Abstract
The effect of Ca substitution for Sr on the phase, microstructure and microwave dielectric properties of the Sr5−x Ca x Nb4TiO17 composition series was investigated using X-ray diffraction (XRD), scanning electron microscopy (SEM), an LCR meter, and vector network analyzer. Below 1450 °C, Sr5−x Ca x Nb4TiO17 (x = 1, 2, 3, or 4) compositions formed single-phase Sr4CaNb4TiO17, Sr3Ca2Nb4TiO17, Sr2Ca3Nb4TiO17, and SrCa4Nb4TiO17 ceramics, respectively. At x = 0 and 5, Sr5Nb4TiO17 and Ca5Nb4TiO17 formed, but along with Sr2Nb2O7 (at x = 0) and CaNbO3 and CaNb2O6 (at x = 5) secondary phases. Above 1450 °C, all the compositions formed two-phase ceramics. At low frequencies, a phase transition was observed in the composition Sr5Nb4TiO17. The substitution of Ca for Sr enabled processing of highly dense Sr2Ca3Nb4TiO17, with εr ~ 53.4, τf ~ −6.5 ppm/°C and Q u × f o ~ 1166 GHz. Further investigations are required to improve the quality factor of these ceramics for possible microwave applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ceramic dielectric resonators (DRs) used at microwave frequencies have been utilized for the last few decades in mobile telecommunications devices. The three key parameters needed for a material to be used as a microwave DR are (a) a high dielectric constant (especially for handsets) to reduce the size of the microwave component, (b) low dielectric loss (implying a high quality factor, Q u , where Q u = 1/tanδ) for fine tuning and (c) a near-zero temperature coefficient of resonant frequency to ensure temperature stability of the microwave component. Only a few materials meet this industrial requirement. Examples include Ba(Zn1/3Ta2/3)O3, BaTi4O9, Ba2Ti9O20, (Zr,Sn)TiO4, and Ba6−3x Re8+2x Ti18O54 (Re = Nd, Sm, La), all of which are used at microwave frequencies in various filters and oscillators in telecommunication devices [1]. Generally, a high dielectric constant material exhibits high loss and vice versa [2, 3]; therefore, it is difficult to have materials exhibiting all the above mentioned three properties simultaneously and there is a constant demand for new materials with better dielectric properties.
A number of dielectric ceramics belonging to the A n B n O3n+2 series are known to exhibit good microwave properties. For example, (CaLa4)Ti5O17 with n = 5 and CaLa8Ti9O31 with n = 4.5 (i.e., (Ca0.5La4)(Ti4.5)O15.5) are layered perovskites, with dielectric constants εr = 55.2 and 54.9, quality factors Q u × f o = 17359 GHz (at 3.67 GHz) and 19345 GHz (at 3.65 GHz), and temperature coefficients of resonant frequency τf = −20 and −6 ppm/°C, respectively [2]. Substitution of 1 wt% of Ca by Zn in CaLa4Ti5O17 has been reported to give εr = 57.6, Q u × f o = 17100 GHz and τf ~ 4.9 ppm/°C [3]. Similarly, the addition of 0.5 wt% CuO as a sintering aid to Ca0.99Zn0.01La4TiO17 resulted in εr = 57, Q u × f o = 15000 GHz and τf ~ −8.16 ppm/°C at 1450 °C in comparison with 1500 °C for pure Ca0.99Zn0.01La4TiO17 [4]. The substitution of 1 wt% of Ca by Mg in CaLa4Ti5O17 gave εr = 56.3, Q u × f o = 12300 GHz and τf ~ −9.6 ppm/°C [5]. Anjana et al. [6] reported that Ca5Nb4TiO17 and Ca3Mg2Nb4TiO17 have εr = 45, Q u × f o = 17600 GHz and τf ~ −113 ppm/°C and εr = 37.5, Q u × f o = 22500 GHz and τf ~ −4.3 ppm/°C, respectively. More recently, Joseph et al. [7] reported Ca5Nb4TiO17 and Ca5Ta5TiO17 to have εr = 44.9, Q u × f o = 17600 GHz and τf ~ −112.9 ppm/°C and εr = 40.1, Q u × f o = 16450 GHz and τf ~ −53.6 ppm/°C, respectively.
