Abstract
Chromium selenide (CrSe) crystalline thin film has been produced via chemical bath deposition on substrates (commercial glass). Transmittance, absorption, optical band gap and refractive index of the films have been examined by UV/VIS. Spectrum. Structural properties have been examined and XRD hexagonal form has been observed. The structural and optical properties of CrSe thin films, produced at different pH levels were analyzed; SEM and EDX analysis have been performed for surface analysis and elemental ratio of the films. It has been found that some properties of the films have been changed with pH and the changes of these properties with respect to pH have been investigated. Tested pH values were between 8 and 11. The optical band gap has been varied between 3.80 and 3.92 eV and film thickness has been changed from 76 nm to 126 nm for tested pH levels. Absorbance values were found to be 0.053, 0.018, 0.012 and 0.069 for pH values of 11, 10, 9 and 8, respectively (550 nm wavelength). The refractive index of CrSe thin films have been changed with film thickness, found as 2.27, 2.24, 2.25 and 2.26.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal selenides, such as CdSe, MnSe and PbSe are very important semiconductors. The researchers intensively worked on producing CdSe, PbSe thin films. CrSe films are rarely produced. However, CrSe alloy is a very interesting material because of its magnetic properties [1]. The chromium selenide analogue Cr(1 − x)Se(x) is well known as antiferromagnetic [2]. The metallic chromium selenides as Cr3Se4 and Cr2Se3 are antiferromagnets and semiconductors [3–5]. Some transition metal chalcogenides are found to be excellent candidates for half-metallic ferromagnets according to literature [6].
It is already known that chromium selenide has a monoclinic structure, located at I2/m space group, and crystallizes as Cr2Se3 and Cr7Se8, Cr3Se4 [5]. The structure of chromium selenides is similar to NiAs and both of them have vacancies in their second metal-atom layer. The researchers found that if the structure is rich in Cr, the material shows antiferromagnetic behavior, whereas meta- or ferromagnetic order was observed in Cr-poor samples [5–11]. It is very important to produce such material which possesses so many features.
The aim of this paper is to produce CrSe thin film using a new method of chemical bath deposition and to examine its structure and optical properties. It is argued that crystal structure and optical properties of CrSe could be controlled by the pH of chemical bath. It is clear that producing CrSe thin films via chemical bath deposition is much more advantageous to the researchers since it is a simple and cheap method to fabricate. Since the production of CrSe via chemical bath deposition, which is a simple and cheap method, is quite difficult, the most appropriate reagents should be selected. Also, this is a new method for producing CrSe thin films. According to the literature mentioned above, CrSe alloys usually result in amorphous structure, thus it is quite difficult to produce crystalline form.
2 Experiment
The preparation of selenium source solution shown in the following procedure is very crucial to this study. 20 mL concentrated 1–4 dioxane and 0.01 mol solid selenium were put in a beaker, with 0.01 mol KOH. The mixture was heated up to 80 °C, where 1–4 dioxane has been completely evaporated. KOH + Se gel was remained at the bottom of the beaker. 0.01 mol LiAlH4 was immediately added into the beaker before the gel get cool. Then, distillated water was slowly added into the beaker and the solution was mixed at 1000 rpm until the solution got a volume of 100 mL. It is required careful attention when LiAlH4 is added since the reaction is flammable. The mixture was filtered before being added to the chemical bath. The pH of the solution was 11 (Fig. 1).
The components of the baths were 8% HCl, 10 mL 0.001 M chromium nitrate 15 mL prepared Se source solution, and 5 mL triethanolamine (TEA). First, 10 ml 0.001 M chromium nitrate and 15 ml Se source solution 5 mL TEA were put into a baker, filled with 40 ml deionized water. 8% HCl was used to adjust the pH of bath. 2, 4, 8 ml of 8% HCL were added to obtain solutions with pH 10, 9, 8, respectively. pH values of the chemical baths were measured using a pH meter (Lenko mark 6230 N). The bath temperature was kept at 50 °C for 3 h. The substrates were dipped in the bath.
