Abstract
In this work, it is reported the necessity to characterize the raw carbon materials before their application in composite electrodes based on multiwall carbon nanotubes (MWCNTs) dispersed in epoxy resin for the development of improved amperometric sensors. These sensors must contain an optimum MWCNT/epoxy ratio for their best electroanalytical response. The main drawback in MWCNTs composite materials resides in the lack of homogeneity of the different commercial nanotubes largely due to different impurities content, as well as dispersion in their diameter/length ratio and state of aggregation. The optimal composite electrode composition takes into account the high electrode sensitivity, low limit of detection, fast response, and electroanalytical reproducibility. These features depend on carbon nanotube physical properties as the diameter. Three different commercial carbon nanotubes with different diameters were characterized by transmission electron microscopy and the results were significantly different from the ones provided by the manufacturers. Then, the three MWCNTs were used for the MWCNT/epoxy sensors construction. After an accurate electrochemical characterization by cyclic voltammetry and electrochemical impedance spectroscopy, they were employed as working electrodes using ascorbic acid as a reference analyte. Percolation theory was applied in order to verify the electrochemical results. It is demonstrated that the optimum interval load of raw carbon material in the optimized-composite electrodes closely depends on the MWCNTs diameter, needing 5 % in carbon content for the narrowest MWCNTs containing composite electrodes versus 12 % for the widest MWCNTs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composite materials based on different forms of carbon as conductive phase, dispersed in polymeric matrix, have enabled important advances in the analytical electrochemistry field, particularly in (bio)sensor devices [1–5].
During the past decades, the electrochemical properties of different carbon paste electrodes have been studied in detail [6–8]. Recently, it has been developed different composite materials based on different types of polymeric matrices, obtaining interesting electrochemical applications by using graphite powder as conductive phase [1, 9–11].
Currently, high interest is focused on composites based on carbon nanotubes (CNTs), because of their remarkable electrical, thermal, and mechanical properties. Furthermore, they exhibit a higher area to volume ratio, lower resistivity and higher mechanical and chemical stability than other carbon allotropic forms such as graphite and fullerenes [12–15]. For these reasons, it is proved that the electrocatalytic activity of CNTs, focused on multiwall carbon nanotubes (MWCNTs), combined with the polymeric matrix properties are very useful for CNT-based amperometric (bio)sensors construction [3, 16, 17], especially for the detection of compounds such as total cholesterol [18], glucose [19], ascorbic acid [20, 21], chlorine [22], and hydrogen peroxide [23, 24].
Some parameters, such as the composite resistivity, the background capacitance current, the material stability, and the heterogeneous electron transfer rate, depend on carbon load within the polymeric matrix [25]. Regarding to the background capacitance current, the electrode capacitance value is directly related to the amount of exposed carbon [26]. Besides, depending on the carbon load, composite electrodes can behave in the same way as microelectrodes array [27, 28]. The carbon load depends on the physical properties of raw CNTs. The lack of homogeneity in the different commercial CNTs is largely due to the metal impurities content and the high dispersion in their diameter/length [29]. One of the main parameters used to determine the percolation behavior and the conductivity of the composites is the length/diameter ratio of CNTs [30–33]. In most studies, the aspect ratio of the CNTs was calculated using the lengths and diameters given by the manufacturer. There are only few studies on the measurement of the MWCNTs length and diameter distributions [34–38]. The low electrochemical reproducibility of (bio)sensors is due to the poor homogeneity of raw MWCNTs. Consequently, a careful characterization and optimization of the raw conductor material results mandatory [26, 39, 40].
Therefore, the aim of this work was to study one of the physical properties of raw MWCNTs, concretely the diameter [41] and its relation in the electrochemical response of the sensor in order to obtain the optimum MWCNT/polymer ratio in composite materials in function of the raw conductor material for their application in amperometric (bio)sensors. Accordingly, three different commercial MWCNTs with a priori distinctive diameters and resin epoxy have been used to fabricate three series of composites from 1 to 20 % in MWCNTs load for each of them.
Finally, the benefits of using this approach in terms of analytical performance are demonstrated by the detection of ascorbic acid as reference analyte.