Slobodyanik et al. [8] synthesized Ca5−x Sr x TiNb4O17 with 0 ≤ x ≤ 5 via co-precipitation of hydroxocarbonates, observed the beginning of Sr5TiNb4O17 formation at temperatures above 1300 °C, and noted the requirement of temperatures well above 1400 °C for the formation of single-phase Sr5TiNb4O17 ceramics. They did not measure the dielectric properties. Iqbal and Reaney [9] reported the dielectric constant εr ~ 57 (at 10 kHz), Q u × f o ~ 1070 MHz and TCε ~ −0.0007 ppm/°C for Sr5Nb4TiO17 with no Ca. The crystal structure of Sr5Nb4TiO17 is reported to be orthorhombic with space group Pnnm and lattice parameters a = 5.6614 Å, b = 32.515 Å, c = 3.9525 Å, and Z = 2 [10].
Keeping in view the high loss of Sr5Nb4TiO17, the effect of Ca substitution on the phase, microstructure and dielectric properties of Sr5Nb4TiO17 has been investigated as an attempt to improve Q u × f o for possible microwave applications.
Experimental procedure
Sr5−x Ca x Nb4TiO17 compositions (x = 0, 1, 2, 3, 4, and 5) were prepared via a mixed-oxide solid-state route. Laboratory reagent grade SrCO3, CaCO3 (Fisher Scientific, > 99%), Nb2O5 (BDH Chemicals Ltd., > 99.9%) and TiO2 (Aldrich Chemicals, 99 + %), powders were weighed according to the stoichiometric ratios. The mixtures were milled for 60 h in disposable polyethylene mill jars with cylindrical Y-toughened ZrO2 balls as grinding media and 2-praponol as lubricant using a conventional horizontal ball mill. The slurry was dried in an oven at ~95 °C overnight and the resulting powder samples were sieved to dissociate agglomerates (if any). Thermo-gravometric (TG) analysis and differential thermal analysis (DTA) were performed from room temperature to 1200 °C at 5 °C/min (for x ≤ 1) and 10 °C/min (for 2 ≥ x ≥ 5) for as-mixed Sr5−x Ca x Nb4TiO17 compositions to determine the weight loss and phase transformation temperatures. The Sr5Nb4TiO17 composition was calcined at 990 °C for 2 h. The other batches were calcined at 1350 °C for 2 h. Heating/cooling rates were 10 °C/min. The calcined powders were re-milled for 30 min and dried before uniaxial pressing into 13 mm diameter pellets at 140 MPa in a stainless steel die. The pellets were sintered in air at 1400–1550 °C for 2 h at heating/cooling rate of 10 °C/min. Sintered pellets were pulverized in a pestle and mortar system and ground into fine powders for XRD. A STOE PSD X-ray diffractometer with CuKα1 radiation (λ = 1.540598 Å) was used for phase analysis and measurement of lattice parameters. The sintered samples were finely polished and thermally etched at temperatures 10% less than the corresponding sintering temperatures and gold coated for microstructural analysis. Microstructural characterization was performed using a JEOL 6400 SEM operating at 20 kV, equipped with an energy dispersive X-ray spectroscopy (EDS) detector (Link, Oxford Instruments). The apparent density of the sintered pellets was measured using the Archimedes method. The theoretical densities of the compositions were calculated by using Eq. (1);
where Z is the number of formula units per unit cell, M is the molecular weight, V is the volume of the unit cell and A g is the Avogadro number (6.022 × 1023 atoms/mole).
