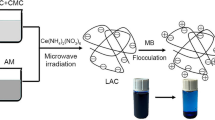
Abstract
How to efficiently utilize most abundant biomass of cellulose, lignin and their derivatives has become an emerging challenge as the anticipative oil depletion. In this paper, the ternary anionic copolymer of carboxymethyl cellulose-acrylamide-lignosulfonate (CAL) was successfully prepared by hydrothermal polymerization. Based on the flocculation characteristics of cationic methylene blue, the optimal polymerization process was confirmed as the raw material ratio of 1:1:1, initiator dosage of 0.9 wt %, the reaction time was 5 h and the reaction temperature was 55 °C. The results showed that the decolorization ratio was 87.5% at the CAL dosage of 600 mg/L for the 500 mg/L methylene blue simulated wastewater. The CAL achieved fast flocculation kinetics and super color removal ratios in the wide ranges of environmental pH, temperature and salt concentration. The flocculation mechanism is single charge neutralization. Moreover, the estimated treatment cost of CAL is 68.3% lower than that of commercial anionic PAM. The prepared anionic CAL flocculant has the characteristics of environmental safety, excellent flocculation performance and cost-effectiveness, which shows great potential in the field of dye wastewater treatment, and also provides a feasible way for the effective utilization of biomass resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the anticipative depletion of petroleum resources, cellulose, lignin and their derivatives have attracted great attentions due to their low cost, wide range of sources and multifunctional properties. They are considered as potential candidates to replace the fossil products in many industries (Luo et al. 2021; Nasrollahzadeh et al. 2021).
Cellulose is most abundant and widely distributed biomass in nature. The derivative of carboxymethyl cellulose (CMC) is a water-soluble anionic linear polysaccharide with rich hydroxyl and carboxyl groups. It can be used to remove the cationic pollutants through the flocculation or adsorption processes (Huang et al. 2019; Jasmani et al. 2016; Kong et al. 2020; Liimatainen et al. 2011). For example, CMC hydrogel could remove the heavy metal ions from the tap water (Hameed et al. 2020). In addition, CMC/acrylamide copolymers with various graft ratios and molecular weight could gain excellent flocculation effects on the printing and dyeing wastewaters (Huang et al. 2019). These previous studies have shown that CMC is a potential raw material of high performance flocculant with the advantages of wide availability and low price.
Lignin is the second-most abundant terrestrial biopolymer in the world. It contains about 20 ~ 30% of the wood dry weight. As the byproduct of pulp industry, lignin is often used in the fields of oil exploration, adhesive, bioplastics and bioenergy (Diop et al. 2017). In recent years, lignin and its derivatives, such as sodium lignosulfonate (LS) and kraft lignin, have been chemically modified as effective adsorbent, flocculant and surfactant due to the anionic property and multiple functional groups (Alipoormazandarani et al. 2021; Guo et al. 2018; Kong et al. 2015; Zhao et al. 2017). As reported by Kong et al., the flocculation ability of the modified kraft lignin was much higher than that of pure kraft lignin, and the maximum removal ratio for reactive black 5 and direct yellow 50 can reach up to 95% (Kong et al. 2015). Moreover, a binary polymer was prepared by copolymerization of lignosulfonate and [2-(Methacryloyloxy) ethyl] trimethylammonium chloride solution under short wave ultraviolet light. The removal ratio of acid black 1 was as high as 99.2% (Wang et al. 2020).
Previous research has shown that the cellulose, lignin and their derivatives are good candidates to prepare the effective flocculants. However, most flocculants prepared have the disadvantages of high flocculant dose, narrow pH window, complex chemical modification process and additional high cost from the chemicals used (Aro et al. 2017; Chen et al. 2020; Dutt et al. 2020; Liu et al. 2020; Oveissi et al. 2016; Yang et al. 2017).
To solve these problems, CMC and LS can be grafted together by the monomer of acrylamide. Grafting these two natural derived polymers would produce high molecular weight flocculant with multiple functional groups, thus improves the flocculation performance and broadens the flocculant window significantly. More importantly, as more contents of CMC and LS could be incorporated, it will not only reduce the cost of raw material, and also avoid the additional cost of the chemicals for the chemical modification process (Guezennec et al. 2014; Okaiyeto et al. 2016, Shewa et al. 2020). In general, the ternary flocculant made from CMC, AM and LS (CAL) has the advantages of environmental safety, excellent flocculation performance and cost-effectiveness.