Experimental
Chemicals and reagents
Three different commercial MWCNTs were used in this research. MWCNT-1 was provided by HELIX Material Solutions (Richardson, TX, USA) and its physical properties are >95 % of carbon purity, 10–20 nm of length and 1–2 μm on diameter. The physical properties of MWCNT-2, provided by SES Research (Houston, TX, USA), were >95 % of carbon purity, 10–30 nm of outer diameter and has about 5–15 μm of length. Finally, MWCNT-3 from Sigma-Aldrich (St. Louis, MO, USA) has >95 % of carbon purity, about 6–9 nm of outer diameter and 5 μm of length. All of them were produced using a chemical vapor deposition (CVD) method. Epotek H77A and its corresponding hardener Epotek H77B, from Epoxy Technology (Billerica, MA, USA), were used as polymeric matrix. All solutions were prepared using deionised water from a Milli-Q system (Millipore, Billerica, MA, USA). Potassium ferricyanide/ferrocyanide (99.8 %), ascorbic acid (99.5 %), potassium nitrate (99.0 %), potassium chloride (99.5 %), and nitric acid (65 %) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Apparatus and procedure
Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) studies were carried out in a conventional three-electrode cell powered by an electrochemical system consisting of a pontentiostat/galvanostat Autolab system (PGSTAT 30 and FRA boards, Eco Chemie, Utrech, The Netherlands). The system was run on a PC using GPES and FRA 4.9 software. The measurements were made in a 10.0 mL of 0.1 M KCl containing 0.01 M K3[Fe(CN)6]/K4[Fe(CN)6] under quiescent condition. The kinetic processes of those species are strongly dependent on the state of the electrode surface. The experiments were performed at room temperature (25 °C). For impedance measurements, a frequency range of 100 kHz–0.1 Hz was employed. The signal amplitude to perturb the system was 10 mV and the equilibrium time was 15 s. Cyclic voltammograms were taken at the same experimental condition (10 mV/s of scan rate). A single junction reference electrode Ag/AgCl Orion 900100 (Thermo Electron Corporation, Beverly, MA, USA) and a platinum-based electrode 52-671 (Crison Instruments, Alella, Barcelona, Spain) were used as reference and auxiliary, respectively. The MWCNT/epoxy composite electrodes, prepared following the conventional methodology previously established [9], were employed as working electrodes. Finally, the MWCNT composite response changing a concentration of ascorbic acid was evaluated by hydrodynamic amperometric measurements using an amperometer LC-4C (Bio Analytical Systems INC., West Lafayette, IN, USA). Ascorbic acid was used as an analyte for evaluating the electroanalytical characteristics of sensors. Measurements were carried out at 600 mV fixed potential.
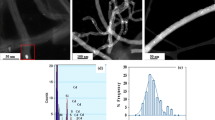
Diameter and length of the different raw MWCNTs were estimated from transmission electron microscopy (TEM) images, using a JEM-1400 unit with an acceleration voltage of 120 kV. A ~1 mg of MWCNTs was dispersed in 5 mL of acetone as organic solvent and then placed in ultrasound bath for 1 h. Finally, a drop of this solution was placed on a grid and let it dry before TEM analysis. Both distributions were compiled from at least 100 tubes. Measurements of composites topography were made by Confocal Microscopy 3D (CM3D). A Leica DCM 3D unit to 150× magnifications was used for measuring fifty-seven profiles in three different areas for each optimum composite electrode (ISO 4287). Electrical resistance measurements were performed by means of a digital multimeter (Fluke, Everett, WA, USA). Lastly, the carbon content was evaluated by thermogravimetric analysis (TGA) [42], using a Netzsch instrument, model STA 449 F1 Jupiter®. A ~20 mg of sample was heated to 1000 °C at 10 °C/min, using flow of air. The mass of the sample was continuously measured as a function of temperature and the rate of weight loss (d.t.g.) was automatically recorded.
