Abstract
Chitosan is an aminopolysaccharide that binds metal ions through different mechanisms such as ion exchange, chelation or formation of ternary complex. The sorption performance depends on the characteristics of the solution (pH, presence of ligands, metal speciation) and the properties of the biopolymer (crystallinity, degree of deacetylation, molecular weight). Sorption performance is also controlled by the accessibility and availability of reactive groups (diffusion properties). These interactions chitosan/metal ions can be used for environmental applications (recovery of toxic or valuable metals) but also for the synthesis of new materials. Hybrid materials (chitosan/metal ion composites) can thus be used for manufacturing new sorbents with improved functionalities, supported catalysts, antimicrobial supports and sensors. The physical versatility of the biopolymer is an important criterion for designing these new materials: The conditioning of the material under the form of hydrogel beads, membranes, fibers and hollow fibers, foams and sponges enhances sorption performance and allows developing new applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The regulations at national or international scales concerning the (a) discharge of heavy metals to the environment and (b) the recycling of waste materials have led to improving metal recovery processes. Conventional processes such as solvent extraction, precipitation, ion exchange or chelating resins and membrane technologies frequently fail to comply with discharge levels (technical limitations), economic constraints (especially for dilute effluents) or environmental issues (toxic sludge production or sub-products, for example). Biosorption is an alternative process to conventional techniques that can be very efficient for the recovery of metal ions from dilute effluents; in this sense, it is frequently considered as a polishing treatment. It consists in using materials of biological origin for the binding of metal ions through mechanisms analogue to those involved in metal uptake by conventional resins (i.e., chelation, ion exchange, etc.) [1]. Though active biosorption mechanisms can be included in these techniques, it is generally easier managing sorption processes that do not require using living microorganisms; in most cases, inactive fungal, bacterial, algal biosorbents are used, as well as waste materials from agriculture. The great variability of the biomass (depending on the age of microorganism culture, the culture medium, etc.) can explain problems to reach reproducible sorption performance. Localizing the metal ions in the cell wall of these microorganisms allows identifying the reactive groups that can be involved in metal uptake and more specifically the active biopolymers (i.e., alginate for algal biomass, chitin-based polymers for fungal biomass). These biopolymers can be more efficient or easier to use than the primary materials. This review focuses on chitosan-based materials for metal binding and shows some examples of new applications based on the synthesis of hybrid materials (associating chitosan and metals).
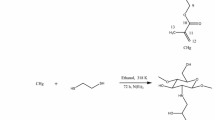
Chitosan is an aminopolysaccharide (poly[β-(1 → 4)-2-amino-2-deoxy-d-glucopyranose]) that is only present in some rare microorganisms (especially Mucorales) (Fig. 1). This polymer is produced, at commercial scale, by the deacetylation of its precursor (i.e., chitin, fully acetylated glucosamine, poly[β-(1 → 4)-2-acetamido-2-deoxy-d-glucopyranose]) [2]. Chitin is the main constituent of the cell wall of fungi; it is the structuring component of the cuticle of insects and the shell of crustacean: It is responsible of the strength of the “shell” of arthropods. Actually, commercial chitosan (being only partially deacetylated) can be considered as a heteropolymer constituted of acetylglucosamine and glucosamine units; the distinction between chitin and chitosan is thus associated with the ability of the biopolymer to be dissolved in acetic acid solution: chitosan being soluble, contrary to chitin. The solubility of chitosan in dilute acidic solutions depends on the degree of acetylation and the type of acid: dilute sulfuric acid and phosphoric acid (to a lesser extent) maintain biopolymer stability [2].
The amino groups of chitosan are responsible of the original properties of this biopolymer; its cationic behavior in acidic solutions is unique among polysaccharides. Protonation of amine groups leads to ion exchange and electrostatic attraction mechanisms. In addition, the free electron doublet of nitrogen can explain the chelating properties of the polymer for metal cations in near neutral solutions [3, 4].
This work describes these sorption mechanisms, the experimental parameters that control metal binding (metal speciation) and identify the critical steps (diffusion properties) in the process for designing the best strategy for process optimization (both in terms of solution properties and polymer conditioning). Finally, some examples of application of the hybrid materials (chitosan–metal) are briefly presented to show how it is possible to offer a “new life” to chitosan after metal binding.
Chitosan, structure and properties
Structure
Chitosan being obtained by the partial deacetylation of chitin, the biopolymer is described as a linear heteropolymer constituted of glucosamine and acetylglucosamine units (Figure AM1, See Additional Material Section). Indeed, the complete deacetylation of chitin requires very drastic deacetylation conditions with a strict control of experimental conditions (non-oxidizing conditions), and this is systematically associated with a depolymerization of the biopolymer [2]. So, the deacetylation degree is a critical characteristic of chitosan, not only in terms of overall percentage but also in terms of distribution along the chains [5]. The deacetylation degree has a direct impact on sorption properties, acid–base characteristics and solubility behavior (which, in turn, may affect the possibility to physically modify the biopolymer). The distinction between chitin and chitosan is made on the solubility of the biopolymer in acetic acid (among other acids, with the exception of sulfuric acid) solution rather than on a formal value of the deacetylation degree.
The second critical parameter is the molecular weight. The extraction process (concentration of reagents, contact time, temperature, redox conditions, etc.) [2] and the deacetylation procedure (chemical vs. enzymatic process) may strongly interfere on the molecular weight of chitosan [6, 7].
The source of chitin may also strongly influence chitosan structure in terms of crystallinity. Indeed, depending on the source of chitin (which is highly ordered and crystalline), the arrangement of biopolymer chains can be significantly changed: In α-chitin, the chains are antiparallel, while in β-chitin, they are parallel. A third rare form has been identified: γ-chitin where two chains are up to each chain down [2]. The deacetylation of chitin completely changes the crystalline structure of the biopolymer. However, though the original polymorphic form of chitin is not retained in the structure of chitosan, significant differences were observed on the X-ray diffraction patterns of chitosan produced from fungal biomass and shrimp (α-chitin) and squid (β-chitin) [8]. This may be attributed to residual zones that were not fully deacetylated and that retain the original arrangement of chitin polymer chains. Post-treatments (reacetylation, dissolving/reprecipitation, drying, etc.) may strongly influence the final crystallinity of the material [9, 10]. In addition, chitosan can be considered as a poorly porous material as shown by its specific surface area (a few m2 g−1) for chitosan flakes or powder.
Properties in relation with metal binding
The solubility of chitosan in acidic solutions (with the remarkable exception of sulfuric acid solutions) is directly related to the protonation of amine groups. The acid/base properties of chitosan (defined by the apparent pKa of amine functions on the polymer) depend on the degree of deacetylation and the degree of dissociation of amine groups [11]. For conventional chitosan samples (degree of acetylation lower than 70 %), the apparent pKa (at full dissociation degree) varies between 6.3 and 6.8. This means that at pH lower than 5 amine groups of chitosan are mainly protonated (more than 90 %) and that the biopolymer will dissolve. This is important for specific applications such as coagulation–flocculation or ultrafiltration assisted by complexation (UFAC) [12]. In UFAC, the macromolecular ligand is mixed with the target metals, a semipermeable membrane retains the metal-loaded macromolecules in the retentate, while the ultrafiltrate (which contains the other solutes) passed through the membrane. When using sulfuric acid solutions for pH control, the biopolymer remains stable (non-dissolved) though amine groups are protonated making possible the binding of metal anions [13–16]. In some cases (such as acidic solutions, other than sulfuric acid media), it is necessary to cross-link the biopolymer to prevent its dissolving. Several agents can be used; the most frequently used being glutaraldehyde, epichlorhydrin and ethylene-diglycidyl-ether [4]. The extent of the cross-linking and the localization of the bonds (chitosan/cross-linking agent) are key parameters since it may affect the availability of reactive functional groups. For example, the cross-linking with glutaraldehyde (formation of a keto–imine between aldehyde groups and amine groups) limits the number of amine groups free for reacting by chelation with metal cations. Similarly, the degree of acetylation also controls the proportion of free amine groups that will be available for metal binding: The degree of deacetylation will impact the sorption performance.
