phoenix EDS system.
Adsorption process
The as-prepared sample was dried in an oven at 80 °C for 6 h for MB adsorption. A series of stock solutions of MB with different concentrations (1–500 ppm) were prepared. The batch adsorption experiments were conducted in a series of flasks containing 100 mL of MB solutions and equilibrated in a temperature-controlled water bath shaker. After adsorption, equilibrium was achieved, the flasks were removed from the shaker. The final concentration of MB in the solution was measured using an UV–Visible spectrophotometer (Genesys 10S, Thermo Electron Co.). The adsorbed amount (Q e) and the removal (R) of MB by HCL were calculated according to the following equations:
where Q e is the adsorption amount at equilibrium (mg g−1), R is the removal ratio (%), C 0 and C e are the initial and equilibrium concentrations of MB in the solution (ppm), V is the volume of solution (L), and M is the mass of HCL (g).
Similar procedures were performed in the solution at pH of 3.0–13.0. The pH was adjusted by adding an appropriate amount of hydrochloric acid or sodium hydroxide.
Adsorption isotherms
Adsorption isotherms of MB on HCL were obtained in temperature range of 25–45°C using the UV-visible spectrophotometer to quantify the equilibrium amount of MB after the adsorption.
Results and discussion
Characteristics of hemicellulose-containing latex
The solid content of hemicellulose-containing emulsion was 16.3 % and the conversion of monomer was 97.6 %, measured gravimetrically.
The particle sizes of HCL and control sample were 195 and 825 nm, respectively (Fig. 1). And the Zeta potential of HCL and the control sample were −37.0 and −13.1 mV, respectively. It is obvious that the particle size of HCL is smaller than that of control sample. The TEM images of HCL and control sample are shown in Fig. 2.
It can be seen that the particle of HCL was very uniform. For control sample, the particle was non-uniform and there were aggregates observed in sample system. In other words, the existence of hemicellulose in the latex improved the stability of the emulsion and lowered the particle size of HCL correspondingly.
Apart from the uniform particle distribution, the dried HCL also has good water absorption, enabling to adsorb 3 times water. Moreover, the HCL particles could be used as absorbent to remove the dyes from waste water. The effect of HCL and control sample on dye removal is shown in Fig. 3.
The amount of MB removal induced by HCL under different MB concentrations was all higher than that of control sample. The maximum dye removal by HCL was 93.8 %, while the control sample only reached 74.1 % under the same conditions. This is attributed to the higher zeta potential and higher surface area of HCL. After added hemicellulose, the particle size of HCL was very uniform and there was no aggregation, which indicated that the hemicellulose acts as auxiliary emulsifying agent in the system. On the other hand, hemicellulose has a negative surface charge, so the zeta potential of HCL was higher than that of control sample. Therefore, the adsorption efficiency of methylene blue onto HCL was higher than that of MB onto control sample for the higher surface area and zeta potential of HCL. Hemicellulose improved the MB removal ability of HCL.
Adsorption of MB on HCL
Effect of HCL dosage
The effect of HCL dosage on MB adsorption at 25 °C was investigated and the results are shown in Fig. 4.
Obviously, the MB removal percentage was increased with the increase of HCL dosage, though the absolute adsorption amount (Q e) was decreased. This is due to fact that the HCL at a higher dosage provides more surface area and active sites for adsorption. However, the MB amount in aqueous solution was definite, with the increase of HCL dosage, the adsorption amount of per gram HCL deceased to some extent.
Effect of initial concentration of MB
Adsorption data collected at various equilibrium concentrations can be used to obtain adsorption isotherms which are important in design of the adsorption system to remove the dyes. The effect of equilibrium concentration of MB on adsorption was investigated and results are shown in Fig. 5.
With the increase of MB equilibrium concentration, the adsorption amount increased. Based on the experimental data, the adsorption isotherm of MB onto HCL at 25°C, based on Langmuir model, is given in Fig. 6. The corresponding equation for the Langmuir model [Eq. (3)] is given as follows [21]:
where Q max is the maximum adsorption amount (mg g−1) and K L is the Langmuir binding constant which is related to the energy of adsorption (L mg−1). Plotting C e/Q e against C e gives a straight line with slope and intercept equal to 1/Q max and 1/K L Q max, respectively (Fig. 6). It was observed that the value of Q max (42.73 mg g−1) obtained from Langmuir plots at 25°C is mainly consistent with the experimentally obtained value (46.85 mg g−1). It can be concluded that the adsorption process is mainly monolayer. According to the R 2 value (0.99904) shown in Fig. 6, the experimental data of adsorption fit the Langmuir model well.