The faces of the sintered pellets were coated with gold paste and heated to 800 °C for 2 h at 10 °C/min. The dielectric constant was measured from 1 kHz to 1 MHz at temperatures ranging from 20 to 800 °C using a HP 4284A LCR meter. For loss tangent measurements, the sintered pellets were mounted on a low-loss quartz single crystal at the centre of a 20 mm diameter cavity made of brass to avoid conduction loss. The microwave energy was coupled to the test piece using two ports. Measurements were taken in transmission mode using a network analyzer (R3767CH, Advantest). The average temperature coefficient of resonant frequency, τ f , between 20 and 70 °C was calculated using Eq. (2);
where f 1 is the resonant frequency at 20 °C and f 2 at 70 °C, and ∆T is the difference between the initial (~20 °C) and final temperature (~70 °C) of measurement.
Results and discussion
TG/DTA analysis
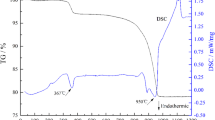
TG/DTA curves from as mix-milled 3SrCO3:2CaCO3:2Nb2O5:1TiO2 powders are shown in Fig. 1. A careful examination of the TGA curve indicated the beginning of mass loss at 650 °C, which continued till 750 °C, due to the decomposition of CaCO3 [11]. Another downward slope was observed just after 750 °C which continued until 902 °C, probably due to the decomposition of SrCO3 [12]. The observed increase in the decomposition temperature of SrCO3 from the previously reported [13] 880–902 °C in this study may be due to the increase in the heating rate from 5 to 10 °C/min [11, 14]. Two endotherms were observed in the DTA curve, at 750 and 902 °C, consistent with the temperatures at which the downward sloping of TGA curves ended. A total of ~18.60% mass loss was recorded in the entire heating cycle from 30 to 1200 °C which is consistent (within ± 1%) with the total CO2 forming during the reaction given in Eq. (3)
X-ray diffraction
The room-temperature XRD patterns recorded for the calcined and sintered (at 1450 and 1500 °C) Sr5−x Ca x Nb4TiO17 (x = 0–5) samples are shown in Figs. 2 and 3. The major phase observed in the sample with x = 0 and calcined at 990 °C/2 h was Sr5Nb4TiO17; however, a few XRD peaks matched JCPDS Card# 28–1247 for Sr2Nb2O7 indicating incomplete reaction. XRD patterns from compositions with x = 1–4 calcined at 1350 °C/2 h were similar and matched JCPDS Card# 87–1170 for Sr5Nb4TiO17 [10] indicating the formation of single-phase ceramics. It is noticeable that no JCPDS cards could be found for Ca5Nb4TiO17 but the observed major XRD peaks for the composition with x = 5, matched JCPDS Card# 51–412 for Ca5Nb5O17. Being isostructural with Ca5Nb5O17, the peaks due to Ca5Nb4TiO17 could be indexed according to a model analogous to the Ca5Nb5O17 reported in JCPDS Card# 51–412. A few low intensity peaks matching the JCPDS Card# 47–1668 for orthorhombic CaNbO3 and JCPDS Card# 39–1392 for CaNb2O6 were also observed which indicated second phase formation at x = 5.