In this paper, an efficient anionic terpolymer flocculant was prepared by hydrothermal copolymerization using CMC, AM and LS as raw materials. The synthesis process was optimized with the flocculation effect of cationic methylene blue simulated wastewater. The effects of initial dye concentration, solution pH, flocculation temperature and inorganic salt were determined. Moreover, the kinetics and flocculation mechanism were discussed in detail.
Materials and method
Materials
Sodium lignosulfonate was provided from Shanghai Yika Biotechnology Co., Ltd., carboxymethyl cellulose and methylene blue (C16H18N3ClS) were obtained from Tianjin Bodi Chemicals. Analytical grade of acrylamide (C3H5NO), ammonium persulfate ((NH4)2S2O8), sodium bisulfite (NaHSO3) and ethanol (C2H5OH) were supplied by Sinopharm Chemicals.
Synthesis of CAL
The typical CAL synthesis process of graft copolymerization was as follows. Firstly, 1.0 g of CMC, AM and LS were dissolved in 50 ml distilled water, respectively. Secondly, the three solutions were mixed in a 250 ml flask, placed in a water bath. After purged with pure N2 atmosphere for 10 min, prescribed amount of polymerization initiators ((NH4)2S2O8 and NaHSO3) were added to initiate the copolymerization. The reaction temperature and time were regulated at 55 °C and 5 h, respectively. The CAL copolymer was precipitated with iced absolute ethanol, and was filtered and washed three times with absolute ethanol to remove unreacted substances. Finally, CAL powder was gained by freeze drying for 48 h. For the process optimization, the raw material ratio, initiator content, reaction time and reaction temperature were varied. All the prepared samples are shown in the Table 1.
Characterization of CAL
The CAL specimen was characterized by scanning electron microscopy (SEM, model: JSM-6390LV, JEOL, Japan) at 10 kV voltage. Before the image acquisition, the specimen was sputtered with gold at 20 mA for 150 s.
FTIR spectra of carboxymethyl cellulose, acrylamide, lignosulfonate and CAL were tested in solid state using a Fourier transform infrared spectrophotometer (model: Nicolet Is50, Thermo Fisher Scientific, USA) between 400 and 4000 cm− 1.
The raw materials and CAL were tested by X-ray diffraction (model: DX-2700, Haoyuan, China) with Cu-Kα radiation (λ = 0.15418 nm) at 40 kV and 30 mA. The scanning speed was 2°/min, and the range of 2 θ was 5° ~ 80°.
The sample of CAL1 was dissolved in pure water with the concentration of 5 mg/L, and the Zeta potential was determined with a Malvern particle size tester (model: Zetasizer Nano ZSE, Malvern Instruments Ltd., England).
Flocculation experiment
In the flocculation experiment, the typical cationic dye of MB was selected as the simulated wastewater. The toxic MB causes nausea, diarrhea, skin irritation and cancer, which is harmful to the natural environment and human health. At room temperature of 25 °C, the prescribed CAL was mixed with 10 ml of simulated wastewater with concentration of 500 mg/L. After mixing the dye solution evenly and standing for 12 h, the suspended flocs in the dyestuff settled down on the bottom of vial completely and the flocculation process reached equilibrium. Then, the supernatant absorbance was determined at 664 nm with a UV/VIS spectrophotometer (Model 721, Shanghai Third Analysis Instrument). The color removal ratio was calculated as follows:
C0 is the initial dye concentration, and Ci is the concentration of dye after flocculation.
The effects of pH (4, 5, 6, 7, 8, 9, 10), initial dye concentration (200, 300, 400, 500 and 600 mg/L), flocculation temperature (10, 20, 30, 40 °C) and salt concentration (0.01 mol/L NaCl, CaCl2 and FeCl3) on flocculation performance were studied. The pH of MB solution was regulated with muriatic acid (0.05 mol/L) or sodium hydroxide (0.05 mol/L) solutions.
Results and discussion
Characterization of CAL
The raw material of CMC displayed the structure of short fiber with the length in the range of 2 ~ 5 μm (Fig. 1a). LS presented the shape of spherical particle with the diameter around 10 ~ 50 μm (Fig. 1b). After the graft reaction, the freeze dried CAL1 copolymer was in state of amorphous fiber. However, they were much longer than the CMC fibers (Fig. 1c). CAL had good solubility in water, same as the raw materials of CMC and LS. The determined solubility was up to 8.6 g/L.