As shown above, chitin is a crystalline material, though chitosan does not retain this crystallinity, residual crystalline zones may contribute to limit the hydration of the biopolymer, which, in turn, controls the accessibility of reactive functions to water and metal ions in the core of sorbent particles [17]. Combined to the poor porosity of the biopolymer, this may contribute to slow down the mass transfer of metal ions and uptake kinetics. So, this is not only the number of reactive groups that are available for metal binding that should be taken into account but the number of reactive groups that are available and accessible [17].
To improve uptake kinetics, it is thus necessary reducing the size of sorbent particles (mass transfer enhancement, larger exchange surface, “diffusion thickness”) but at the expense of operating limitations (solid/liquid separation at the end of the sorption step in batch reactor or hydrodynamic constraints in fixed-bed column systems). These limitations may explain the development of alternative conditionings of the biopolymer to overcome the problems associated with diffusion restrictions, operating constraints, etc.
Conditioning
The dissolving of chitosan in acid solutions (hydrochloric acid, acetic acid, in most cases) is used for elaborating new shapes and forms of chitosan-based materials. This step contributes to (a) decrease the crystallinity of the raw material (mobility of polymer chains) and (b) expand the porous structure of the material (high water content). However, regardless of the conditioning mode, it is necessary strictly controlling the drying of the hydrogel at the end of the process. Indeed, an uncontrolled drying leads to partial restoration of initial crystallinity. Freeze-drying, drying in the presence of “spacer” (such as saccharose) and, better, drying under supercritical CO2 conditions, allows maintaining at least partially the porosity of gelled materials [18, 19]: The transition from hydrogel to aerogel/alcogel/xerogel is a critical step in the optimization of biopolymer macrostructure.
Different physical forms of chitosan have been developed (Fig. 2): (a) hydrogel beads, (b) films and membranes, (c) fibers and hollow fibers, and (d) foams and sponges. The biopolymer is dissolved in acetic solution and then shaped using different techniques. Gel beads are prepared by pumping the viscous chitosan solution through a thin nozzle, and the beads are neutralized or gelled in alkaline solutions (or hexametaphosphate solution) for acid neutralization [20]. More sophisticated systems can be used (Encapsulator Büchi, for example) for high-flow production of standardized and well-separated beads (using electrostatic facilities, vibrator system, etc.). Fibers are extruded through a spinneret before falling in a neutralization/gelling bath (wet spinning) or into ammonia atmosphere (dry spinning) [10, 21, 22]. For hollow fibers, it is necessary using a concentric spinneret system with circulation of the neutralizing solution in the central nozzle. Films and membranes are generally produced by (a) pouring the viscous solution into a mold, (b) drying (completely or partially) and (c) final neutralization or gelling [23–25]. It is also possible to directly extrude the viscous biopolymer solution into the neutralizing/gelling solution through a specially designed injector system (thin slot). For sponges and foams, the concept is based on: (a) the pouring of the viscous solution into a mold, (b) the freezing of the system and (c) the neutralization/coagulation step (preceded or followed by a freeze-drying) [26, 27]. New technologies for the preparation of hydrogel foams have been recently developed using supercritical fluids [28, 29]: The hydrogels that were initially prepared with conventional processes (biopolymer dissolving followed by cross-linking with glutaraldehyde, for example) are submitted to a CO2 atmosphere under high pressure. Afterward, the pressure was released rapidly, causing the formation of highly porous foam; complementary treatment may be applied, such as freeze-drying.
These new conditionings allow developing alternative modes of application. For example, hollow fibers, immersed in the metal ion solution, have been used for the binding of chromate anions by ion exchange/electrostatic attraction, while an extractant (quaternary ammonium salt, Aliquat 336) was circulated in the lumen of the fiber to desorb chromate. The system allows simultaneous sorption and desorption, while the fiber plays the role of a reactive membrane: affinity of protonated amine groups for chromate anions and physical separation of aqueous and organic phases [30]. Hyper porous foams can be used as self-supported reactive “filtration” units: The solution is pumped through the monoliths (designed as tubes, disks) with almost no hydrodynamic restrictions [27]. Based on the encapsulation properties of chitosan, it was possible to immobilize micro- or nanoparticles of ion exchangers into chitosan foams for preparing hybrid sorbents for radionuclides [31]. In the last part of this review, the use of hybrid chitosan/metal materials is considered for designing new applications. The conditioning of the biopolymer under different forms can be also useful for these new materials for enhancing the efficiency of the system or improving its mode of application.
Interactions of chitosan with metal ions: sorption mechanisms
Chelation/complexation
Though some authors reported the contribution of OH groups for the stabilization of metal bonds on chitosan, the amine groups represent the most active functional groups on the biopolymer. The free electron doublet on nitrogen is responsible of metal cation complexation. The mechanisms involved in metal sequestration remain much discussed [32, 33]; however, it is generally accepted that metal ion binds trough the formation of a “pending complex” (vs. the “bridge” model that suggests that metal ion is bound with two or more nitrogen atoms from the same or from vicinal chains) [34] (Figure AM2, See Additional Material Section). According to the HSAB principle (hard and soft acid and base theory), amine groups are considered as “intermediary” ligands that have greater affinity for transition metal cations [Cu(II), Pb(II), Zn(II)] and soft-acid metals [i.e., high polarizability and low electronegativity: Hg(II), Cd(II), Au(III), Ag(I)] compared to alkaline and alkaline-earth metals and more generally hard-acid metals (i.e., low polarizability and “hard spheres”: Sr(II), La(III) etc.) [35].
The sorption is strictly controlled by the pH of the solution due to the competition of protons with metal cations: The sorption increases with the pH of the solution [36, 37]. The degree of acetylation (and more generally the environment of amine groups) is also an important criterion: (a) reacetylated chitosan (or chitin) is not able to bind significant quantities of metal cations [34] and (b) the sorption of metal cations is strongly decreased for glutaraldehyde cross-linked chitosan (where aldehyde groups react with amine) [37].
Ion exchange/electrostatic attraction
The protonation of amine groups on chitosan in acid solutions (providing that chitosan can be maintained stable using sulfuric acid for pH control, or using cross-linked polymer) opens the possibility to bind anionic compounds by an electrostatic attraction/ion exchange mechanism (Figure AM2, See Additional Material Section): This was well documented for anionic dyes, but also for metal anions such as molybdate [15], vanadate [16]. Contrary to the chelation mechanism, the environment of amine groups has less influence on metal binding properties because this is the charge of protonated amine groups that is responsible for metal anion uptake. The cross-linking with glutaraldehyde hardly decreases molybdate sorption at least for small particles [14]. In the case of large particles, the cross-linking affects metal accessibility by decreasing the porosity (supplementary linkages between the chains of the polymer); the elaboration of glutaraldehyde cross-linked chitosan beads (with expanded structure) limits the impact of glutaraldehyde on diffusion properties and accessibility [15]. For these ion exchange mechanisms, the pH effect is mainly related to the competition effect of the counter anions (dissociation of the acid used for pH control) and to its impact on the speciation of target metals.