Composite preparation
A resin Epotek H77 and its corresponding hardener compound were mixed in the ratio of 20:3 (w/w). Three series of MWCNT/epoxy composites were prepared by loading different amounts of CNTs, from 1 to 20 % (w/w) into the epoxy resin before hardening. The composites were homogenized for 1 h. The mixture was blended thoroughly and placed in the hollow end of a PVC tube to form the body of the electrode [9]. The composite paste thickness in the electrode was 3 mm. Then, the composite paste electrodes were allowed to harden during 24 h at 80 °C [5]. Afterward, composite electrodes surfaces were polished with different sandpapers of decreasing grain size. The final electrode dimensions were 28 mm2 of physical circular area. The three series of MWCNT/epoxy composites were made in the same way but modifying the MWCNT nature. Thus, the first series was MWCNT-1/epoxy composites and they contain MWCNT-1, C1. The second series consists of MWCNT-2/epoxy composites, C2. Finally, the third series of composites was made dispersing MWCNT-3 into epoxy resin, C3.
Results and discussion
Physical properties characterization of raw MWCNTs
Diameter and length of the three different MWCNTs (MWCNT-1, MWCNT-2, and MWCNT-3) were calculated from TEM images (Fig. 1). For the measurements, a greater dispersion of these tubes was needed. After an accurate data compilation, an important difference between the experimental results and the specifications provided by the manufacturers were found as it is shown in Table 1.
Firstly, it was observed a shorter MWCNT length than the specified one by the manufacturers which were above 5 µm for MWCNT-3 and above 1 µm for the rest. Both lower and upper length limit values obtained by TEM images were shorter than the specified by the manufactures, as shown in Table 1. Thus, 0.5 µm was the estimated lower length limit value for the three kinds of MWCNTs. Regarding to the upper length, 1.5, 2.0, and 2.5 µm were the values obtained for MWCNT-1, MWCNT-2, and MWCNT-3, respectively.
Secondly, the diameter was another characteristic that shows really different results as well. MWCNT-1 and MWCNT-2 obtained superior diameters for both lower and upper limit. Moreover, while MWCNT-1 was expected to be narrower than MWCNT-2, the result was contrary and MWCNT-1 became the widest. Finally, MWCNT-3 had the diameter value closer to the provided by Sigma-Aldrich, emerging the narrowest.
On the other hand, TGA analysis reveals information about purity of MWCNTs, as to percentage of carbon referred. It has been observed that all the carbon was oxidized to CO2 at 600 °C in air atmosphere [43]. The background line is because of the metallic catalyst residues. In this case, the purity obtained by TGA for each MWCNT was slightly higher than the specified one.
In brief, length, diameter, and purity values obtained by TEM and TGA for all studied MWCNTs were different from the values given by the manufacturers. Considering length and purity experimental values were homogeneous for each MWCNT, the study was focused on the effect of the diameter in the electroanalytical response of the composite sensors.
Electrical characterization
The electrical properties of the MWCNT composite electrodes were investigated by percolation theory [22]. In order to estimate the maximum conductivity value of the composite materials with the minimum conductive particle loading, each series of MWCNT/epoxy composites was tested following the experimental procedure previously presented. While C1, C2, and C3 composites contained more than 20 % of MWCNTs had a poor mechanical stability, those composites contained less than 1 % exhibited resistivity values that tend to infinity. Analytically, a lower resistivity value produces a higher sensitivity. The percolation threshold (PT) zone was achieved around 1 % in conductor material because at this point the first conductive network is formed throughout the matrix. Resistivity decreases rapidly below PT by several orders of magnitude to a minimum value, where variations in resistivity are negligible. It is important to highlight that MWCNTs loading has to be high enough to guarantee a conducting pathway and sufficiently low bulk resistivity. Due to this fact, the study was based on the zone right below PT zone, see Fig. 2. After the experiments, three different conductive regions (Fig. 2b) were determined regarding the electrical behavior: high resistance zone composite (HRC), near-percolation zone or second percolation zone composite (NPC), and low resistance zone composite (LRC).