In the FTIR spectra of CMC, the new peaks occurred at 3334 cm−1, 1323 cm−1 are attributed to the tensile and flexural vibrations of -OH, respectively (Fig. 2). Peaks at 1591 cm−1 and 1043 cm−1 are characteristic peaks of -COOH and C-O, respectively (Mishra et al. 2012). The characteristic peaks of AM are at 3173 cm−1, 1667 cm−1, 1612 cm−1 and 960 cm−1, corresponding to the tensile vibration of N–H, the bending vibration of C = O, C = C and C-H, respectively (Chen et al. 2018). Peaks of 1031 cm−1 and 689 cm−1 from LS correspond to the stretching and bending vibration of—SO3, peaks of 1577 cm−1 and 1423 cm−1 are attributed to the skeleton vibration of aromatic ring (Guo et al. 2019). CAL copolymer presented most of the characteristic peaks from CMC and LS (Fig. 2). In addition, the peak intensity decreased significantly at 1612 cm−1, indicating that C = C from AM participated in the copolymerization. Concurrently, 689 cm−1 (− SO3) from LS and 3334 cm−1 (− OH) from CMC also decreased significantly, indicating that the grafting reaction might take place on the − SO3, C = C and − OH functional groups of LS, AM and CMC, respectively (Zhang et al. 2018).
The crystal structures of lignosulfonate, carboxymethyl cellulose and CAL were characterized by XRD (Fig. 3) to show the construction of the final product further. A major crystalline peak (2θ = 20.06°) appeared in the pattern of CMC, which is the characteristic of II crystal structure (Dharani et al. 2016). Moreover, the three peaks from LS (28.52°, 34.78° and 42.52°) did not occur, and the diffraction peak at 20.06° from CMC was significantly reduced in the XRD pattern of CAL, indicating that the original construction of CMC and LS were wrecked after AM was incorporated into the ternary polymer, and a new disordered polymer was successfully synthesized by graft copolymerization (He et al. 2015).
Determination of the optimal synthesis process
The optimal synthesis process was determined by changing the raw material ratio, initiator content, reaction time and reaction temperature. The ratio of monomers leads to the difference of flocculation performance through introduction of various functional groups and charge properties. The initiator content affects the free radical quantity and the molecular weight of the final copolymer. Normally, the excessive free radicals generated by overdosed initiator lead to high rate of termination reaction, resulting in lower molecular weight products and weakened flocculation performance (Li et al. 2010; Polunin et al. 2021). Previous studies have shown that the increases of reaction time and temperature promote the copolymerization process. However, the copolymer would undergo thermal or chemical decomposition in case of prolonged reaction time and higher temperature, which decreases the copolymerization rate and leads to lower reaction yield and flocculation performance (Lou et al. 2017; Wang et al. 2016). In our study, the synthesis conditions were optimized by comparing the flocculant performance towards MB simulated wastewater (500 mg/L). As the results of Fig. 4a, b, c, d, it can be determined that the optimal synthesis conditions of CAL are material ratio of 1:1:1, initiator content of 0.9%, reaction time of 5 h and reaction temperature of 55 °C.
Effect of initial dye concentration on flocculation performance
The optimal removal ratio increased from 60.9 to 91.3% with the initial MB concentration in the range of 200 ~ 600 mg/L. The typical decolorization curve is obviously divided into two stages (Fig. 5a). The first stage is the rising stage, with the increase of flocculant concentration, the decolorization ratio reaches the optimal removal ratio. During this process, the anionic flocculant (the determined Zeta potential is −69.9 mV) completely neutralizes the opposite charge of MB dye to form an insoluble complex. Furthermore, after correlating the best dosage and the initial dye concentration, a good linear relation was found between them, indicating that flocculation is mainly controlled by charge neutralization (Fig. 5b). In the second stage, as the flocculant concentration continues to increase, the decolorization ratios decrease gradually. This is due to the charge reversal phenomenon of excessive flocculants, which leads to the enhancement of electrostatic repulsion between flocs and the reduction of flocculation ability (Cai et al. 2015).