Formation of ternary complex
The binding of alkaline and alkaline-earth metal cations (considered as “hard acids” in the HSAB theory) is reported to be very low due to the weak affinity of these metal ions for amine groups. However, a third binding mechanism has been identified for the sorption of these metal ions [38]. For example, in the case of Sr(II), the formation of an ion pair between Sr(II) and CO3 2− (Sr2+, CO3 2−) allows the formation of a ternary complex with chitosan (Figure AM2, See Additional Material Section). Strict experimental conditions are required for efficient Sr(II) uptake: high pH value (above pH 11) and high relative concentration of carbonate (0.01 M). Obviously, these experimental conditions limit the interest of the process to very specific applications such as the treatment of effluents containing radionuclides.
Main parameters and criteria to be considered for biosorption on chitosan
The effect of pH on metal sorption has been briefly discussed above in relation to competition effects (chelation mechanism) and necessary protonation of amine groups (ion exchange/electrostatic attraction mechanism). The sorption performance depends on a great number of experimental parameters and biopolymer properties. However, in most cases, these different parameters are related to (a) crystallinity and diffusion properties of the biopolymer and (b) metal speciation in solution.
Crystallinity and diffusion properties
Depending on the origin of chitosan (squid vs. shrimp, for example), the characteristics of the biopolymer (degree of acetylation, etc.) the residual crystallinity may be significantly changed [8, 14]. The crystallinity strongly impacts the swelling properties of the biopolymer; this is playing a critical role on the accessibility of water and metal ions to internal reactive groups. The uptake kinetics are strongly affected by the crystallinity of the material: The squid chitosan (issued from the deacetylation of β-chitin) is characterized by very slow kinetics [39]. Under similar experimental conditions (metal concentration, pH, sorbent dosage), squid chitosan required more than 1 week for reaching equilibrium, while shrimp or fungal chitosan needed only 1–2 days. The arrangement (and the mobility) of the chains in the compact structure of chitosan flakes is controlling the accessibility and the availability of reactive groups for both water and metal ions [17]; this effect is reinforced when the biopolymer is cross-linked with glutaraldehyde [14]. X-ray diffraction analysis of samples allows calculating a crystalline index but also identify the presence of specific peaks that were correlated with “poor” sorption performances [14].
The diffusion properties are thus directly correlated with the structural parameters of the biopolymer. However, the conditioning of the biopolymer under the form of hydrogel allows reducing the impact of this parameter [15]. Hydrogel beads show a much expanded structure that contributes to improve diffusion properties: The specific surface area of glutaraldehyde cross-linked chitosan beads (after freeze-drying) has been reported to reach 150–250 m2 g−1 [15] and up to 330 m2 g−1 for chitosan aerogel (dried under supercritical CO2 conditions) [40]. It is noteworthy that the specific surface area of chitosan aerogel produced from squid chitosan (under the same experimental conditions) did not exceed 150 m2 g−1. The effect of diffusion properties is also observed in the comparison of Pd(II) and Pt(IV) uptake kinetics for four lots of glutaraldehyde cross-linked chitosan beads that were submitted to different drying procedures [18]. The uncontrolled drying (oven-drying) drastically reduced kinetic rates, and the preliminary rehydration of the dried beads contributed to slightly enhance uptake kinetics. However, the best results were obtained with beads that were dried in the presence of saccharose (acting as a spacer) and rehydrated before adsorption test: The kinetic profiles were close to the profiles obtained with un-dried beads. This is a clear demonstration that the uncontrolled drying causes the irreversible collapse of the porous structure of the hydrogel, losing the benefit of the conditioning of the biopolymer. Drying in the presence of a “spacer,” freeze-drying, and even better drying under supercritical CO2 conditions are powerful solutions to maintain substantial porosity in the sorbent [40].
Hence, playing with the crystallinity (dissolving, reprecipitation, and controlled drying …) and playing with the diffusion properties (expansion of the porous network, crystallinity disruption, etc.) are key parameters for controlling both accessibility and availability of reactive functional group, which, in turn, are enhancing both equilibrium performance (thermodynamics) and reaction rates (kinetics).
Metal speciation
Another critical point is the effect of the composition of the solution on the affinity of metal ions for chitosan. The speciation of the metal, associated with pH effect, may affect not only the sorption performance but also the mechanism involved in metal binding. For example, the pH controls the hydrolysis of metal ions such as molybdate [13] or vanadate [16] (See Additional Material Section). The comparison of the speciation diagram (which depends both on pH and metal concentration, for metals that form polynuclear species) with the sorption isotherms allows correlating the affinity of the material for the sorbent at different pHs. For a given pH, the sorption isotherm may have an unfavorable form: the sorption capacity being negligible on the range of low concentration and only begins to increase when the metal concentration reaches a given value that corresponds to the formation of the metal ions that have a good affinity for the sorbent. In the case of molybdate and vanadate, a clear correlation was established between the sorption efficiency and the presence of significant amounts of hydrolyzed polynuclear molybdate species and decavanadate species, respectively [13, 16]. Plotting the sorption isotherm q (sorption capacity) versus the concentration of adsorbable metal species allowed drawing very favorable (almost irreversible) isotherm (i.e., very steep initial slope followed by a saturation plateau reached at low residual concentration) instead of the unfavorable isotherm found with the plot of q versus total metal concentration (Figure AM3, See Additional Material Section).
The presence of ligands may also have a strong impact on the affinity of chitosan for metal ions. A number of papers have documented the effect of citrate, EDTA on the binding of metal ions and even on the mechanism involved in metal binding. For example, in the case of Cu(II), the presence of citrate completely changed the speciation of the metal (See Additional Material Section) and the uptake mechanism [41]. In conventional systems, copper ions are bound to chitosan through chelation mechanism at near neutral pH; in the presence of citrate anions and at appropriate pH (i.e., acidic pH), copper ions are present in the solution under the form of anionic complexes that can bind to chitosan through electrostatic attraction/ion exchange mechanism. The plot of maximum sorption capacity (q max) versus pH shows that the sorption (a) begins to be significant when the fraction of anionic copper complexes exceeds the concentration of free anionic ligand, (b) continues to increase with pH while amine groups remain protonated and (c) begins to decrease when the chitosan begins to deprotonate (above pH 5) [42]. In addition, the curve q max versus pH overlaps with the distribution curve of the complex Cu(OH)L2−: This suggests that copper is bound (under this specific form) on protonated amine groups of chitosan (Fig. 3). The presence of ligands may also influence the selectivity of chitosan for metal ions in complex solutions: In the presence of nitrotriacetic acid, chitosan binds preferentially Co(II) versus Cu(II) at pH 2.9, while at pH 6, the selectivity is reversed [43].
Cu(II) sorption in the presence of citrate ions (L ligand): a copper speciation (ACuC anionic copper citrate complex, CuFAL copper-free anionic citrate ligand), protonation of amine groups of chitosan (−RNH3 +) and maximum sorption capacity at different equilibrium pHs (q max); b distribution of copper citrate complexes and maximum sorption capacity (q max) [reprinted with permission from Elsevier, Int. J. Biol. Macromol., 2003, 33(1–3) 57]
The understanding of the speciation phenomena and the identification of predominating species can be used for predicting the best operative conditions for the binding of a specific metal taking into account the type of reactive groups present on the sorbent, as well as its acid–base properties.