Percolation curve obtained for the rigid conducting composites based on the polymer epoxy and raw MWCNTs for a C1, b C2, and c C3. b describes HRC High resistivity zone composites, and thus lowly conducting; PC percolation zone composites, NPC Near-percolation zone composites, and LRC Low resistivity zone composites, and thus highly conducting
Comparing the electrical differences between the three series of MWCNT/epoxy composites, a displacement in the NPC is observed in Fig. 2. This fact is due to the differences of the MWCNTs diameter. For composites loaded with MWCNT-1 which have a greater diameter, around 12 % in MWCNTs was the needed load to fall on the second PT, Fig. 2a. However, composites containing MWCNT-3 had the lowest diameter and the first point that fell on the NPC was around 5 % in MWCNTs, Fig. 2c. Finally, for MWCNT-2, the first composition that fell on the second PT zone was 10 % in MWCNTs, as it is shown in Fig. 2b. Thus, as diameter of raw MWCNTs decreases, a leftward displacement on the second PT zone was observed, due to the increase of surface area for the same percentage of MWCNTs in the composite, see Table 2. Consequently, a lower load of MWCNTs is required for those composites that contain the narrowest nanotubes (C3).
Electrochemical impedance spectroscopy measurements
Ohmic resistance (RΩ), charge transfer resistance (Rct), and double-layer capacitance (Cdl) for composites with different MWCNT loadings were evaluated for the three series of MWCNT/epoxy composites by EIS technique. These parameters were obtained by fitting the impedance spectra to a simple equivalent circuit: RΩ·(Rct·Cdl), using the equilibrium potential previously obtained by CV. This circuit was sufficiently suitable to interpret the RΩ, Rct, and Cdl values in terms of the interfacial phenomena that occur at the electrochemical cell, Fig. 3. If in a previous study was demonstrated a general trend of these physical parameters as a function of MWCNT loading [26], in this work is attempted to obtain the relation between raw MWCNTs diameter and the electrochemical response of the composite electrodes.
Impedance spectra for different MWCNT-3 loading electrodes (C3) in the presence of 0.01 M [Fe(CN)6]3−/4− and 0.1 M KCl. RΩ·(Rct·Cdl) were the equivalent circuit used for the impedance spectra fitting. MWCNT-3 containing electrodes a from 3 to 20 % in nanotubes and b zoom of the low impedance composite electrodes (from 10 to 20 %)
Accordingly, 15 different compositions of MWCNT/epoxy composite electrodes were studied for each series by changing the MWCNT loading from 1 to 20 %. For each MWCNT composition, three equal electrodes were prepared and evaluated in order to obtain the reproducibility of the composites. From NPC to LRC (>10 % of MWCNT loading for C1, >9 % for C2, and >5 % for C3) impedance values showed a kinetically controlled response for those composites with low resistivity due to the impedance behavior was dominated by a small diameter semicircle. In some cases, the diffusion-controlled process started to be discerned at low frequencies. On the other hand, the impedance plots for composites from HRC to near-percolation resistance (<10 % for C1, <9 % for C2, and <5 % for C3, percentage expressed in MWCNTs content) appeared to be dominated by a big diameter semicircle and only the kinetic-controlled electrode process was present in the recorded frequency range. An example of this behavior is shown in Fig. 3 for MWCNT-3/epoxy composite electrodes (C3).
RΩ parameter, which is dependent on the ionic concentration, the type of ions and also the electrode area, consists of the solution resistance in series with the contact or the ohmic composite resistance. C1, C2, and C3 showed a RΩ decrease at the same time that the MWCNT loaded increased, as shown in the black plots in Fig. 4. Whereas at low carbon loads the ohmic resistance is dominated by ohmic composite resistance, at higher carbon loads the ohmic resistance is more dominated by the solution resistance.