Effect of pH on flocculation performance
In the realistic application of flocculant, the environment pH has an important influence on the flocculation efficiency. As indicated in Fig. 6, the optimal removal efficiencies of MB in the test pH range of 4 ~ 10 are higher than 70%, suggesting that CAL can be used in a wide pH window. This phenomenon is attributed to the multi-functional groups in the terpolymer, such as carboxyl, sulfonic and hydroxyl groups (Yang et al. 2014; Zahrim et al. 2010). The optimal flocculation pH was 8 with the decolorization ratio of 87.5%. This may be explicated as: MB is a cationic dye and has excellent affinity with anionic substances, the decolorization ratio of MB declines with the rise of acidity. Moreover, the level of dye ionization also has great effect on the flocculation performance. In alkaline environment, CAL with negative charge is closely combined with the cationic functional group of MB molecule by charge neutralization, which affects the ionization of the dye and leads to a decrease in decolorization ratio (Guibal et al. 2006; Zhao et al. 2021). In summary, CAL can deal with complex water environment with excellent removal effect in the practical application.
Effect of temperature on flocculation efficiency
When the flocculation temperature increased from 20 to 40 °C, the optimal removal ratio increased from 78.6 to 92.5% (Fig. 7). This might be due that the collision probability is enhanced and the electrostatic interactions between the copolymer and dye are accelerated, resulting in the improvement of flocculation performance. Previous studies also reported that the flocculation process was endothermic and the increase of temperature was conducive to flocculation (Chen et al. 2007; Ma et al. 2019). Hence, the region and season have significant influence on the flocculation performance, and the superior flocculation effect can be achieved near the equator or in summer.
Effect of inorganic salts
The existence of inorganic salt affects the electrostatic effect and reduces the flocculation performance by shielding the charge of flocculant (Cai et al. 2015). The effects of three common salts (NaCl, CaCl2 and FeCl3, 0.01 mol/L) on the performance of CAL flocculation were researched. The results indicated that the presence of NaCl and CaCl2 decreased the flocculation performance slightly compared with the unsalted samples. However, the presence of FeCl3 decreased the removal ratio from 83.9 to 20.9% (Fig. 8). This can be attributed to two facts. One is that with the increase of ionic strength, the positive charge of CAL is partially shielded and the effect of trivalent metal ion is the strongest, which weakens the electrostatic interaction between the flocculant and the dye (Jiang et al. 2011). Another reason is that the Fe3+ cations compete with the positive charged MB to bind the active sites from CAL.
Dynamics of flocculation process
Figure 9 shows that the flocculation process is divided into two stages. In the first stage of the rising trend, the decolorization ratio reaches 60% in about 4.8 min, where the flocculation process is mainly controlled by charge neutralization. In the second stage, with the raise of the amount of flocculant, the removal ratio increases slowly until it reaches equilibrium. This is because the concentration of dye decreases quickly and the charge neutralization slows down. As the discussion of the initial dye concentration effect, only the flocculation mechanism of charge neutralization was presented in the test ranges of dye concentration and flocculant dose. The excellent flocculation kinetics might relate with the pure charge neutralization effect, since the bridge and sweep effects normally need long time to accomplish the flocculation process (Yang et al. 2016). As reported by the previous studies, the removal ratio of the flocculant synthesized by the copolymerization of AM and methyl acrylloxyethyl trimethylammonium chloride was 60% after 10 min (Ma et al. 2017). The graft copolymer with acrylamide and magnetic cellulose needed 30 min to achieve the removal ratio of 66.4% (Mohamed Noor et al. 2020). Compared with other flocculants, CAL has the advantages of fast treatment speed and excellent flocculation effect. The fast kinetics makes the flocculation process effectively and saves the time cost.
Comparison of flocculation performance
Figure 10 shows the flocculation effect of CAL and commercial anionic PAM on MB solution. Commercial anionic PAM (molecular weight: 15 million) is a kind of water-soluble polymer with high molecular weight and good solubility. It is mainly used for flocculation and sedimentation of various cationic industrial wastewaters (Mohamed Noor, Ngadi et al. 2020). Due to the electrostatic and hydrogen bonding interaction between PAM and suspended particles, the dye particles combine with PAM to settle down and accomplish the flocculation process (Bao et al. 2011; Zheng et al. 2020). As shown in Fig. 10, the decolorization ratios of MB reach to 57% and 81.5% with PAM and CAL dosage of 600 mg/L, the flocculation performance of PAM is 30.1% lower than that of CAL. The results shows the CAL is more potential to be widely used in practical production than the commercial anionic PAM.