Hybrid metal–chitosan materials for advanced applications
Apart the environmental application (and the valorization of secondary resources, for valuable metals), the affinity of chitosan for metal ions can be used for elaborating new materials and designing new applications. These materials are applicable in high-added value fields; this makes the cost of the biopolymer less sensitive than for the large-scale treatment of dilute effluents containing non-valuable metals. The following sections give some examples of new hybrid materials in the field of specific sorption, supported catalysis, antimicrobial supports and sensors.
New sorbents
The concept is based on the immobilization on chitosan of a metal ion that has a specific affinity or reaction with a target molecule (other metal, organic compound, etc.). The challenge is selecting the appropriate system for reaching both the stability of the metal on chitosan (oxidation state, metal desorption/release, etc.) and the interaction of the metal with the target molecule (bending strength, selectivity, etc.), especially in terms of pH and composition of the solution.
Molybdate can be used for the spectrophotometric determination of As(V) in solution (formation of the arsenomolybdate complex, followed by reduction of the complex). Since chitosan has a strong affinity for molybdate (sorption capacity can reach up to 800 mg Mo g−1), it was possible using the composite chitosan/molybdate under the form of gel beads [molybdate impregnated chitosan beads (MICB)] for the binding of As(V) in solution [44]. Surprisingly, As(III) (though not reactive with molybdate) was slightly bound to MICB. XPS analysis did not show any change in the oxidation state, and the mechanism of sorption was not fully explained. The material is very selective to arsenic due to the specific interaction mode between As(V) and molybdate; and only compounds that form complex with molybdate can interfere on As(V) removal; phosphate, and to a lesser extent silicate, reduce arsenic binding. This affinity of phosphate was used for selective desorption of As(V), maintaining molybdate on the sorbent with an appropriate selection of pH and phosphate concentration. Other metals [such as Cu(II), Fe(III), La(III), Zr(IV)] were also immobilized on chitosan for binding As(V): Best results were obtained with Fe(III) [45].
Another concept was recently developed for the binding of chromate with chitosan/Cu hybrid material [46]. Copper (II) is bound to chitosan before being reduced to Cu(I) with a chemical reducing agent (i.e., sodium borohydride). The composite material bound Cr(VI) by reduction to Cr(III) [and simultaneous oxidation of Cu(I) to Cu(II)]. It is noteworthy that though the reduction potentials for Cr(VI)/Cr(III) and V(V)/V(IV) are close to each over (i.e., +1.33 and +1.00, respectively), in the case of V(V), the sorption mainly proceeded through direct sorption on protonated amine groups of chitosan.
Silver is readily sorbed to cross-linked chitosan, and the affinity of silver for sulfur-bearing compounds was utilized to prepare a sorbent for the removal of a pesticide: methyl parathion [47]. Chitosan was cross-linked with either glutaraldehyde or epichlorhydrin and the resulting material bound Ag(I) by coordination at pH 5.5 in acetate buffer. The thiophosphate group of the pesticide coordinated to Ag(I). Though Ag(I) sorption was maximum with the epichlorydrin cross-liked chitosan (probably because amine groups were protected by copper-template formation before cross-linking), methyl parathion was better sorbed on glutaraldehyde cross-linked chitosan. This was explained by the direct binding of the pesticide on vacant coordination sites of Ag(I) due to the lower steric hindrance of the metal for pesticide access for glutaraldehyde cross-linked chitosan. On the opposite hand, in the case of epichlorhydrin cross-linked chitosan, occupied coordination sites of Ag(I) limit the access of the pesticide by steric hindrance. The pesticide can be released from loaded sorbent using different eluents such as acetate/acetic acid buffer (at different pHs), alkaline solutions of ammonium thiocyanide or ammonia. The challenge consisted in achieving pesticide removal while stabilizing silver on the hybrid material. The sorption/desorption was found optimal using ammonium thiocyanide solution. Despite the decrease in sorption and desorption efficiencies along sorbent reuse, the material remained very efficient for a minimum of five cycles.
Enzyme immobilization on biopolymer frequently proceeds by encapsulation or by covalent binding through linking agent (for example glutaraldehyde) with drawbacks such as mass transfer resistance (for encapsulation) or loss of enzymatic activity (case of covalent binding). In addition, it is generally difficult recovering the enzyme at the end of the process. An alternative process was reported for preparing metal-immobilized affinity chromatographic supports for the binding of trypsin [48]. The enzyme is immobilized on chitosan (supported on silica gel particles) through metal–protein interactions. Zn(II) and Ni(II) revealed the most efficient for the preparation of the supports while taking into account all the criteria: (a) amount of trypsin immobilized, (b) activity of the enzyme, (c) stability (in terms of operating time and storage) and (d) enzyme release (using acidic solutions or metal-chelating agents, such as EDTA). Using Cu(II) resulted in a loss of activity attributed to multipoint attachment between the enzyme (imidazo groups of histidine, mercapto and indoyl groups of the polypeptide backbone) and the metal that induces a conformational change in the enzyme, which loses its activity. Another example of use of chitosan-Ni(II) hybrid material for the synthesis of affinity chromatographic support has been reported [49]. Chitosan was deposited on polymeric support, and Ni(II) was chelated to amine groups (Chit–Ni); a hexahistidine-tagged green fluorescent protein was bound to the material (His-GFP/Chit-Ni). The composite material was thus tested for the immobilization of the green fluorescent protein antibody and compared to a similar material [where the hexahistidine-tagged green fluorescent protein was immobilized on chitosan through glutaraldehyde, instead of Ni(II)] [49]. The composite chitosan-Ni(II) material allowed increasing the amount of protein bound and the affinity constant for the antibody. This better reactivity of the support for the antibody was associated with the proper orientation given by Ni(II) to the histidine-tagged green fluorescent protein (compared to the reference material; i.e., glutaraldehyde cross-linked chitosan). Lysozyme recovery was also developed for the separation and purification of proteins using magnetic carboxymethyl chitosan nanoparticles loaded with Zn(II), Cu(II) and Fe(III) [50]. Carboxymethyl chitosan was used for encapsulating [by co-precipitation, in the presence of poly(ethyleneglycol)] freshly prepared magnetic iron nanoparticles (Fe3O4, 15 nm in size) before binding Zn(II), Cu(II) and Fe(III). The best results were obtained using Fe(III) and Zn(II) as the linking metal–protein agent. Indeed, in the case of Cu(II), after lysozyme desorption, a slight denaturation and conformational change in the enzyme was observed contrary to the two other metal ions. These paramagnetic particles can be efficiently used for protein separation and purification.
Supported catalysts
For the last decade, a number of new supported catalysts have been developed making profit of the affinity of catalytic metals such as Cu(II), Co(II) and more specifically Pd(II) and Pt(IV) for chitosan or chitosan derivatives [51–53]. These materials have been tested for hydrogenation reactions [54, 55], oxidation reactions [56, 57], cross-coupling reactions [58, 59], cyclo-addition reactions [59], hydroamination of alkynes [60]. Different modes of synthesis were reported depending on the oxidation state required for catalytic metal, its chemistry and affinity for chitosan: (a) adsorption, (b) precipitation and (c) co-precipitation (encapsulation) [57]. The catalytic metal ions tend to agglomerate and form clusters with a more or less important decrease of catalytic activity, and it is generally necessary to prevent this agglomeration using ligands. Chitosan can be used to stabilize catalytic metals and to prevent both the formation of aggregates (enhancement of the dispersion of nanoparticles) and the leaching of the metal [60]. The physical and chemical versatility of chitosan allows designing by adsorption different catalytic systems: flakes and beads (the sorbent is dropped into the metal ion solution) [61], hollow fibers (the metal ion solution is pumped into the lumen of the fiber) [62].