XRD patterns from Sr5−x Ca x Nb4TiO17 samples with x = 0–5 sintered at 1450 °C for 2 h, showing the presence of single-phase compounds for x = 0, 1, 2, 3, and 5 and a mixture of layered perovskite (Sr1−x Ca x ) n (Nb,Ti) n O3n+2 phases with n = 5 and 6 for the Sr5−x Ca x Nb4TiO17 composition with x = 4
The XRD of the samples sintered at 1450 °C for 2 h revealed the formation of single phases for the compositions with x = 0, 1, 2, 3, and 5, while a mixture of n = 5 and 6 layered perovskites was found for the composition with x = 4. No reference data cards could be found for the Ca versions of the compounds expected from the compositions with x = 4; however, these XRD peaks matched JCPDS Card# 87–1170 for Sr5Nb4TiO17 and 49–544 for Sr6Nb4Ti2O20 with appropriate shifting of peak positions due to Ca substitution. Thus, SrCa4Nb4TiO17 and (Sr,Ca)6Nb4Ti2O20, being isostructural with the compounds Sr5Nb4TiO17 and Sr6Nb4Ti2O20, respectively, were indexed according to the models analogous to these compounds reported in JCPDS Card# 87–1170 and 49–544 (Fig. 2). Above 1450 °C, even the compositions with x = 1, 2, 3, and 5 were not single phase and XRD of samples sintered at 1500 °C for 2 h revealed the presence of a mixture of (Sr1−x Ca x ) n (Nb,Ti) n O3n+2 perovskite compounds with n = 5 and 6, i.e., Sr5Nb4TiO17 (JCPDS Card# 87–1170) and Sr6Nb4Ti2O20 or Ca6Nb4Ti2O20 (JCPDS Card# 49–544) for x = 0 or 5, respectively. It is noticeable that at 1500 °C, the major phases observed in the compositions with x = 1, 2, 3, and 4 were Sr4CaNb4TiO17, Sr3Ca2Nb4TiO17, Sr2Ca3Nb4TiO17, and SrCa4Nb4TiO17, respectively; however, the nature of the secondary phases, i.e., the layered perovskites with n = 6 in compositions with x = 1−4, could not be identified. The crystal structure of the compounds with x = 0−4 was orthorhombic (Pnnm) and the compound with x = 5 crystallized into a monoclinic structure with space group P21/b or P21/c [8]. Manan et al. [13, 15] and Levin et al. [16] have also reported the formation of a mixture of Sr n (Nb,Ti) n O3n+2 compounds with n = 5 and 6 in the SrTiO3–Sr2Nb2O7 system with no Ca+2 substitution. The decomposition into secondary phases is more visible in the compositions with x = 4 and 5 as compared to the compositions with x = 0−3 sintered at 1500 °C/2 h (Fig. 3).
The compounds in the system Sr5−x Ca x Nb4TiO17 crystallize into A5B5O17 structure where the large Sr/Ca cations occupy the A site with coordination of 12 and the Ti/Nb cations occupy the B site with coordination of 6. The resulting structure consists of perovskite-like blocks each of which is five octahedra thick and separated by an extra layer of oxygen. With increasing Ca+2 content from x = 1 to 5, the XRD peaks shifted toward higher diffraction angles, i.e., smaller interplanar spacings due to the smaller size of Ca+2 (1.34 Å) substituted for the larger Sr+2 (1.44 Å) ion [17, 18] resulting in a decrease in the lattice constants of the unit cell (Fig. 4).
The unit cell parameters of the Sr5−x Ca x Nb4TiO17 ceramics refined by the least squares method are given in the Table 1. The lattice parameters ‘a’, ‘b’, and ‘c’ decreased from 5.6614 to 5.5360 Å, 32.515 to 32.151 Å, and 3.9525 to 3.8727 Å, respectively, with increase in ‘x’ from 0 to 4. For x = 5, ‘a’ and ‘c’ increased to 7.6889 and 32.253 Å, respectively, and ‘b’ decreased to 5.4763 Å. This appears consistent with the observed change in structure from orthorhombic (Pnnm) [10] to monoclinic (P21/c) [19]. Consequently, the normalized cell volume (V m = V unit cell /Z) of the Sr5−x Ca x Nb4TiO17 unit cell decreased from 363.788 (x = 0) to 339.515 Å3 (x = 5) due to the substitution of the small Ca+2 ion in the unit cell for the larger Sr+2 ion as shown Fig. 5. The alternative orthorhombic arrangement can be obtained by reorienting the monoclinic unit cell with a m || −a o, b m || c o and c m || b o, where the subscripts ‘m’ and ‘o’ refer to the modified and original unit cell parameters.
Density measurement
The variation in the apparent density of Sr5−x Ca x Nb4TiO17 compounds (x = 0–5) with increase in sintering temperature is shown in Fig. 6. The density of each composition first increased with increase in the sintering temperature from 1400 to 1500 °C and then decreased with further increase in the sintering temperature to 1550 °C. This indicated that the density of each composition reached a maximum at ~1500 °C. A previous study [15] reported a maximum density of ~4.46 g/cm3 for Sr5Nb4TiO17 at ~1450 °C. In this study, a maximum density of ~4.74 g/cm3 was achieved for the composition with x = 2, indicating an increase in the density due to the substitution of Ca for Sr.