We also compared the flocculants based on lignin and carboxymethyl cellulose for the past few years (Table 2). Most of flocculants are binary system and are prepared with the chemical modification. In this study, ternary CAL synthesized from inexpensive natural polymers of CMC and LS exhibits the virtues of low cost and eco-friendliness. Moreover, the CAL flocculant shows fast flocculant kinetics and excellent flocculation performance in broad ranges of environmental pH, temperature and salt concentration. The prospect of CAL in practical dye wastewater treatment is viable due to its ecological friendliness, cost-effective performance and high flocculation performance.
Cost effectiveness evaluation
In terms of cost, the price of CMC, AM and LS gained from wholesale suppliers in China is 1.2, 1.4 and 0.4 USD/kg respectively. The cost of raw materials is 1.77 USD/kg based on the mass ratio of 1:1:1(CMC: AM: LS), the yield of 80%, and additional manufacturing cost (42% of the raw material cost) (Alsbaiee et al. 2015). The treatment costs of CAL and PAM (molecular weight: 15 million, 2.8 USD/kg) are 0.533 USD/ton and 1.68 USD/ton, respectively (dye wastewater: 100 mg/L, removal ratio of 50%). It can be seen that the cost of CAL is 68.3% lower than that of PAM in treating the same wastewater. It is reasonable to expect that CAL is economically competitive with PAM in the practical application.
Conclusions
In this study, the anionic copolymer of carboxymethyl cellulose, acrylamide and lignosulfonate was prepared by hydrothermal polymerization method. The optimal synthesis conditions were determined by the flocculation properties towards MB. The results showed that the decolorization ratio towards 500 mg/L cationic MB wastewater could reached 87.5% at the flocculant dosage of 600 mg/L. The flocculant has fast flocculation kinetics and good flocculation effect in broad ranges of environmental pH, salt concentration and temperature. The flocculation mechanism is single charge neutralization. Moreover, cost evaluation shows the treatment cost of CAL is 68.3% lower than that of commercial anionic PAM. The anionic flocculant CAL has the advantages of easily available materials, cost effectiveness and excellent flocculation performance. It has a bright future in the field of dye wastewater treatment.
References
Alipoormazandarani N, Zhang Y, Fatehi P (2021) Super functional anionic hydrolysis lignin for capturing dyes. Ind Crop Prod 162:113243
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR (2015) Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 529:190–194
Aro T, Fatehi P (2017) Production and Application of lignosulfonates and sulfonated lignin. Chemsuschem 10:1861–1877
Bao Y, Ma J, Li N (2011) Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr Polym 84:76–82
Cai T, Li H, Yang R, Wang Y, Li R, Yang H, Li A, Cheng R (2015) Efficient flocculation of an anionic dye from aqueous solutions using a cellulose-based flocculant. Cellulose 22:1439–1449
Chen Y, Liu S, Wang G (2007) A kinetic investigation of cationic starch adsorption and flocculation in kaolin suspension. Chem Eng J 133:325–333
Chen L, Zhu H, Sun Y, Chiang P-C, Sun W, Xu Y, Zheng H, Shah KJ (2018) Characterization and sludge dewatering performance evaluation of the photo-initiated cationic flocculant PDD. J Taiwan Inst Chem Eng 93:253–262
Chen N, Liu W, Huang J, Qiu X (2020) Preparation of octopus-like lignin-grafted cationic polyacrylamide flocculant and its application for water flocculation. Int J Biol Macromol 146:9–17
Cui G, Wang X, Xun J, Lou T (2017) Microwave assisted synthesis and characterization of a ternary flocculant from chitosan, acrylamide and lignin. Int Biodeter Biodegr 123:269–275
Dharani M, Balasubramanian S (2016) Synthesis, characterization and application of acryloyl chitosan anchored copolymer towards algae flocculation. Carbohydr Polym 152:459–467
