Abstract
Crystalline lead-free piezoelectric potassium niobate (KNbO3) powders have been synthesized through a modified solid-state reaction method. The thermal behavior of the K2C2O4·H2O and Nb2O5 raw material mixture was investigated by thermogravimetric analysis (TGA) and differential thermal analysis (DTA). The X-ray diffraction technique (XRD) was used to investigate the phase formation and purity. The morphology of the powder obtained was characterized using a scanning electron microscope (SEM). The XRD pattern showed that the monophasic perovskite phase of KNbO3 could be synthesized successfully at a temperature as low as 550 °C for 240 min, with an average crystallite size of 36 ± 8 nm. The SEM images suggested that the average particle size of the powder obtained was 278 ± 75 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead zirconate titanate (PZT) ceramics are used widely in piezoelectric applications, due to their superior piezoelectric properties near the morphotropic phase boundary (MPB) [1, 2]. However, more than 50% of the lead-based piezoelectric material contains poisonous lead, which is a major drawback [3]. It has been reported that the use of lead-based ceramics causes serious environmental problems and numerous physical symptoms [3]. Furthermore, EU legislation will enforce draft directives for waste from electrical and electronic equipment (WEEE), and restrictions on the use of certain hazardous substances in electrical and electronic equipment (RoHS) and end-of life vehicles (ELV) [4–6]. According to these issues, lead and other heavy metals should be phased out, and alternative lead-free piezoelectric materials are receiving considerable attention.
Among various alternative families, perovskite type (ABO3) ceramics have attracted much consideration. Among alkali metal niobates, potassium niobate (KNbO3) is a well-known perovskite oxide that possesses attractive physical and piezoelectric properties [6–9]. Furthermore, the electromechanical coupling factor of the thickness-extensional mode, k t, was reported to reach as high as 0.69 for the 49.5°-rotated X-cut on the Y-axis. This value of k t is the highest among current lead-free piezoelectrics [10]. However, the main hindrance regarding this alkali niobate-based material lies in the difficulty of preparing dense and stoichiometric controlled ceramics using the conventional solid-state reaction and ordinary air sintering methods [11, 12]. These difficulties are caused by potassium volatility at high temperatures and excessive reactivity with moisture [13, 14]. Thus, different additive methods, hot pressing and spark plasma sintering have been used to improve ceramic densification [12, 15–19].
Several alternative ways for preparing alkali niobates have been investigated and developed: the hydrothermal [20] and hydrothermal-assisted sol–gel method [22], and glycothermal [23], nitrate–tartarate precursor technique [21], etc. However, most chemical synthesis routes require high purity reactants, which are more expensive and demand complicated procedures and specific apparatus. A modified solid-state reaction method has been used to synthesize the NaTaO3 perovskite type material successfully, with reduced reaction temperature [24]. In this method, the carbonate compound was replaced by oxalate, and the addition of urea played an important role. Recently, this method also has been applied to synthesize lead-free sodium niobate (NaNbO3) powders (without fuel) [25]. By replacing sodium carbonate using oxalate as the raw material, a lower calcination temperature and fine powders with an average crystallite size of 31.45 ± 5.28 nm were achieved.
In this study, a modified solid-state reaction method, with an expected lower reaction temperature, was used to synthesize KNbO3 particles, using potassium oxalate as raw material without the addition of any fuel. Effects of the calcination conditions on the KNbO3 phase development were investigated by the X-ray diffraction technique (XRD) and a scanning electron microscope (SEM).
Experiment
KNbO3 was synthesized by a modified solid-state reaction method. Reagent-grade potassium oxalate monohydrate (K2C2O4·H2O, 99.9%) and niobium oxide (Nb2O5, 99.9%) were employed as the starting material. The raw materials were weighed in stoichiometric quantities following the equation below.