For hydrogenation reactions, palladium is the most frequently used catalytic metal. Palladium(II) is loaded on chitosan by adsorption in aqueous solutions before being reduced, using NaBH4 [55], formic acid or in situ produced hydrogen [61]. Another method consists in the mixing of chitosan with the catalytic metal in ethanol under reflux: The binding occurs by impregnation and simultaneous reduction [56]. The concentration of the catalytic metal controls the dispersion and the size of metal crystallites [63] (Figs. 4 and 5), which, in turn, influence catalytic activity. Hydrogenation reaction can take place in either aqueous solutions [61] or solvent media [55]. The hydrogen donor may be hydrogen gas or a reducing agent such as formic acid. The challenge is the stability of the chitosan support in aqueous solutions: The biopolymer is sensitive to acid conditions and a special attention has to be paid to the cross-linking treatment. The cross-linking may impact the mechanical stability of the biopolymer, especially when chitosan has been conditioned under the form of gel beads or hollow fibers [63].
Effect of Pd(II) loading on the distribution of metal nanoparticles on catalytic chitosan hollow fibers, agglomeration effect [TEM analyses and Pd(loading): a 0.25 mg/fiber; b 0.75 mg/fiber; c 1.75 mg/fiber, magnification 100000; d 1.75 mg/fiber, magnification 150000, fiber weight: 23 mg]; X-ray diffraction analysis (e) and correlation between Pd loading and size of the crystallites (calculated by the Scherrer equation) (f) [reprinted with permission from Elsevier, J. Membr. Sci., 2009, 329(1–2) 30]
SEM microphotographs of chitosan hollow fibers cross-linked with glutaraldehyde, dried under supercritical CO2 conditions (a and b internal surface; c and d cross-section) before (I) and after (II) Pd loading (1.5 mg Pd/fiber, fiber weight: 23 mg) [reprinted with permission from Elsevier, J. Membr. Sci., 2009, 329(1–2) 30]
Oxidation reactions have been reported using palladium, copper or cobalt as catalytic metals [56]. Metal binding may proceed through different mechanisms including direct sorption on chitosan, but also through co-precipitation and incorporation into chitosan hydrogels [57]. The choice of the oxidizing agent is critical and should take into account not only its reactivity (and accessibility) but also the stability of the support: For example, hydrogen peroxide may contribute to depolymerize chitosan and then induce its dissolving and degradation.
Based on the poor thermal stability of chitosan, these supports are generally limited to catalysis in liquid phase: The catalysis in gaseous phase frequently requires temperatures where the biopolymer can be significantly degraded (above 200 °C, the material begins to degrade). Another possibility would consist in (a) the binding the metal to the biopolymer (different possibilities may exist: sorption, gelling biopolymer beads into the metal solution), (b) the calcination (or pyrolysis) of the composite material to produce the metal oxide catalyst. The biopolymer serves as template to obtain morphology-controllable materials with structural specificity, complexity, dispersion of metal centers and unique functions [64]. The main problem consists in the loss of structuration of the catalyst after calcination/pyrolysis step. This problem could be probably overcome using reinforcing inert and thermally stable fibers in the encapsulating matrix.
Depending on the size of the particles and their shape, the mode of application in static or dynamic regime may vary: batch reactor for particles and gel beads, fixed-bed columns for gel beads, membrane holder for foams and disks. Hollow fibers offer the possibility to use original reactors: The catalytic metal is immobilized on the fiber (preferentially on the side in contact with the substrate to be converted, generally the inner side), and the substrate is circulated in the lumen of the fiber, while the reagent (hydrogen donor, oxidizing agent, etc.) is flowed outside the fiber [63]. The reagent (either in the liquid or in the gaseous state) diffuses through the fiber, binds to catalytic sites and reacts with the substrate.
Antimicrobial supports
The antimicrobial activity of chitosan was characterized against fungi, algae and some bacteria. Compared to conventional disinfectants, it has a higher antimicrobial activity, a broader spectrum of activity and a lower toxicity [65]. This antibacterial effect can be improved by chemical modification (grafting of carboxymethyl, sulfate moieties, etc.) and by binding metal ions such as Zn(II), Cu(II), Fe(II), Mn(II) or Ag(I) [65, 66]. The antimicrobial activity strongly depends on the properties of the biopolymer (molecular weight and degree of deacetylation), the type of metal and the environmental parameters (more specifically pH). The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MCB) strongly decreased by one or two orders of magnitude for composite materials (metal-complexed chitosan) compared to chitosan or metal alone. Another important parameter for the evaluation of antimicrobial activity is the molar ratio between the metal and the amine groups of chitosan [65, 67]. This was explained by the impact of this stoichiometric ratio on the structure of the complex, which, in turn, controls its activity [68]. A direct correlation was also observed between the antibacterial activity and the zeta potential of metal–chitosan nanoparticles [66].
Composite chitosan–silver films were prepared in the presence of poly(ethylene glycol) (PEG) and glutaraldehyde. Both chitosan and PEG contribute to reduce Ag(I) to Ag nanoparticles (12 nm by TEM analysis) [69]. In addition, PEG could be removed at the end of the synthesis procedure (heating at 80 °C in water for 5 h); this leaded to the formation of pores and holes in the composite film. Both the mechanical properties (uniaxial strain–stress tests: stress at maximum load, elongation at break) and the antimicrobial activity were improved compared to chitosan blend (silver-free) and chitosan–silver nanocomposite (non-porous) films. Obviously, the presence of the metal and the porosity/accessibility properties of the support are strongly impacting the performance of the hybrid material. This makes these materials appropriate for water purification and wound dressing.
Chitosan foams were used for immobilizing silver (100 nm nanoparticles; after exposure to UV light the nanoparticles tended to aggregate); cellulose fibers were added to the matrix for increasing the mechanical properties of the support [27]. The highly porous structure allowed using these materials for antimicrobial tests in static mode (inoculation of petri dishes and contact with Ag/chitosan/cellulose fiber disks—Antibiogram tests) and dynamic mode (circulation of the bacterial suspension through the disks immobilized in a membrane holder, with recirculation of the suspension). The results showed a very efficient antibacterial effect against Gram + and Gram-bacteria (Pseudomonas putida ≫ Escherichia coli > Staphylococcus hominis ≫ S. aureus) in static mode even with very low silver loads on the foams. In the dynamic mode (with flow recirculation), a cumulative contact time of 40–55 s was sufficient for decreasing the E. coli charge by 7 log-units (silver load on the foam 0.1 mg/foam; i.e., <0.6 % w/w) (Fig. 6).