Diop CIK, Tajvidi M, Bilodeau MA, Bousfield DW, Hunt JF (2017) Isolation of lignocellulose nanofibrils (LCNF) and application as adhesive replacement in wood composites: example of fiberboard. Cellulose 24:3037–3050
Dutt MA, Hanif MA, Nadeem F, Bhatti HN (2020) A review of advances in engineered composite materials popular for wastewater treatment. J Environ Chem Eng 8:104073
Feng X, Wan J, Deng J, Qin W, Zhao N, Luo X, He M, Chen X (2020) Preparation of acrylamide and carboxymethyl cellulose graft copolymers and the effect of molecular weight on the flocculation properties in simulated dyeing wastewater under different pH conditions. Int J Biol Macromol 155:1142–1156
Guezennec AG, Michel C, Bru K, Touze S, Desroche N, Mnif I, Motelica-Heino M (2014) Transfer and degradation of polyacrylamide-based flocculants in hydrosystems: a review. Environ Sci Pollut R 22:6390–6406
Guibal E, Van Vooren M, Dempsey BA, Roussy J (2006) A review of the use of chitosan for the removal of particulate and dissolved contaminants. Sep Sci Technol 41:2487–2514
Guo Y, Gao W, Fatehi P (2018) Hydroxypropyl sulfonated kraft lignin as a coagulant for cationic dye. Ind Crop Prod 124:273–283
Guo K, Gao EB, Wang W, Yue Q, Xu X (2019) Evaluation of molecular weight, chain architectures and charge densities of various lignin-based flocculants for dye wastewater treatment. Chemosphere 215:214–226
Hameed A, Khurshid S, Adnan A (2020) Synthesis and characterization of carboxymethyl cellulose based hydrogel and its applications on water treatment. Desalin Water Treat 195:214–227
He K, Lou T, Wang X, Zhao W (2015) Preparation of lignosulfonate–acrylamide–chitosan ternary graft copolymer and its flocculation performance. Int J Biol Macromol 81:1053–1058
Huang T, Shao YW, Zhang Q, Deng YF, Liang ZX, Guo FZ, Li PC, Wang Y (2019) Chitosan-cross-linked graphene oxide/carboxymethyl cellulose aerogel globules with high structure stability in liquid and extremely high adsorption ability. ACS Sustain Chem Eng 7:8775–8788
Jasmani L, Eyley S, Schütz C, Van Gorp H, De Feyter S, Thielemans W (2016) One-pot functionalization of cellulose nanocrystals with various cationic groups. Cellulose 23:3569–3576
Jiang X, Cai K, Zhang J, Shen Y, Wang S, Tian X (2011) Synthesis of a novel water-soluble chitosan derivative for flocculated decolorization. J Hazard Mater 185:1482–1488
Kong F, Parhiala K, Wang S, Fatehi P (2015) Preparation of cationic softwood kraft lignin and its application in dye removal. Eur Polym J 67:335–345
Kong Q, Wang X, Lou T (2020) Preparation of millimeter-sized chitosan/carboxymethyl cellulose hollow capsule and its dye adsorption properties. Carbohydr Polym 244:116481
Li M, Zhu Z, Jin E (2010) Graft copolymerization of granular allyl starch with carboxyl-containing vinyl monomers for enhancing grafting efficiency. Fiber Polym 11:683–688
Liimatainen H, Sirvio J, Sundman O, Visanko M, Hormi O, Niinimaki J (2011) Flocculation performance of a cationic biopolymer derived from a cellulosic source in mild aqueous solution. Bioresource Technol 102:9626–9632
Liu D, Liu J, Zhou Y, Chen J, Zhan P, Yang G, Wu Z (2020) Assembly of lignin-based colloidal particles: effects of cationic surfactants, molecular weight, and solvent on morphology. RSC Adv 10:18594–18600
Lou T, Wang X, Song G, Cui G (2017) Synthesis and flocculation performance of a chitosan-acrylamide-fulvic acid ternary copolymer. Carbohydr Polym 170:182–189
Luo J, Ma XT, Zhou X, Xu Y (2021) Construction of physically crosslinked cellulose nanofibrils/alkali lignin/montmorillonoite/polyvinyl alcohol network hydrogel and its application in methylene blue removal. Cellulose 28:5531–5543
Ma J, Fu K, Fu X, Guan Q, Ding L, Shi J, Zhu G, Zhang X, Zhang S, Jiang L (2017) Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification. Sep Purif Technol 182:134–143