These starting materials were mixed by the ball-milling method using ethyl alcohol and partially stabilized zirconia balls for 18 h. Then, the mixture was dried on a hot plate with regular stirring for a suitable period. After drying, the precursor mixture was determined by thermo gravimetric analysis (TGA, Perkin Elmer) and differential thermal analysis (DTA, Perkin Elmer) for investigating the thermal behavior during heat treatment and finding the appropriate calcination temperature. Based on TG–DTA results, the mixture was placed subsequently in a closed alumina crucible, and calcined for different periods of time at various temperatures ranging from 300 to 700 °C, to investigate formation of the KNbO3 phase.
Subsequently, calcined powders were inspected by room temperature X-ray diffraction (XRD, Advance D8), using Ni-filtered CuKα radiation to examine the effect of thermal treatment on the phase development and optimal calcination condition of crystalline KNbO3 powder formation. The room temperature FTIR spectra were recorded in the range of 4,000–400 cm−1 (Perkin-Elmer, Spectrum GX spectrometer), with eight scans and a resolution of 4 cm−1 using KBr pellets. Powder morphologies and particle size were figured directly using a scanning electron microscope (SEM, Hitachi S4700).
Results and discussion
Figure 1 shows the TG–DTA curves of the stoichiometric precursor of KNbO3. The thermogravimetric (TG) curve of the KNbO3 precursor shows three stages of weight loss from room temperature to 1,300 °C. Four endothermic peaks at 123, 398, 524 and 1,066 °C were observed in the differential thermal analysis (DTA) curve. Three weight-loss steps were observed in the ranges of 50–121, 121–172, and 416–532 °C. The corresponding weight losses seen were 3.92, 1.07, and 16.00%. The overall weight loss was found to be about 21%, which is close to the theoretical value of 20.01%, and corresponds to the release of 1 mol H2O, 1 mol CO, and 1 mol CO2 related to Eq. 1. In the temperature range from 50 to 121 °C (first stage), the initial weight loss of 3.92% showed decomposition of the oxalate molecule releasing water molecules (0.98 mol H2O), which concurred with the theoretical value for releasing 1.00 mol H2O (4.00%). This weight-loss corresponded to the endothermic peak, centered at 123 °C.
The second and third weight-loss steps illustrated the highest weight loss (~17%), which indicated a large elimination of organic compound that could be related to the release of CO and CO2 by combustion reactions according to Eqs. 2 and 3 (16% theoretically). In the temperature range from 121 to 532 °C, the DTA curve shows corresponding endothermic peaks (398 and 524 °C) that agree with the TG result.
However, an exothermic DTA peak was found centered at 565 °C. This implied that the third decomposition stage could lead to the formation of potassium niobate compound, which could be expected from the exothermic peak at 565 °C (as confirmed by XRD analysis in Fig. 2). As the temperature increased to 695 °C, weight loss was found to start again in the TGA curve, which could be correlated to the decomposition of the activated-K2CO3 residue. It is well known that K2C2O4 decomposes to K2CO3 at a higher temperature; however, this carbonate residue could decompose at a lower temperature when its degree of arrangement is lower than its initial state [26]. When heating further, an endothermic peak (without the observed weight-loss stage) could correspond to the phase transformation at 1,066 °C. Therefore, temperatures from the above TG–DTA analysis, which ranged from 300 to 700 °C, were selected for calcinations and investigation of the phase formation. The mixture of raw materials in the required stoichiometric ratio was calcined in air using a heating/cooling rate of 20 °C/min at various temperatures and followed by the phase analysis using an X-ray diffractometer.