3 Measurement
The crystalline structure of the CrSe was confirmed by X-ray diffraction (XRD) with a CuKα1 radiation source (Rikagu RadB model, λ = 1.5406 Å) over the range 10° < 2θ < 90° at a speed of 3° min− 1 with a step size of 0.02°. The surface properties of the films were examined using an EVO40-LEO computer controlled digital scanning electron microscope (SEM). Chemical analysis by EDX was performed with an EDX spectrometer attached to the SEM. The optical measurements were performed by Hach Lange 500 Spectrophotometer at room temperature by placing an uncoated identical commercial glass substrate in the reference beam. The optical spectrum of thin films was recorded in the wavelength range of 300–1100 nm.
4 Result and discussion
XRD patterns of CrSe films deposited by CBD at different pH values are shown in Fig. 2 (a: pH 11, b: pH 10, c: pH 9, d: pH 8) and XRD data of the films were compared with ASTM values in Table 1. The peaks were hidden in amorphous structures for pH: 11 and 8 but the peak observed for pH 9 is sharper than the others. Crystalline structures were observed for pH: 10 and 9. The sharper peak was 23.45° for pH: 9. The structures that we have obtained were indexed with monoclinic structure Cr7Se8 for pH: 9 and monoclinic structure Cr7Se8 for pH: 10. This result is not surprising because the amount of HSe− or Se−2 dissolved in the bath vary according to the pH of the environment. Thus, a specific method was developed to produce Cr7Se8 at pH: 9. According to literature, structures of Cr2Se3, Cr7Se8, and Cr3Se4 are very close to each other and can be easily transformed into each other. So we have achieved this by changing the pH of the bath. Ohtani et al. produced solid Cr3Se4 at 900 °C, yielding a defect-CdI2 structure [7]. Ivanova et al. and Maurer and Collin found that Cr3Se4 is a material with weak conductivity [8, 3]. Chevreton M. et al. achieved to synthesize Chromium selenide at Cr7Se8 phase [5]. Therefore, the method that we have tried was pretty good; we can control the phase of CrSe thin films by controlling the pH of the bath [5]. In our method, we can produce CrSe thin films in various phases, such as Cr7Se8, whereas many researchers had exerted a lot of effort to produce each of these phases one by one [5, 7–9]. The important point is that pH: 9 and 10 can be special values for producing CrSe thin films.
The structural parameters such as grain size (D) and dislocation density (δ) were computed by XRD patterns for all films and they are presented in Table 2. The grain size of the thin films was calculated by XRD patterns using Debye Scherrer’s formula [12],
where D is the grain size, λ is the X-ray wavelength used, β is the angular line width at half-maximum intensity in radians and θ is Bragg’s angle. The grain size and dislocation density of the films were calculated using the FWHM of (103) and (020) peaks obtained through the Scherrer’s method. The dislocation density (δ), which is calculated by the formulas below, gives more information about the amount of defects in the films [12].
Higher δ values indicate lower crystallization levels for the films and indicate the amount of defects in the structure. Larger D and smaller δ values indicate better crystallization of the films [12].
where N is the number of crystallites per unit area, t is the film thickness. The higher N value indicates abundance of crystallization.
D, δ, N values of the film produced at pH 11 and 8 could not be identified because of their amorphous structure. The highest value of grain size was 25 nm at pH 10, whereas the lowest value was 15 nm at pH: 9. The amount of defects was calculated to be 1.50 × 10− 3 lines/m2 and 3.99 × 10− 3 lines/m2 at pH: 10 and 9, respectively. The number of crystallites per unit area was 25.59 × 10− 4 and 126.10 × 10− 4 m− 2 at pH: 10 and 9. The number of crystallites per unit area is increasing while grain size gets smaller. Similarly, the amount of defects in the structure increases while grain size decreases.