The essential features of Langmuir adsorption isotherm can be expressed in terms of a dimensionless constant called separation factor or equilibrium parameter (R L), which is given below [Eq. (4)] [22, 23]:
The value of R L indicates the nature of adsorption isotherm where 0 < R L < 1 shows favorability of the process, R L = 1 and R L > 0 define unfavorable and irreversible processes, respectively. The value of K L was 0.06963 L mg−1 obtained from the equation in Fig. 6. Therefore, the R L can be calculated using Eq. (4), which was in the range of 0.5660–0.1154. The values of R L implied that the adsorption of MB on HCL was favorable under the given conditions.
Effect of temperature
The temperature is one of the most important factors influencing the process of adsorption. The effect of temperature on the adsorption of MB onto HCL is shown in Fig. 7.
Results showed that the adsorption amount increased as the temperature increasing. This might be attributed to the increasing rate of diffusion of the methylene blue molecules, owing to the decrease in the viscosity of the solution. On the other hand, the rate of dye adsorption on HCL could also be influenced by the temperature. In fact, both the diffusivity of dye and the rate of adsorption are sensitive to temperature; and the rate-determining step depends on the activation energy. As can be seen from the Figure, the equilibrium adsorption amount was also affected by temperature. The amount of methylene blue adsorbed was increased from 32.8 to 46.5 mg g−1 as the temperature was raised from 25 to 45°C. The adsorption amount of methylene blue onto HCL increases with the increase of temperature, suggesting that adsorption is endothermic.
Effect of pH
The pH of aqueous solution is also one of the most important factors to study the adsorption property of an adsorbent. The effect of pH on the adsorption of MB onto HCL at 25 °C is shown in Fig. 8.
It can be seen that the adsorption amount (Q e) of MB on HCL was almost constant in the pH range of 3.0–7.0 and an increase was observed after pH 7.0; and reached 30.38 mg g−1 at pH 3.0 and increased to 97.27 mg g−1 at pH 13.0. The increase may be related to the formation of negative surface charges at higher and higher pH. Increasing the pH of the adsorbing medium modified the negative charges of HCL surface and this might be responsible for the increasing uptake of methylene blue at higher pH condition. Furthermore, as the pH value increased, the methylene blue cationic ions got closer to the HCL surface for the increase of negative charges and the thickness of the electrical double layer around the HCL decreased. That meant more methylene blue was adsorbed in Stern layer [24, 25]. The surface charge became more negative as the pH increased favoring the adsorption of methylene blue cationic ions.
Effect of contact time
The effect of contact time on adsorption of MB onto HCL was carried out at two initial MB concentrations (50 and 100 ppm) at 25 °C. Results are shown in Fig. 9.
With the prolonging of contact time, the adsorption amount of HCL increased gradually. The time required to reach the equilibrium was about 12 and 24 h for 50 and 100 ppm, respectively. The corresponding adsorption amounts at the equilibrium were 19.5 and 27.7 mg g−1, respectively. There is only slight increase in the adsorption amount as the concentration increased from 50 to 100 ppm. It is obvious that the saturated adsorption occurred at a low dye concentration.
Conclusion
The functional latex was successfully prepared in the presence of bio hemicellulose which plays a significant role in stabilizing the emulsion and lowering the particle size of latex. The resulting latex is very effective in absorbing dyes, methylene blue in particular. The investigation on the equilibrium adsorption indicated that the incorporation of hemicellulose into the latex increased their removal capacities toward the dye. The adsorption of MB onto the HCL was dependent on MB initial concentration, absorbent dosage, temperature, pH, and contact time. The equilibrium data fit the Langmiur isotherm model well (R 2=0.99904); and the adsorption process was an endothermic process. The maximum adsorption capacity obtained according to Langmuir model at 25 °C was 42.73 mg g−1, demonstrating that the latex prepared with hemicellulose is an effective and promising absorbent in industrial applications for the removal of dyes from waste water.