Ma J, Wang R, Wang X, Zhang H, Zhu B, Lian L, Lou D (2019) Drinking water treatment by stepwise flocculation using polysilicate aluminum magnesium and cationic polyacrylamide. J Environ Chem Eng 7:103049
Mishra S, Usha Rani G, Sen G (2012) Microwave initiated synthesis and application of polyacrylic acid grafted carboxymethyl cellulose. Carbohydr Polym 87:2255–2262
Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS (2021) Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: a review. Carbohydr Polym 251:116986
Noor MHM, Ngadi N, Inuwa IM, Opotu LA, Nawawi MGM (2020) Synthesis and application of polyacrylamide grafted magnetic cellulose flocculant for palm oil wastewater treatment. J Environ Chem Eng 8:104014
Okaiyeto K, Nwodo UU, Okoli SA, Mabinya LV, Okoh AI (2016) Implications for public health demands alternatives to inorganic and synthetic flocculants: bioflocculants as important candidates. MicrobiologyOpen 5:177–211
Oveissi F, Sitter T, Fatehi P (2016) PDADMAC as a flocculant for lignosulfonate of NSSC pulping process. Biotechnol Prog 32:686–691
Polunin Y, Burns TJ, Serum EM, Sibi MP, Voronov A (2021) Evaluation of 3-Allyl-5-vinylveratrole in Latex Copolymerization with an Acrylic Monomer from High Oleic Soybean Oil. ACS Sustain Chem Eng 9:7003–7011
Shewa WA, Dagnew M (2020) Revisiting chemically enhanced primary treatment of wastewater: a review. Sustainability 12:5928
Wang D, Zhao T, Yan L, Mi Z, Gu Q, Zhang Y (2016) Synthesis, characterization and evaluation of dewatering properties of chitosan-grafting DMDAAC flocculants. Int J Biol Macromol 92:761–768
Wang B, Wang H-M, Sun D, Yuan T-Q, Song G-Y, Shi Q, Zheng L, Wang S-F, Sun R-C (2020) Chemosynthesis, characterization and application of lignin-based flocculants with tunable performance prepared by short-wavelength ultraviolet initiation. Ind Crop Prod 157:112897
Yang Z, Wu H, Yuan B, Huang M, Yang H, Li A, Bai J, Cheng R (2014) Synthesis of amphoteric starch-based grafting flocculants for flocculation of both positively and negatively charged colloidal contaminants from water. Chem Eng J 244:209–217
Yang R, Li H, Huang M, Yang H, Li A (2016) A review on chitosan-based flocculants and their applications in water treatment. Water Res 95:59–89
Yang XG, Zhang LW, Jin X, Liu L, Zhang Y, Ni QQ, Yao JM (2017) Synthesis of hydrophobically modified cellulose-based flocculant and its application in treatments of kaolin suspension and machining wastewater. Cellulose 24:5639–5647
Zahrim AY, Tizaoui C, Hilal N (2010) Evaluation of several commercial synthetic polymers as flocculant aids for removal of highly concentrated C.I. acid black 210 dye. J Hazard Mater 182:624–630
Zhang X, Wang M, Ji C-H, Xu X-R, Ma X-H, Xu Z-L (2018) Multilayer assembled CS-PSS/ceramic hollow fiber membranes for pervaporation dehydration. Sep Purif Technol 203:84–92
Zhang W, Wang X, Xu Q, Peng J, Lou T (2019) Synthesis of lignosulfonate-acrylamide-dimethyldiallylammonium chloride copolymer and its flocculation performance. J Appl Polym Sci 137:48560
Zhao J, Zheng K, Nan J, Tang C, Chen Y, Hu Y (2017) Synthesis and characterization of lignosulfonate- graft-poly (acrylic acid)/hydroxyethyl cellulose semi-interpenetrating hydrogels. React Funct Polym 115:28–35
Zhao X, Wang X, Lou T (2021) Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J Hazard Mater 403:124054
Zheng X, Zheng H, Xiong Z, Zhao R, Liu Y, Zhao C, Zheng C (2020) Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem Eng J 392:123706
Acknowledgments
This study was supported by Department of Science and Technology, Shandong province, China (No. ZR2020MB142).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, G., Gao, T., Wang, X. et al. A cost-effective anionic flocculant prepared by grafting carboxymethyl cellulose and lignosulfonate with acrylamide. Cellulose 28, 11013–11023 (2021). https://doi.org/10.1007/s10570-021-04232-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04232-8