Concerning Rct, this parameter is well known as a useful parameter reflecting the facility of electrode reaction, which can be measured by EIS from the semicircle diameter in the Nyquist plots [44]. Moreover, the charge transfer resistance was inversely proportional to the heterogeneous charge transfer rate and also affected the sensitivity and response time of the electrode. From Fig. 4, the light gray plots show the decrease of Rct with the increasing of MWCNTs load because of the rise of electroactive sites. This increase also exhibits an enhancement on the electrodes kinetics. It is known that the electrode kinetics of CNTs electrodes is dependent on the CNTs nature and structure. Thus, the electroactive area becomes different from a same percentage of MWCNTs, depending on the raw MWCNTs nature; as a matter of fact it was the diameter for this study. However, electroactive area was very similar for MWCNT/epoxy near-percolation composites, motivating to think that they should have a similar electroanalytical behavior, see Table 2. This fact demonstrated again the importance of characterizing both raw carbon material and CNT/resin ratio in order to make the best electrochemical sensor. Moreover, it was observed that composites with low charge transfer resistances were the most appropriate to be used in electrochemical measurements. According to the results, composites between NPC and LRC presented lower charge transfer values. The use of near-percolation composite electrodes guarantees a fast electron exchange. In spite of the enhanced kinetics, high load of conducting material can increase the background current and smear the Faradaic signal response, especially when the electroactive species are present in low concentration. As a consequence, it is important to consider the remaining impedance parameter represented by the double-layer capacitance which is directly related to the charging or background current.
An increase of Cdl values was obtained for electrodes comprising high surface area of conducting material. In general, composites contain only a fraction of conductive area exposed to the solution and the other fraction is occupied by the insulating polymer. The electrode capacitance, which is determined nearly exclusively by the exposed carbon, becomes low and because of that the background current decreases. This fact enhances the signal to noise ratio and consequently decreases the analyte detection limits (LOD). The decrease of the double-layer capacitance values with the decrease of the MWCNT loadings can be observed in Fig. 4 (dark gray plots).
An electrode for electroanalytical purpose must have a rapid response time, low limit of detection, and high sensitivity. According to the impedance results and taking that into account, the interval between 10 and 12 % of MWCNT-1 loading fulfill all these requirements for MWCNT-1/epoxy composites, C1. For C2, the optimum ratio was between 9 and 10 % of MWCNT-2. Those intervals are similar because of the proximity of their diameter. Finally, an interval around 5–6 % in MWCNT-3 load was considered the optimum to obtain the best electroanalytical response for MWCNT-3/epoxy composites, C3. These results demonstrated that the optimum interval of MWCNT/epoxy ratio was closely tied to raw MWCNTs nature.
It is worthy to highlight that the manufactured composites in the optimum carbon load intervals mentioned above presented similar electrochemical performance. The benefit of working within these intervals led to an increase of the electrochemical reproducibility. Furthermore, it is assured the compromise to work with electrodes with low resistivity and high signal-noise ratio.
Finally, it was observed that the optimum interval of MWCNT/epoxy ratio for each series was in agreement with the obtained results in the percolation curve.
Cyclic voltammetry characterization
CV was used in order to obtain the equilibrium electrode potential of each composite composition, necessary parameter to make EIS measurements. Moreover, it was also used to compare the results obtained with the EIS technique. Different parameters were calculated at the same experimental conditions from the cyclic voltammograms, such as the equilibrium electrode potential (Eeq), peak separation potential (ΔE), and peak current (Ip). The relative electroactive area (A) was estimated from the peak-shaped voltammograms by quantifying the peak current with the use of this relationship, \( {\text{I}}_{\text{p}} = 3.0 1\cdot 10^{ 5} {\text{n}}^{ 3/ 2} \left( {\alpha {\text{D}}_{\text{red}} \upsilon } \right)^{ 1/ 2} {\text{AC}}_{\text{red}}^{*} \) [45], which is appropriate for electron transfer-controlled processes, Table 2. An increase of the peak current with the MWCNT loading was observed for each series, due to an increase of the electroactive area, together with a decrease of peak separation related to an enhancement of the electron transfer rate. Figure 5 shows the cyclic voltammogram of the three optimized carbon load composites previously selected for each series (5 % for C1, 10 % for C2, and 12 % for C3). Non-significative changes of ΔE and Ip were observed, demonstrating again that the optimum composites have the same electrochemical behavior and that their performance depends on the raw MWCNTs diameter.