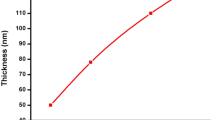
Film thickness of CrSe thin films is shown in Fig. 3. The film thickness was changed as a function of pH, to be found 820, 541, 645 and 983 nm at pH 8, 9, 10, and 11, respectively. A parabolic curve has been observed for various pH values. Higher values of 820 and 983 nm were obtained from amorphous structures produced at pH: 8 and 11. Smaller thickness of 541 and 645 nm were obtained from the crystalline structure at pH 9 and 10. These thicknesses were in line with grain sizes.
The optical band gap energy (Eg) was determined from the absorption spectra of the films using the following relation [13, 14]:
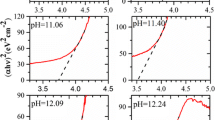
The plot of (αhυ)2 vs. hυ for CrSe films at different pH is shown in Fig. 4. The optic band gap of CrSe thin films decreased down to 3.80 and 3.75 eV at pH: 11 and 8. Optical band gap were higher in crystalline structures than in amorphous structures. The quantum size effect seemed to affect the optical band gap at pH 9 and 10. It is quite expected for amorphous films to have lower optical band gap. The defects in the structure generate a tail from the allowed band to the conductive band at the localized states (Urbach tail). This tail leads to shrinkage of the band gap [12].
The transmittance (T) of CrSe thin film can be calculated using reflectivity (R) and absorbance (A) spectra from the expression [13, 14]:
Transmission measurements are performed at room temperature in the range of 300–1100 nm. The transmission and reflectance of films deposited at different pH are shown in Fig. 5.
Transmission values, which were 88.51, 95.94, 97.27 and 85.31% (at 550 nm wavelength), were in line with film thicknesses, that were 820, 541, 645 and 983 nm, respectively. The highest transmission has been observed in the film produced at pH: 9, where the best crystallization has also been occurred. As expected, reflectance, which was proportional to film thickness, was inversely correlated with transmission and gets the following values 3.39, 1.16, 0.77 and 4.39% (at 550 nm wavelength). Figure 6 is shown absorbance curves of CrSe thin films for different pH levels. Absorbance values were 0.053, 0.018, 0.012 and 0.069 for pH 11, 10, 9 and 8, respectively (550 nm wavelength). The light was absorbed by amorphous structure much more than crystalline structure. This is mostly due to the film thickness. Since thick film contains more particles, it absorbs the light more than thinner films.
Refractive index and extinction coefficient are usually given by the following equations [13, 14]:
However, this equation can be derived by assuming the dielectric constant to be 1. So, the material that we measure should not have any magnetic feature, since n = √µ.ε. Usually, it is assumed that µ = 1 for the materials lacking magnetic properties and Eq. 6 is derived. Since we know the dielectric constants of the chromium-selenide derivatives, it makes more sense to use the Moss equality.
There are various empirical and semi-empirical rules and expressions that relate n to Eg. In Moss’ rule, n and Eg are related by n 4 Eg = K = constant (≈100 eV) [15]. The refractive indexes of the films are given in Fig. 7. Similar to reflectance, refractive indexes of CrSe thin films were also proportional to film thickness and get the following values with the increase of pH: 2.27, 2.24, 2.25 and 2.26. It seems that film thickness has an impact on refractive index. This is because the increase of film thickness allow light to interact with more particles. The refraction index of the film produced at pH 10 was not coherent with film thickness. The reason is that the film produced at pH 10 is lack of uniformity in film structure. At this pH level, chromium selenate was also formed in the film.
-
(a)
pH: 11
-
(b)
pH: 10
-
(c)
pH: 9
-
(d)
pH: 8
SEM images of CrSe thin films are given in Fig. 8. Amorphous structures were observed for pH: 8 and 11, whereas nano-rods CrSe structure was observed for pH: 9. The grains were irregular for pH: 8 and the cracks were observed for pH: 11. CrSeO4 grains were observed in the film produced at pH: 10, although the amount of CrSeO4 grains is very little, nano-rods were detected in the film produced a pH 9. These results were in agreement with XRD patterns.