Microstructural analysis
The secondary electron images (SEI) from thermally etched Sr5−x Ca x Nb4TiO17 (x = 0–5) samples sintered at ~1500 °C are shown in Fig. 7. The edges of the grains observed in sintered Sr5−x Ca x Nb4TiO17 ceramics with x = 0 and 1 appeared less sharp in comparison to those with x ≥ 2. As evident from Fig. 6, the density of these ceramics (with x ≤ 1) was also observed to be lower than the other ceramics sintered at the same temperature, indicating the effect of calcium substitution upon sintering. In general, the grains were irregular in shape with varying size. The average grain size observed in the microstructure of the compositions with x = 2 and 3 was ~1 × 2–1.5 × 5 μm2; however, a small number of elongated (~2.5 × 23 μm2) grains were also observed. The number of elongated grains increased with increase in x (i.e., x = 4, 5) consistent with the XRD results showing the splitting of the compound into layered n = 5 and 6 perovskites. The grains in the microstructure of the compositions with x = 2 and 3 appeared more compact than the other compositions, consistent with the observed higher density of these compounds in comparison to the others.
Dielectric properties at low frequencies
In spite of forming multi-phasic ceramics, the dielectric properties of the compositions sintered at 1500 °C were better due to their relatively higher densities than those sintered at 1450 and 1550 °C. The variation in the dielectric constant (εr) and loss tangent (tanδ) with temperature measured at 1 kHz–1 MHz for Sr5−x Ca x Nb4TiO17 (with x = 0–5) sintered at 1500 °C/2 h is shown in Figs. 8 and 9. Strong anomalies in εr and tanδ for the x = 0 compound, which may be due to the ferroelectric to para electric phase transition at ~531.3 °C, were observed. Similar behavior has been reported in εr and tanδ measured for Ba5−x Sr x DyTi3V7O30 (with x = 0–5) ceramics at 435, 355, 326, 82, and 46 °C [20]. The observed decrease in the dielectric constant with increase in frequency may be due to the vanishing of the contribution from interfacial and ionic polarization. Furthermore, a decrease in εr was observed with increase in the Ca content due to the reduced ionic polarizability of Ca+2 (3.17 Å3) than the Sr+2 (4.25 Å3) [21]. The observed increase in tanδ with increase in temperature may be due to the increased conductivity of the compounds. [20]. The tanδ was observed to be the lowest for the composition with x = 5.
Microwave dielectric properties
The microwave dielectric properties of the Sr5−x Ca x Nb4TiO17 (x = 0−5) ceramics are given in Table 1. The unexpectedly low dielectric constant (48) observed for pure Sr5Nb4TiO17 with no substitution may be due to its having the lowest density of all the compositions in this study. It is evident from Table 1 that the substitution of Ca decreased the dielectric constant and temperature coefficient of resonant frequency; however, the Q u × f o was not highly improved under the processing conditions employed in this study. The temperature coefficient of resonant frequency, τ f , for the composition with x = 1 was positive (+12.9 ppm/°C) when sintered at 1400 °C while negative (−2.5 and −14.5 ppm/°C) when sintered at 1450 and 1500 °C, respectively. The nature of the negative τ f is not known. The substitution of Ca for Sr increased the Q u × f o value from 447 (x = 0) to 3087 GHz (x = 5), decreased the dielectric constant from 48 (x = 0) to 37.6 (x = 5). The substitution of Ca for Sr is known to cause tilting of octahedra [1] which may be the possible reason for the observed decrease in τ f from 162.4 ppm/°C for Sr5Nb4TiO17 to −132.5 ppm/°C for Ca5Nb4TiO17.