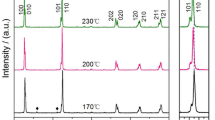
The X-ray diffraction (XRD) patterns of potassium niobate (KNbO3) powders, calcined for 4 h at different temperatures, are illustrated in Fig. 2. The diffraction pattern of the powder calcined at 300 °C suggests a composition of potassium oxalate (◊) (JCPDS no.22-1232) and niobium oxide (●) (JCPDS no.30-0873) raw materials. No evidence of the KNbO3 perovskite phase was found. As calcination temperatures increased to 500 °C, diffraction peaks of un-reacted raw materials were also found, but with lower intensity. This could demonstrate that the completed reaction cannot occur at such a low temperature range. As the diffusion coefficient is a temperature dependent parameter, the rate of diffusion is affected greatly by higher temperatures [27], which also could improve higher atomic mobility [28]. Nonetheless, the powders calcined from 550 to 700 °C showed diffraction peaks that could correspond to the orthorhombic potassium niobate perovskite phase (KNbO3) JCPDS no.32-0822 (▼). Amplified peak intensities can be seen after calcinations at increased temperatures. However, this result indicates the formation of KNbO3 perovskite phase powder, which passes through the calcination temperatures from 550 to 700 °C in 4 h. These temperatures were lower than those in the chemical synthesis of KNbO3, which used the polymerized complex method (PC method). This technique achieved the KNbO3 compound after the calcination step at 900 °C [29], or once the citrate precursor route had obtained KNbO3 nanopowder after heat treatment at 800 °C [30]. In addition, other chemical methods always require high purity reagent, which is more expensive, and involves complex procedures.
For a verdict on fine KNbO3 nucleation condition, a temperature of 550 °C was chosen to find the effect of calcination dwell time. The mixture of raw material powder was calcined at 550 °C for 15–360 min. The XRD analysis of calcined powder, with a different dwell time (Fig. 3), revealed an amorphous phase for a calcination period of 15 min, and no distinct crystalline phase could be detected. The absence of reflection peaks that correspond to K2C2O4·H2O and Nb2O5 indicated the amorphous nature of the powder obtained. The presence of reflection peaks for the XRD pattern of powder calcined at 550 °C for 20 min or longer could be ascribed to the crystalline phase of the sample. The different diffraction pattern of the powder, calcined for 20 min, suggests the nucleation condition of the KNbO3 phase, which was confirmed by further soaking time. After the calcination step at 550 °C for 20 min or longer, the powder showed an XRD pattern that could be matched with the perovskite potassium niobate (KN) phase JCPDS no.32-0822. These XRD analyses agreed with the TG–DTA analysis, in which crystallization of the KNbO3 phase was found around the previously mentioned temperature range. During the course of calcinations, the rise in calcination temperature and dwell time resulted in increased diffraction peak intensities, which related to higher crystallinity of the powder. This was supported by the increase in lattice parameters and average crystallite size, as revealed below. Nevertheless, it has been confirmed that this modified solid state reaction method can synthesize pure KNbO3 phase powder by using potassium oxalate monohydrate as starting material at the calcination temperature of 550 °C for 20 min. This calcination temperature is much lower than that used in a mixed oxide powder process, which lies in the range of 800 °C [9, 11, 13, 31, 32], or solution process (sol–gel and precipitation methods) that requires calcination temperatures of over 600 °C [33, 34]. Since XRD analysis suggested an orthorhombic crystal structure for preparing KNbO3 powder, lattice parameters of the sample could be deliberate by means of the UnitCell program package (ftp://rock.esc.cam.ac.uk/pub/minp/UnitCell/). The corresponding cell parameters, which are close to those reported from JCPDS file No.32-0822 (a = 5.695 nm, b = 5.721 nm, and c = 3.973 nm) are given in Table 1. The suggested orthorhombic crystal structure, obtained from matching with the JCPDS file, could be supported by this correlation of lattice parameters.
The average crystallite size of KNbO3 powders was considered as a function of calcination temperature, and time for broadening the X-ray line of the reflection peak using Scherrer’s equation [35]: D = kλ/βcosθB, where D is the average crystallite size, k a constant taken as 0.89, λ the wavelength of X-ray radiation, β the full width at half maximum (FWHM), and θB the diffraction angle. The corresponding values are reported in Table 2. The average crystallite size of powders, calcined from 550 to 700 °C for 4 h, was found to be about 36 ± 8 to 58 ± 6 nm. As the dwell time increased, it was found that the average crystallite size of calcined powders was increasing from 33 ± 9 to 36 ± 8 nm. The low D values suggest that the surface area of calcined powder was high enough to support high sinterability sufficiently [36]. The increase in crystallinity of the KNbO3 phase was affected by increasing dwell time and calcination temperature. This consequence may confirm that the dwell time and calcination temperature also play an important role in developing the pure phase creation.