Silver/chitosan hybrid foam material (reinforced by cellulose fiber) for antibacterial applications (SEM and SEM–EDX analyses, and effect of mean residence time and silver load on the decrease of E. coli load in the recirculation mode of bacterial suspension through the hybrid material) (reprinted with permission from Elsevier, J. Colloid Interface Sci., 2013, 393, 411)
Another example of the beneficial effects of metal on the antimicrobial effect of chitosan has been demonstrated in the agriculture field. Mixed solutions of chitosan (in acetic acid solution) and copper (under the form of nitrate salt) were sprayed on four-true-leaf cucumber seedlings, and the resistance to Botrytis cinerea rot development was analyzed and compared to plants protected with either copper or chitosan solution [70]. Though chitosan alone has an elicitor effect (contributing to stimulate the defense of the plant to pest), its association with copper allows reducing the dosages of reagents to be applied for plant protection. The synergistic effect consists in (a) a better efficiency of the copper–chitosan complex than chitosan alone, (b) a longer effect (the presence of copper is suspected to prevent the early degradation of chitosan by the natural flora) and (c) copper enhances the direct elicitation effect of chitosan. The adhesion properties of chitosan on the leaves probably contribute to immobilize the copper on the leaf for a longer time of contact. A potential improvement could consist in separating the copper–chitosan complex from free chitosan and free copper using, for example, UFAC: The Cu-loaded macromolecule is retained in the retentate and can be recovered by drying, or, better, freeze-drying. This would help in reducing the excess of free copper sprayed on the leaves (weakly bound copper).
Sensors
The incorporation of chitosan in the manufacturing of sensors has also retained a great attention for the last decade. In most cases, chitosan was selected for its properties: (a) encapsulation facilities, (b) stabilization effect on nanoparticles and (c) electrodeposition by cathodic potential. The acid–base properties of chitosan (pKa of amine groups between 6.3 and 6.8) lead to the precipitation/deposition of the biopolymer (dissolved in acid media) when the pH increases [71]. When a chitosan solution is disposed at the surface of an electrode, the application of a cathodic potential leads to (1) the conversion of protons to hydrogen, (2) the localized increase of the pH and finally (3) the deposition of the hydrogel film at the surface of the electrode. This cathodic electrodeposition can typically be used for the encapsulation of enzymes, cells nanoparticles, reagents at the contact of the electrode. In addition, the cationic behavior of the biopolymer in solution contributes to stabilize the micro- or nanoparticles that constitute or participate to the elaboration of the sensor. As reported earlier in the case of catalytic nanoparticles, the efficiency of the system is generally improved by the small size of particles (nanometric size); an encapsulating media that contributes to prevent the aggregation of nanoparticle is thus a significant advantage for the manufacturing of sensors.
Colorimetric sensors have been developed using the facility of chitosan to entrap and stabilize gold nanoparticles [72]. Chloroauric acid is heated under boiling before addition of monosodium glutamate (similar to the role of citrate ions in several other systems), which is supposed to contribute to both reduce Au(III) to gold crystals and cap gold nanoparticles (the excess of glutamate adsorbed at the surface of gold particles brings charges and induces sufficient electrostatic repulsion to keep the particles from agglomerating). The color turned from yellow to deep red color (formation of gold nanoparticles, 10–20 nm in size, by TEM analysis). Chitosan is added to the suspension after quenching at room temperature. The absorption spectra of the mixture at the different steps of the synthesis clearly show that the chitosan has a true interaction with gold nanoparticles and is not playing only the role of encapsulating material. The colloidal chitosan-stabilized gold nanoparticles have been used as a detector for Cu(II) and Zn(II) following the optical absorption of the suspension at 650 nm, in the low concentration range [1–5 mM for Cu(II) and 2–20 mM for Zn(II)]. The presence of chitosan strongly improved the sensitivity of the gold nanoparticle system for detection of Cu(II) and Zn(II): With chitosan-free nanoparticles, the spectral changes between metal-exposed and metal-free suspensions are not very marked. Another procedure was described for the detection of Pb(II) through gold–chitosan nanocomposite electrodeposited on a gold foil [73]. Chitosan was directly mixed with chloroauric acid solution and sodium borohydride before being electrodeposited. The presence of chitosan allowed improving the stability (for storage), preventing nanoparticle agglomeration. FT-IR spectroscopy confirmed the interaction of gold with amine groups. TEM analyses showed the uniform entrapment of gold–chitosan nanocomposite in the gel (at high pH). The composite gels can be deposited at the surface of gold electrodes that can be used for the analysis of Pb(II) by cyclic voltammetry. The sensitivity depends on the pH: about 1 mM at low pH and as lows as 1 µM at high pH (for the colorimetric method) and about 10 µM for the electrochemical system. The system being selective to lead in the presence of competitor metal ions [such as Cd(II) or Zn(II)] can be used for the detection of metal traces.
Another system has been developed for the electrochemical sensing of trichloroacetic acid using silver nanoparticles immobilized in chitosan hydrogel deposited by electrodeposition at the surface of the glassy carbon electrode [74]. Silver nanoparticles were produced by mixing silver nitrate with ascorbic acid (as reductant) and poly(vinylpyrrolidone (as the stabilizer, but chitosan would probably have the same effect and could substitute). Finally, the silver nanoparticles were removed by centrifugation, washed and added to the chitosan solution for cathodic electrodeposition at the surface of the glassy carbon electrode. Voltammograms were obtained for the detection of dichloroacetic acid and trichloroacetic acid. The amperometric responses for successive additions of trichloroacetic acid solutions show that the analytical system is linear in the range 3–56 µM (the detection limit being close to 1.1 µM). In addition, the tests showed that the current response of the system was quite fast (<5 s), which indicate that this is a quite fast catalytic system and that the mass transfer of substrate (and products) in the chitosan hydrogel film is not rate limiting. The electrostatic interaction between positively charged chitosan and negatively charged trichloroacetic acid may contribute to this fast mechanism.
A non-enzymatic sensor has been designed for the analysis of hydrogen peroxide using a composite made of silver nanoparticles (in situ generated) and hemoglobin [75]. Silver nanoparticles (size: 12 nm) were synthesized by reaction with glucose and chitosan in an ultrasonic batch at 80 °C. Graphite powder was mixed with melted paraffin oil before adding silver nanoparticles. The carbon paste electrode was polished and then coated with hemoglobin, while a final layer of chitosan was deposited at the surface of the hybrid material. The electrochemical properties of the sensor were characterized by cyclic voltammetry before being tested for hydrogen peroxide detection: Very low detection limit was obtained (i.e., 5 × 10−8 M, lower than conventional systems), and the response was linear on a wide range of concentrations (i.e., 8 × 10−8 M–2.5 × 10−4 M).
Conclusion
Chitosan is very efficient at removing metal ions from dilute solutions through two main mechanisms (electrostatic attraction/ion exchange and chelation). The physical versatility of the biopolymer (playing with chitosan dissolving and appropriate shaping) allows producing original materials to enhance diffusion properties, accessibility and availability of reactive groups and sorption capacities. The combination of this versatility with the high affinity of the biopolymer for transition metals can be used for metal recovery but also for designing new materials and new applications of high-added value (specific biosorbents, supported catalysts, antimicrobial supports, sensors, and so on).
The comparison of these new alternative materials is made difficult by the limited number of comparative studies performed under similar experimental conditions, especially for some of the new applications cited in this work. However, the main interest of these materials is related to the fact that: (a) they are issued from renewable resources and (b) their elimination (for example thermally) is generally less hazardous for the environment than for conventional synthetic materials. In the case of adsorption systems, the performances of these materials are generally of the same order of magnitude than those of conventional systems under mild conditions. One of the limitations that can explain their limited transfer to industry remains the difficulty for commercial suppliers to guarantee a reproducible production: Characteristics of the biopolymer may significantly change from one batch to the other with potential impact on the physicochemical characteristics of the final product. In some cases, this variability may hardly change the performance of the material, but this remains a limitation for industrial users that would prefer standardized primary resources. Another potential drawback may be related to the limited physicochemical stability of the material (against aging, bacterial degradation or chemical deterioration), though this may be in some case an advantage (easy degradation of the polymer at the end of its life cycle).