References
Sun RC, Lawther JM, Banks WB (1996) Fractional and structural characterization of wheat straw hemicelluloses. Carbohydr Polym 29:325–331
Ren JL, Xu F, Sun RC, Peng B, Sun JX (2008) Studies of the lauroylation of wheat straw hemicelluloses under heating. J Agric Food Chem 56:1251–1258
Goksu EI, Karamanlioglu M, Bakir U, Yilmaz L, Yilmazer U (2007) Production and characterization of films from cotton stalk xylan. J Agric Food Chem 55:10685–10691
Lindblad MS, Ranucci E, Albertsson AC (2001) Biodegradable polymers from renewable sources. New hemicellulose-based hydrogels. Macromol Rapid Commun 22:962–967
Tan IAW, Hameed BH, Ahmad AL (2007) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127:111–119
Al-Qodah Z (2000) Adsorption of dyes using shale oil ash. Water Res 34:4295–4303
Lee JW, Choi SP, Thiruvenkatachari R, Shim WG, Moon H (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigments 69:196–203
Sheng JW, Xie YA, Zhou Y (2009) Adsorption of methylene blue from aqueous solution on pyrophyllite. Appl Clay Sci 46:422–424
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Wang SB, Zhu ZH (2006) Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J Hazard Mater 136:946–952
Lee JW, Choi SP, Thiruvenkatachari R, Shim WG, Moon H (2006) Submerged microfiltration membrane coupled with alum coagulation/powdered activated carbon adsorption for complete decolorization of reactive dyes. Water Res 40:435–444
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Senthilkumaar S, Varadarajan PR, Porkodi K, Subbhuraam C (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interface Sci 284:78–82
Hong S, Wen C, He J, Gan FX, Ho YS (2009) Adsorption thermodynamics of methylene blue onto bentonite. J Hazard Mater 167:630–633
Pagga U, Taeger K (1994) Development of a method for adsorption of dyestuffs on activated-sludge. Water Res 28:1051–1057
Wang KY, Furney TD, Hawley MC (1995) Modeling the Hf adsorption process on wood chips in a packed-bed reactor. Chem Eng Sci 50:2883–2897
Yao Y, Xu F, Zhu Z, Xu Z, Chen M (2010) Adsorption of methyl violet onto multi-walled carbon nanotubes: equilibrium, kinetics and modeling. Fresenius Environ Bull 19:854–861
Nassar MM, Magdy YH (1997) Removal of different basic dyes from aqueous solutions by adsorption on palm-fruit bunch particles. Chem Eng J 66:223–226
Wu GW, Koliadima A, Her YS, Matijevic E (1997) Adsorption of dyes on nanosize modified silica particles. J Colloid Interface Sci 195:222–228
Liu ZH, Ni YH, Fatehi P, Saeed A (2011) Isolation and cationization of hemicelluloses from pre-hydrolysis liquor of kraft-based dissolving pulp production process. Biomass Bioenerg 35:1789–1796
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Atia AA, Donia AM, Al-Amrani WA (2009) Adsorption/desorption behavior of acid orange 10 on magnetic silica modified with amine groups. Chem Eng J 150:55–62
Weber TW, Chakravo Rk (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238
Krajewski A, Piancastelli A, Malavolti R (1998) Albumin adhesion on ceramics and correlation with their Z-potential. Biomaterials 19:637–641
Drzymala J, Sadowski Z, Holysz L, Chibowski E (1999) Ice/water interface: zeta potential, point of zero charge, and hydrophobicity. J Colloid Interf Sci 220:229–234
Acknowledgements
Authors are grateful to Atlantic Innovation Fund (AIF), Innovative Green Wood Fibre Products—NSERC Strategic Network (Canada), NSF China (No. 31200456 and No. 51379077) and program for high school excellent talent of Liaoning Province China (No. LJQ2013060) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Xiao, H. & Yang, Y. Preparation of hemicellulose-containing latex and its application as absorbent toward dyes. J Mater Sci 50, 1673–1678 (2015). https://doi.org/10.1007/s10853-014-8728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8728-8