Figure 4 shows the FT-IR spectroscopic studies of the crystalline potassium niobate (KNbO3) before and after the calcination step. The IR band for the uncalcined precursor was observed at 3,253 cm−1, due to O–H asymmetric stretching (ν3), which related to the moisture content of the KBr pellet and scissor bending mode (ν2) of HO–H at 1,600 cm−1 and 1,310 cm−1. When KNbO3 powders were calcined at 550 °C for 4 h, the absorption of bands at a low wave number range of 620 cm−1 suggested occurrence of Nb–O bond formation, which was believed to be the vibration (ν3) mode in the corner-shared NbO6 octahedron, according to the reported IR spectra of niobate glass ceramics [37]. This result shows that the perovskite KNbO3 phase was synthesized, which correlated with other results. The TG result indicated that the mass loss in the TG curve at around 700 °C could be the result of the K2CO3 residue decomposition, however, the FTIR band corresponding to the C–O stretching mode of carbonate at 1,450 cm−1 [38] was not found in KNbO3 powders calcined at 550 °C for 4 h. This observation could be described as the effect of dwell time.
Figure 5 shows SEM micrographs of KNbO3 powder prepared using a modified solid state reaction method at 550 and 700 °C for 240 min. The KNbO3 powder was found to be polyhedral in shape, with uniform features. The secondary phase could not be observed, which suggested the homogeneous character of the powder prepared. The mean particle sizes, which can be estimated from the micrographs, were found to be 278 ± 75 and 341 ± 80 for powder obtained at 550 and 700 °C, respectively. Particle growth was detected in powder calcined at a higher temperature. This value is greater than the average crystallite size, calculated from X-ray line broadening. It was believed that this contradictory value could indicate the agglomerate of the calcined powders. As reported by other studies [39, 40], the firing process tends to produce agglomerated particles and grain growth. No evidence of a different or pyrochlore phase was found. This outcome relates to the XRD result, in which the monophasic perovskite phase of KNbO3 can be established after calcinations at 550 °C for 240 min.
Conclusion
Crystalline KNbO3 powder was prepared from a modified solid state reaction of K2C2O4·H2O and Nb2O5. The final product was confirmed by XRD and SEM techniques. This is a simple cost- and time-saving method for synthesizing stoichiometric, homogeneous, and fine KNbO3 powder, with a low calcination temperature of 550 °C for 240 min. This temperature is about 250 °C lower than others used, even in conventional methods. The powder obtained was found to be a uniform agglomerated particle that possesses an average crystallite size (defined by XRD) of between 36 ± 8 and 58 ± 6 nm, and a mean particle size (defined by SEM micrograph) of 278 ± 75 nm.