References
Volesky B (2003) Sorption & Biosorption. BV Sorbex Inc, Montréal (Canada)
Roberts GAF (1992) Chitin chemistry. The Macmillan Press Limited, London
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym 55:77–93
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74
Varum KM, Anthonsen MW, Grasdalen H, Smidsrod O (1991) High-field NMR-spectroscopy of partially N-deacetylated chitins (chitosans). 3. C-13-NMR studies of the acetylation sequences in partially N-deacetylated chitins (chitosans). Carbohydr Res 217:19–27
Jung J, Zhao Y (2011) Characteristics of deacetylation and depolymerization of beta-chitin from jumbo squid (Dosidicus gigas) pens. Carbohydr Res 346:1876–1884
Pacheco N, Garnica-Gonzalez M, Gimeno M, Barzana E, Trombotto S, David L, Shirai K (2011) Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 12:3285–3290
Jaworska M, Sakurai K, Gaudon P, Guibal E (2003) Influence of chitosan characteristics on polymer properties. I: crystallographic properties. Polym Int 52:198–205
Qun G, Ajun W, Yong Z (2007) Effect of reacetylation and degradation on the chemical and crystal structures of chitosan. J Appl Polym Sci 104:2720–2728
Notin L, Viton C, David L, Alcouffe P, Rochas C, Domard A (2006) Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: influence of the dry-jet-stretching step and ageing. Acta Biomater 2:387–402
Sorlier P, Denuziere A, Viton C, Domard A (2001) Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2:765–772
Kuncoro EP, Roussy J, Guibal E (2005) Mercury recovery by polymer-enhanced ultrafiltration: comparison of chitosan and poly(ethylenimine) used as macroligand. Sep Sci Technol 40:659–684
Guibal E, Milot C, Roussy J (2000) Influence of hydrolysis mechanisms on molybdate sorption isotherms using chitosan. Sep Sci Technol 35:1021–1038
Milot C, McBrien J, Allen S, Guibal E (1998) Influence of physicochemical and structural characteristics of chitosan flakes on molybdate sorption. J Appl Polym Sci 68:571–580
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Ind Eng Chem Res 37:1454–1463
Guzman J, Saucedo I, Navarro R, Revilla J, Guibal E (2002) Vanadium interactions with chitosan: influence of polymer protonation and metal speciation. Langmuir 18:1567–1573
Piron E, Accominotti M, Domard A (1997) Interaction between chitosan and uranyl ions. Role of physical and physicochemical parameters on the kinetics of sorption. Langmuir 13:1653–1658
Ruiz M, Sastre A, Guibal E (2002) Pd and Pt recovery using chitosan gel beads. I. Influence of the drying process on diffusion properties. Sep Sci Technol 37:2143–2166
Valentin R, Molvinger K, Quignard F, Brunel D (2003) Supercritical CO2 dried chitosan: an efficient intrinsic heterogeneous catalyst in fine chemistry. New J Chem 27:1690–1692
Sicupira D, Campos K, Vincent T, Leao V, Guibal E (2010) Palladium and platinum sorption using chitosan-based hydrogels. Adsorption 16:127–139
Desorme M, Montembault A, Lucas J-M, Rochas C, Bouet T, David L (2013) Spinning of hydroalcoholic chitosan solutions. Carbohydr Polym 98:50–63
Modrzejewska Z, Eckstein W (2004) Chitosan hollow fiber membranes. Biopolymers 73:61–68
Kaminski W, Modrzejewska Z (1997) Application of chitosan membranes in separation of heavy metal ions. Sep Sci Technol 32:2659–2668
Meneghetti E, Baroni P, Vieira RS, Carlos MG, da Silva MM, Beppu (2010) Dynamic adsorption of chromium ions onto natural and cross linked chitosan membranes for wastewater treatment. Mater Res 13:89–94
Vieira RS, Beppu MM (2006) Interaction of natural and crosslinked chitosan membranes with Hg(II) ions. Colloids Surf A 279:196–207
Madihally SV, Matthew HWT (1999) Porous chitosan scaffolds for tissue engineering. Biomaterials 20:1133–1142
Guibal E, Cambe S, Bayle S, Taulemesse J-M, Vincent T (2013) Silver/chitosan/cellulose fibers foam composites: from synthesis to antibacterial properties. J Colloid Interface Sci 393:411–420
Ji C, Annabi N, Khademhosseini A, Dehghani F (2011) Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater 7:1653–1664
Tsioptsias C, Paraskevopoulos MK, Christofilos D, Andrieux R, Panayiotou C (2011) Polymeric hydrogels and supercritical fluids: the mechanism of hydrogel foaming. Polymer 52:2819–2826
Vincent T, Guibal E (2000) Non-dispersive liquid extraction of Cr(VI) by TBP/Aliquat 336 using chitosan-made hollow fiber. Solvent Extr Ion Exch 18:1241–1260
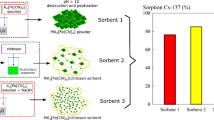
Vincent C, Hertz A, Vincent T, Barré Y, Guibal E (2014) Immobilization of inorganic ion-exchanger into biopolymer foams—application to cesium sorption. Chem Eng J 236:202–211
Terreux R, Domard M, Viton C, Domard A (2006) Interactions study between the copper II ion and constitutive elements of chitosan structure by DFT calculation. Biomacromolecules 7:31–37
Webster A, Halling MD, Grant DM (2007) Metal complexation of chitosan and its glutaraldehyde cross-linked derivative. Carbohydr Res 342:1189–1201
Rhazi M, Desbrieres J, Tolaimate A, Rinaudo M, Vottero P, Alagui A, El Meray M (2002) Influence of the nature of the metal ions on the complexation with chitosan. Application to the treatment of liquid waste. Eur Polym J 38:1523–1530
Pearson RG (1966) Acids and bases. Science 151:172–177
Miretzky P, Fernandez Cirelli A (2009) Hg(II) removal from water by chitosan and chitosan derivatives: a review. J Hazard Mater 167:10–23
Dzul Erosa MS, Saucedo Medina TI, Navarro Mendoza R, Avila Rodriguez M, Guibal E (2001) Cadmium sorption on chitosan sorbents: kinetic and equilibrium studies. Hydrometallurgy 61:157–167
Piron E, Domard A (1998) Formation of a ternary complex between chitosan and ion pairs of strontium carbonate. Int J Biol Macromol 23:113–120
Jaworska M, Kula K, Chassary P, Guibal E (2003) Influence of chitosan characteristics on polymer properties: II. Platinum sorption properties. Polym Int 52:206–212
Quignard F, Di Renzo F, Guibal E (2010) From natural polysaccharides to materials for catalysis, adsorption, and remediation. In: Rauter AP, Vogel P, Queneau Y (eds) Carbohydrates in sustainable development I: renewable resources for chemistry and biotechnology. Topics in current chemistry, vol 294. Springer, Berlin, Heidelberg, pp 165–197
Wu FC, Tseng RL, Juang RS (1999) Role of pH in metal adsorption from aqueous solutions containing chelating agents on chitosan. Ind Eng Chem Res 38:270–275