Morphological characterization
Roughness surfaces of NPC electrodes were compared for the three different MWCNT/epoxy composites. Thus, the topography of each optimum composite was measured by CM3D. Figure 6 presents the most significant images obtained during the electrode surface study. Based on these results showed in Table 3, it was determined that the three optimum electrode topographies were strongly similar, in spite of the differences with carbon loading. These results were the expected due to their electroactive areas (A) are much similar, as it is shown in Table 2. Once again it is corroborated that the physical properties of raw nanotubes are crucial in the electrochemical response of the sensor.
Electroanalytical performance
Ascorbic acid was used as a reference analyte for evaluating the electroanalytical characteristics of sensors. The MWCNT/epoxy composite response to the change of ascorbic acid concentration was evaluated by hydrodynamic amperometric measurements. The analytical parameters as the LOD and sensitivity were evaluated for the optimum composite electrode composition of each series and compared with the most concentrated composites prepared (20 % in carbon load), see Table 2. Furthermore, 20 % in carbon load is the convectional composition used in the vast majority of previous studies reported by our research group [5, 46]. Comparing optimum composite sensors with the ones which contain 20 % in carbon load, the best LOD was obtained for optimized-composite sensors, although the most concentrated composites prepared contain a higher number of transducer particles. A lower MWCNTs load results in a increase of the signal to noise ratio, Cdl. C3 sensors containing 20 % in carbon load could not be amperometrically stabilized due to their high value of Cdl. 9 % of MWCNT-3 was the highest carbon load which could be measured in this series. The LOD obtained for this electrode composition was 0.68 ppm, higher than optimized-composite electrode composition (5 % for C3).
On the other hand, comparing among series, the lower LOD was obtained for the optimum composite composition which contains nanotubes with the narrowest diameter (MWCNT-3). MWCNT-1 and MWCNT-2 optimum composites loading are closely similar that the same LOD was obtained. These results showed again the Cdl effect on the amperometry measurements. Finally, in agreement with the percolation theory and the electrochemical results, the best sensor for each series of MWCNTs in terms of LOD is the one which just falls on second PT zone: 5 % for C1, 10 % for C2, and 12 % for C3.
Conclusions
Purity and dimensions of raw MWCNTs (in powder form) were studied and compared with the provided values by the manufacturers. While the carbon content is in good agreement with the manufacturer specifications, the length is significantly shorter and the diameter is proved to be strongly different. Considering the purity and length as constant parameters, the diameter value could be considered as the only variable. One of the most important parameters that determines the composite conductivity and the percolation behavior of the MWCNTs is the aspect ratio. Thus, a leftward displacement on the NPC could be observed, only needing 5 % in carbon load the narrowest MWCNT containing composites (MWCNT-3) in comparison with 12 % for the widest, MWCNT-1. Therefore, the CNT diameter is a key parameter to optimize the composite ratio, needing less carbon load for the narrowest nanotubes.
The electrochemical measurements (EIS and CV) were in concordance with the results achieved by electrical experiments (PT), obtaining the same optimum MWCNT/epoxy ratio for each series. A minimum LOD for 5 % MWCNT-3/epoxy electrode was obtained when it was used as a working electrode for ascorbic acid detection as reference analyte, due to the Cdl value turned out to be the lowest and this parameter is directly connected with the signal to noise ratio. On the other hand, non-optimized composites (20 % in carbon load) presented a higher LOD, showing the necessity of optimizing the MWCNT/polymer ratio. Accordingly, an optimum characterization of the raw carbon material before their application for amperometric sensors is required to obtain an optimal composite electrode composition which contains high sensitivity, low limit of detection, fast response, and electroanalytical reproducibility.