Conclusions
The effect of Ca substitution for Sr on the phase, microstructure and dielectric properties of Sr5Nb4TiO17 was investigated. XRD revealed the formation of single-phase ceramics for the compositions with x = 1–4. Compositions with x = 0 and 5 showed the formation of secondary Sr2Nb2O7 (x = 0), and CaNbO3 and CaNb2O6 (x = 5) phases along with the parent Sr5Nb4TiO17 and Ca5Nb4TiO17 phases, respectively, upon calcination. XRD of the sintered samples with x = 0–5 showed the decomposition into n = 5 and 6 layered perovskites with the general formula (Sr1−x Ca x ) n (Nb,Ti) n O3n+2 at 1500 °C. Such decomposition was most evident in the compositions with x = 4 and 5. The layered perovskite with n = 6 in the compositions with x = 0 and 5 were Sr6Nb4Ti2O20 and Ca6Nb4Ti2O20, respectively; however, the nature of the layered perovskites with n = 6 in the compositions with x = 1–4 could not be identified. The normalized cell volume of the unit cell decreased with increase in the Ca content. The Ca substitution decreased the dielectric constant and τf and increased the Q u × f o (GHz) value.
References
Reaney IM, Idles D (2006) J Am Ceramic Soc 89(7):2068
Jawahar IN, Santha N, Sebastian MT, Mohanan P (2002) J Mater Res 17:3084
Zhao F, Yue Z, Gui Z, Li L (2006) J Am Ceram Soc 89(11):3421
Chen YC, Tsai JM (2008) J Appl Phys 47:7959
Chen YC, Yao SL, Jie R, Chen KC (2009) J alloys Compd 486:410
Anjana PS, Tony J, Sebastian MT (2007) IEEE electromagnetic conference, Kolkata, India
Tony J, Anjana PS, Letourneau S, Ubic R, Smaalen SV, Sebastian MT (2010) Matt Chem Phys 121(1–2):77
Slobodyanik NS, Titov YA, Chumak (2005) Theor Exp Chem 41(1):53
Iqbal Y, Reaney IM (2004) Ferroelectrics 302:259
Drews AR, Wong-Ng W, Roth RS, Vanderah TA (1996) Mat Res Bull 31(2):153
Sinton CW (2006) Raw materials for glass and ceramics sources, processes and quality control, John Willey & Son Inc, New York, p 151
Hungria T, Lisoni JG, Castro A (2002) Chem Mater 14:1747
Manan A, Iqbal Y, Qazi I (2008) J Pak Matt Soc 2(2):77
Zorel HE Jr, Guinesi LS, Ribeiro CA, Crespi MS (2000) Matt Lett 42:16
Manan A, Iqbal Y, Qazi I (2010) J Phys Conf Series 241:012028
Levin I, Leonid A, Bendersky A, Vanderah TA (2000) Phil Mag A 80(2):411
Pei J, Yue Z, Zhao F, Gui Z, Li L (2008) J Alloy Compd 459:390
Bijumon PV, Sebastian MT, Dias A, Moreira RL, Mohanan P (2005) J Appl Phys 97:104
Guevarra J, SchÖnleber A, Smaalen SV, Lichtenberg F (2007) Acta Cryst B 63:183
Sahoo PS, Panigrahi A, Patri SK, Choudhary RNP (2009) J Alloy Compd 484:832
Shannon RD (1993) J Appl Phys 73(1):348
Acknowledgements
The authors acknowledge the financial support of the Higher Education Commission of Pakistan (NRPU 20-569, IRSIP and Development of Materials Connection Centre Pak-US Project ID 131) and the enormous support provided by Prof. I. M. Reaney and his group in facilitating the authors at the Electroceramics laboratory, Department of Engineering Materials, University of Sheffield (UK) and Dr. Rick Ubic, Boise State University (USA) for typographical corrections and guidance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manan, A., Iqbal, Y. & Qazi, I. Phase, microstructural characterization and dielectric properties of Ca-substituted Sr5Nb4TiO17 ceramics. J Mater Sci 46, 3415–3423 (2011). https://doi.org/10.1007/s10853-010-5230-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-5230-9