Guzman J, Saucedo I, Revilla J, Navarro R, Guibal E (2003) Copper sorption by chitosan in the presence of citrate ions: influence of metal speciation on sorption mechanism and uptake capacities. Int J Biol Macromol 33:57–65
Padala AN, Bhaskarapillai A, Velmurugan S, Narasimhan SV (2011) Sorption behaviour of Co(II) and Cu(II) on chitosan in presence of nitrilotriacetic acid. J Hazard Mater 191:110–117
Dambies L, Vincent T, Guibal E (2002) Treatment of arsenic-containing solutions using chitosan derivatives: uptake mechanism and sorption performances. Water Res 36:3699–3710
Shinde RN, Pandey AK, Acharya R, Guin R, Das SK, Rajurkar NS, Pujari PK (2013) Chitosan-transition metal ions complexes for selective arsenic(V) preconcentration. Water Res 47:3497–3506
de Godoi FC, Rodriguez-Castellon E, Guibal E, Beppu MM (2013) An XPS study of chromate and vanadate sorption mechanism by chitosan membrane containing copper nanoparticles. Chem Eng J 234:423–429
Yoshizuka K, Lou ZR, Inoue K (2000) Silver-complexed chitosan microparticles for pesticide removal. React Funct Polym 44:47–54
Wu J, Luan M, Zhao J (2006) Trypsin immobilization by direct adsorption on metal ion chelated macroporous chitosan-silica gel beads. Int J Biol Macromol 39:185–191
Ahmed SR, Kelly AB, Barbari TA (2006) Controlling the orientation of immobilized proteins on an affinity membrane through chelation of a histidine tag to a chitosan-Ni++ surface. J Membr Sci 280:553–559
Sun J, Rao S, Su Y, Xu R, Yang Y (2011) Magnetic carboxymethyl chitosan nanoparticles with immobilized metal ions for lysozyme adsorption. Colloids Surf A 389:97–103
Macquarrie DJ, Hardy JJE (2005) Applications of functionalized chitosan in catalysis. Ind Eng Chem Res 44:8499–8520
Guibal E (2005) Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci 30:71–109
Adlim M, Abu Bakar M, Liew KY, Ismail J (2004) Synthesis of chitosan-stabilized platinum and palladium nanoparticles and their hydrogenation activity. J Mol Catal A 212:141–149
Vincent T, Guibal E (2004) Chitosan-supported palladium catalyst. 5. Nitrophenol degradation using palladium supported on hollow chitosan fibers. Environ Sci Technol 38:4233–4240
Schuessler S, Blaubach N, Stolle A, Cravotto G, Ondruschka B (2012) Application of a cross-linked Pd–chitosan catalyst in liquid-phase-hydrogenation using molecular hydrogen. Appl Catal A 445:231–238
Mekhaev AV, Pestov AV, Molochnikov LS, Kovaleva EG, Pervova MG, Yaltuk YG, Grigor'ev IA, Kirilyuk IA (2011) Structure and characteristics of chitosan cobalt-containing hybrid systems, the catalysts of olefine oxidation. Russ J Phys Chem A 85:1155–1161
Kramareva NV, Stakheev AY, Tkachenko OP, Klementiev KV, Grunert W, Finashina ED, Kustov LM (2004) Heterogenized palladium chitosan complexes as potential catalysts in oxidation reactions: study of the structure. J Mol Catal A 209:97–106
Leonhardt SES, Stolle A, Ondruschka B, Cravotto G, De Leo C, Jandt KD, Keller TF (2010) Chitosan as a support for heterogeneous Pd catalysts in liquid phase catalysis. Appl Catal A 379:30–37
Martina K, Leonhardt SES, Ondruschka B, Curini M, Binello A, Cravotto G (2011) In situ cross-linked chitosan Cu(I) or Pd(II) complexes as a versatile, eco-friendly recyclable solid catalyst. J Mol Catal A 334:60–64
Corma A, Concepcion P, Dominguez I, Fornes V, Sabater MJ (2007) Gold supported on a biopolymer (chitosan) catalyzes the regioselective hydroamination of alkynes. J Catal 251:39–47
Vincent T, Guibal E (2002) Chitosan-supported palladium catalyst. 1. Synthesis procedure. Ind Eng Chem Res 41:5158–5164
Guibal E, Vincent T, Spinelli S (2005) Environmental application of chitosan-supported catalysts: catalytic hollow fibers for the degradation of phenolic derivatives. Sep Sci Technol 40:633–657
Peirano F, Vincent T, Quignard F, Robitzer M, Guibal E (2009) Palladium supported on chitosan hollow fiber for nitrotoluene hydrogenation. J Membr Sci 329:30–45
Behar S, Gonzalez P, Agulhon P, Quignard F, Swierczynski D (2012) New synthesis of nanosized Cu–Mn spinels as efficient oxidation catalysts. Catal Today 189:35–41
Wang XH, Du YM, Fan LH, Liu H, Hu Y (2005) Chitosan-metal complexes as antimicrobial agent: synthesis, characterization and structure-activity study. Polym Bull 55:105–113
Du W-L, Niu S–S, Xu Y-L, Xu Z-R, Fan C-L (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym 75:385–389
Adewuyi S, Kareem KT, Atayese AO, Amolegbe SA, Aldnremi CA (2011) Chitosan-cobalt(II) and nickel(II) chelates as antibacterial agents. Int J Biol Macromol 48:301–303
Wang X, Du YM, Liu H (2004) Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr Polym 56:21–26
Vimala K, Mohan YM, Sivudu KS, Varaprasad K, Ravindra S, Reddy NN, Padma Y, Sreedhar B, MohanaRaju K (2010) Fabrication of porous chitosan films impregnated with silver nanoparticles: a facile approach for superior antibacterial application. Colloids Surf B 76:248–258
Ben-Shalom N, Fallik E (2003) Further suppression of Botrytis cinerea disease in cucumber seedlings by chitosan–copper complex as compared with chitosan alone. Phytoparasitica 31:99–102
Wu LQ, Lee K, Wang X, English DS, Losert W, Payne GF (2005) Chitosan-mediated and spatially selective electrodeposition of nanoscale particles. Langmuir 21:3641–3646
Sugunan A, Thanachayanont C, Dutta J, Hilborn JG (2005) Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci Technol Adv Mater 6:335–340
Mathew M, Sureshkumar S, Sandhyarani N (2012) Synthesis and characterization of gold-chitosan nanocomposite and application of resultant nanocomposite in sensors. Colloids Surf B 93:143–147
Liu B, Deng Y, Hu X, Gao Z, Sun C (2012) Electrochemical sensing of trichloroacetic acid based on silver nanoparticles doped chitosan hydrogel film prepared with controllable electrodeposition. Electrochim Acta 76:410–415
Tian L, Feng Y, Qi Y, Wang B, Chen Y, Fu X (2012) Non-enzymatic amperometric sensor for hydrogen peroxide based on a biocomposite made from chitosan, hemoglobin, and silver nanoparticles. Microchim Acta 177:39–45
Acknowledgements
E.G. acknowledges the support of all PhD., master-level students and academic partners that collaborated with the research group over the last two decades for the development of biopolymer-based materials.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guibal, E., Vincent, T. & Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J Mater Sci 49, 5505–5518 (2014). https://doi.org/10.1007/s10853-014-8301-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8301-5