References
Céspedes F, Martinez-Fàbregas E, Alegret S (1996) New materials for electrochemical sensing I. Rigid conducting composites. Trends Anal Chem 15:296–304
Zhao Q, Gan Z, Zhuang Q (2002) Electrochemical sensors based on carbon nanotubes. Electroanalysis 14:1609–1613
Vashist SK, Zheng D, Al-Rubeaan K, Luong JHT, Sheu F-S (2011) Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol Adv 29:169–188
Yang X, Feng B, He XL, Li FP, Ding YL, Fei JJ (2013) Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim Acta 180:935–956
Pumera M, Merkoçi A, Alegret S (2006) Carbon nanotube-epoxy composites for electrochemical sensing. Sensors Actuat B: Chem 113:617–622
Švancara I, Vytřas K, Barek J, Zima J (2001) Carbon paste electrodes in modern electroanalysis. Crit Rev Anal Chem 31:311–345
Zima J, Švancara I, Barek J, Vytřas K (2009) Recent advances in electroanalysis of organic compounds at carbon paste electrodes. Crit Rev Anal Chem 39:204–227
Navratil T, Barek J (2009) Analytical applications of composite solid electrodes. Crit Rev Anal Chem 39:131–147
Alegret S, Morales A, Céspedes F et al (1996) Hydrogen peroxide amperometric biosensor based on a peroxidase-graphite-epoxy biocomposite. Anal Chim Acta 332:131–138
Lermo A, Fabiano S, Hernández S et al (2009) Immunoassay for folic acid detection in vitamin-fortified milk based on electrochemical magneto sensors. Biosens Bioelectron 24:2057–2063
Orozco J, Fernández-Sánchez C, Mendoza E, Baeza M, Céspedes F, Jiménez-Jorquera C (2008) Composite planar electrode for sensing electrochemical oxygen demand. Anal Chim Acta 607:176–182
Wang D, Li Z-C, Chen L (2006) Templated synthesis of single-walled carbon nanotube and metal nanoparticle assemblies in solution. J Am Chem Soc 128:15078–15079
Gao C, Li W, Morimoto H, Nagaoka Y, Maekawa T (2006) Magnetic carbon nanotubes: synthesis by electrostatic self-assembly approach and application in biomanipulations. J Phys Chem B 110:7213–7220
Balasubramanian K, Burghard M (2005) Chemically functionalized carbon nanotubes. Small 1:180–192
Guadagno L, De Vivo B, Di Bartolomeo A et al (2011) Effect of functionalization on the thermo-mechanical and electrical behavior of multi-wall carbon nanotube/epoxy composites. Carbon 49:1919–1930
Valentini F, Amine A, Orlanducci S, Terranova ML, Palleschi G (2003) Carbon nanotube purification: preparation and characterization of carbon nanotube paste electrodes. Anal Chem 75:5413–5421
Shi J, Wang Z, Li H-l (2007) Electrochemical fabrication of polyaniline/multi-walled carbon nanotube composite films for electrooxidation of methanol. J mater sci 42:539–544
Solanki PR, Kaushik A, Ansari AA, Tiwari A, Malhotra B (2009) Multi-walled carbon nanotubes/sol-gel-derived silica/chitosan nanobiocomposite for total cholesterol sensor. Sensors Actuat B: Chem 137:727–735
Noonan M (2005) Glucose biosensor based on carbon nanotube epoxy composites. Nanosci Nanotechnol 5:1694–1698
Liu M, Wen Y, Xu J et al (2011) An amperometric biosensor based on ascorbate oxidase immobilized in poly(3,4-ethylenedioxythiophene)/multi-walled carbon nanotubes composite films for the determination of L-ascorbic acid. Anal Sci 27:477–482
Liu Y, Su Z, Zhang Y et al (2013) Amperometric determination of ascorbic acid using multiwalled carbon nanotube-thiolated polyaniline composite modified glassy carbon electrode. J Electroanal Chem 709:19–25
Olivé-Monllau R, Baeza M, Bartrolí J, Céspedes F (2009) Novel amperometric sensor based on rigidi near-percolation composite. Electroanalysis 21:931–938
Shobha Jeykumari DR, Ramaprabhu S, Sriman Narayanan S (2007) A thionine functionalized multiwalled carbon nanotube modified electrode for the determination of hydrogen peroxide. Carbon 45:1340–1353
Liang M, Jin F, Liu R et al (2013) Enhanced electrochemical detection performance of multiwall carbon nanotubes functionalized by aspartame. J Mater Sci 48:5624–5632