References
Miclea C, Tanasoiu C, Miclea CF, Amarande L, Gheorghiu A, Spanulescu I, Plavitu C, Miclea CT, Cioangher MC, Trupina L, Iuga A (2007) J Eur Ceram Soc 27:4055
Setter N (2002) Piezoelectric materials in devices. Ceramic lab, EPFL, Switzerland
Lead and you: a guide to working safely with lead (2003) HSE (UK Health and Safety Executive). http://www.hse.gov.uk/pubns/indg305.pdf. Accessed 25 Apr 2004
Commission of the European Communities (2003) Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on waste electrical and electronic equipment (WEEE). Off J Eur Union, L37, 46:24
Commission of the European Communities (2003) Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off J Eur Union, L37, 46:19
Ichiki M, Zhang L, Tanaka M, Maeda R (2004) J Eur Ceram Soc 24:1693
Liu J-F, Li X-L, Li Y-D (2003) J Cryst Growth 247:419
Paula AJ, Parra R, Zaghete MA, Varela JA (2008) Mater Lett 62:2581
Yamanouchi K, Odagawa H, Kojima T, Matsumura T (1997) Electron Lett 33:193
Nakamura K, Kawamura Y (1999) Proc IEEE Ultrason Symp 2:1013
Matsumoto K, Hiruma Y, Nagata H, Takenaka T (2008) Ceram Int 34:787
Makovec D, Pribošič I, Drofenik M (2008) Ceram Int 34:89
Lu CH, Lo SY, Lin HC (1998) Mater Lett 34:172
Muthurajan H, Kumar HH, Samuel V, Gupta UN, Ravi V (2008) Ceram Int 34:671
Kim MS, Lee DS, Park EC, Jeong SJ, Song JS (2007) J Eur Ceram Soc 27:4121
Mgbemere HE, Herber R-P, Schneider GA (2009) J Eur Ceram Soc 29:1729
Wang R, Xie R, Sekiya T, Shimojo Y, Akimune Y, Hirosaki N, Itoh M (2002) Jpn J Appl Phys 41:7119
Ahn ZS, Schulze WA (1987) J Am Ceram Soc 70:18
Jaeger RE, Egerton L (1962) J Am Ceram Soc 45:209
Chang Y, Yang Z, Wei L, Liu B (2006) Mater Sci Eng A 437:301
Bhattacharyya K, Tyagi AK (2009) J Alloys Compd 470:580
Amini MM, Mirzaee M (2009) Ceram Int 35:2367
Lu C-H, Lo S-Y, Wang Y-L (2002) Mater Lett 55:121
Xu J, Xue D, Yan C (2005) Mater Lett 59:2920
Chaiyo N, Boonchom B, Vittayakorn N (2010) J Mater Sci 45:1443. doi:10.1007/s10853-009-4098-z
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M (2008) J Anal Appl Pyrolysis 81:253
Hsiao Y-J, Chang Y-H, Chang Y-S, Fang T-H, Chai Y-L, Chem G-J, Huang T-W (2007) Mater Sci Eng B 136:129
Callister WD (2007) Materials science and engineering: an introduction. Wiley, New York
Pribošič I, Makovec D, Drofenik M (2005) J Eur Ceram Soc 25:2713
Kakimoto K, Ito T, Ohsato H (2008) Jpn J Appl Phys 47:7669
Malic B, Bernard J, Bencan A, Kosec M (2008) J Eur Ceram Soc 28:1191
Chang Y, Yang Z-P, Ma D, Liu Z, Wang Z (2008) J Appl Phys 104:024109
Amini MM, Sacks MD (1991) J Am Ceram Soc 74:53
Kim KJ, Matijevic E (1992) J Mater Res 7:912
Klug HP, Alexander LE (1974) X-ray diffraction procedure of polycrystalline and amorphous materials. Wiley, New York
Lanfredi S, Dessemond L, Rodrigue ACM (2000) J Eur Ceram Soc 20:983
de Andrade JS, Pinheiro AG, Vasconcelos IF et al (1999) J Phys Condens Matter 11:4451
Böke HA, Akkurt S, Özdemir S, Göktürk EH, Saltik ENC (2004) Mater Lett 58:723
Terashi Y, Purwanto A, Wang WN, Iskandar F, Okuyama K (2008) J Eur Ceram Soc 28:2573
Wongmaneerung R, Chaisan W, Khamman O, Yimnirun R, Ananta S (2008) Ceram Int 34:813
Acknowledgements
This study was supported by the Thailand Research Fund (TRF), Thailand Graduate Institute of Science and Technology (TGIST), and the National Nanotechnology Center (NANOTEC) NSTDA, Ministry of Science and Technology, Thailand, through its “Center of Excellence Network” Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaiyo, N., Ruangphanit, A., Muanghlua, R. et al. Synthesis of potassium niobate (KNbO3) nano-powder by a modified solid-state reaction. J Mater Sci 46, 1585–1590 (2011). https://doi.org/10.1007/s10853-010-4967-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4967-5