McCreery RL (2008) Advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687
Olivé-Monllau R, Esplandiu MJ, Bartrolí J, Baeza M, Céspedes F (2010) Strategies for the optimization of carbon nanotube/polymer ratio in composite materials: applications as voltammetric sensors. Sensors Actuat B: Chem 146:353–360
Arrigan DW (2004) Nanoelectrodes, nanoelectrode arrays and their applications. Analyst 129:1157–1165
Weisshaar DE, Tallman DE (1983) Chronoamperometric response at carbon-based composite electrodes. Anal Chem 55:1146–1151
Castillo FY, Socher R, Krause B et al (2011) Electrical, mechanical, and glass transition behavior of polycarbonate-based nanocomposites with different multi-walled carbon nanotubes. Polymer 52:3835–3845
Martin C, Sandler J, Shaffer M et al (2004) Formation of percolating networks in multi-wall carbon -nanotube-epoxy composites. Composites Sci Technol 64:2309–2316
Li J, Ma PC, Chow WS, To CK, Tang BZ, Kim JK (2007) Correlations between percolation threshold, dispersion state, and aspect ratio of carbon nanotubes. Adv Funct Mater 17:3207–3215
Jiang M-J, Dang Z-M, Xu H-P, Yao S-H, Bai J (2007) Effect of aspect ratio of multiwall carbon nanotubes on resistance-pressure sensitivity of rubber nanocomposites. Appl Phys Lett 91:072907-1–072907-3
Pegel S, Pötschke P, Petzold G, Alig I, Dudkin SM, Lellinger D (2008) Dispersion, agglomeration, and network formation of multiwalled carbon nanotubes in polycarbonate melts. Polymer 49:974–984
Song W, Windle AH (2005) Isotropic-nematic phase transition of dispersions of multiwall carbon nanotubes. Macromolecules 38:6181–6188
Krause B, Boldt R, Pötschke P (2011) A method for determination of length distributions of multiwalled carbon nanotubes before and after melt processing. Carbon 49:1243–1247
Rosca ID, Hoa SV (2009) Higly conductive multiwall carbon nanotube and epoxy composites produced by three-roll milling. Carbon 47:1958–1968
Chen Z, Appenzeller J, Knoch J, Lin Y-m, Avouris P (2005) The role of metal-nanotube contact in the performance of carbon nanotube field-effect transistors. Nano Lett 5:1497–1502
Krause B, Villmow T, Boldt R, Mende M, Petzold G, Pötschke P (2011) Influence of dry grinding in a ball mill on the length of multiwalled carbon nanotubes and their dispersion and percolation behaviour in melt mixed polycarbonate composites. Composites Scie Technol 71:1145–1153
Zhao H, O’Hare D (2008) Characterization and Modeling of Conducting Composite Electrodes. J Phys Chem C 112:9351–9357
Carabineiro S, Pereira M, Nunes-Pereira J et al (2012) The effect of nanotube surface oxidation on the electrical properties of multiwall carbon nanotube/poly (vinylidene fluoride) composites. J Mater Sci 47:8103–8111
Cadek M, Coleman J, Ryan K et al (2004) Reinforcement of polymers with carbon nanotubes: the role of nanotube surface area. Nano Lett 4:353–356
Mansfield E, Kar A, Hooker SA (2010) Applications of TGA in quality control of SWCNTs. Anal Bioanal Chem 396:1071–1077
Pang LS, Saxby JD, Chatfield SP (1993) Thermogravimetric analysis of carbon nanotubes and nanoparticles. J Phys Chem 97:6941–6942
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley, New York
Pacios M, Del Valle M, Bartroli J, Esplandiu M (2008) Electrochemical behavior of rigid carbon nanotube composite electrodes. J Electroanal Chem 619:117–124
Acknowledgements
We are sincerely grateful to all our associates cited throughout the text for making this publication possible. J. Muñoz thanks Universitat Autònoma de Barcelona (UAB) for the award of PIF studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muñoz, J., Bartrolí, J., Céspedes, F. et al. Influence of raw carbon nanotubes diameter for the optimization of the load composition ratio in epoxy amperometric composite sensors. J Mater Sci 50, 652–661 (2015). https://doi.org/10.1007/s10853-014-8624-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8624-2