EDX technique was used to identify the composition of the CrSe thin films. Figure 9 shows the average atomic ratio of Cr/Se as a function of pH. It was seen that Cr/Se ratio draw a parabolic curve as 0.065, 0.438, 0.500 and 0.074 with increasing pH. Although EDX results are not an indicator for amorphous structures (pH: 11 and 8), they show that the average atomic ratio of Cr/Se is nearly stoichiometric for pH: 10 and 9. The theoretical stoichiometric ratios of Cr/Se are 0.493 and 0.576 for pH 10 and 9. The difference between the theoretical and experimental ratios may be due to the very little amount of CrSeO4, sustained in the structure. EDX results were in agreement with the XRD and SEM measurements.
5 Conclusion
A novel method was developed for producing CrSe thin films at different pH. Crystalline structures were observed at pH: 10 and 9, whereas amorphous structure was observed at pH: 11 and 8. Cr7Se8 structure was dominant at pH: 10, whereas Cr7Se8 structure was dominant at pH: 9. The properties, such as refractive index, transmission, optical band gap, and film thickness were changed according to pH. Consequently, we developed a novel method for producing CrSe films via CBD. It has been shown that CrSe thin films can be produced via chemical bath deposition, which is a simple and cheap method. Further improvement in quality and uniformity is possible if this reaction is realized in an autoclave or in an environment of purging air. It is also possible to improve the crystallization through annealing at elevated temperature.
References
Bettinger, J.S., Chopdekar, R.V., Liberati, M., Neulinger, J.R., Chshiev, M., Takamura, Y., Alldredge L.M.B., Arenholz, E., Idzerda, Y.U., Stacy, A.M., Butler, W.H., Suzuki, Y.: Magnetism and transport of CuCr2Se4 thin films. J. Magn. Magn. Mater. 318, 65–73 (2007)
Lotgering, F.K., Gorter, E.W.: Solid solutions between ferromagnetic and antiferromagnetic compounds with NiAs structure. J. Phys. Chem. Solids. 3, 238–249 (1957)
Maurer, A., Collin, G.J.: Structural and physical properties of Cr3 ± x Se4(x ≤ 0.20). J. Solid State Chem. 34, 23–25 (1980)
Adachi, Y., Ohashi, M., Kaneko, T., Yuzuri, M., Yamaguchi, Y., Funahashi, S., Mori, S.: Magnetic structure of rhombohedral Cr2Se3. J. Phys. Soc. Jpn. 63, 1548–1559 (1994)
Chevreton, M., Murat, M., Eyraud, C., Bertaut, E.F.: Structure et conductibilité électrique des composés à lacunes ordonnées du système chrome-sélénium, Le. J. Phys. 24, 443–446 (1963)
Xie, W.H., Xu, Y.Q., Liu, B.G., Pettifor, D.G.: Half-metallic ferromagnetism and structural stability of zincblende phases of the transition-metal chalcogenides. Phys. Rev. Lett. (2003). http://journals.aps.org/prl/abstract/10.1103/PhysRevLett.91.037204#fulltext
Ohtani, T., Fujimoto, R., Yoshinaga, H., Nakahira, M., Ueda, Y. J.: The Intralayer Order-disorder Transition in Monoclinic Cr3 ± xSe4. Solid State Chem. 48, 161–167 (1983)
Ivanova, V. A., Abdinov, D. S., Aliev, G. M.: On some characteristics of chromium selenides. Phys. Status Solidi, 24, K145–K147 (1967)
Babot, D., Chevreton, M., Buevoz, J. L., Langnier, R., Lambert-Andron, B., Winterberger, M.: Nouvelles donnes experimentales sur les proprietes magnetiques de Cr3_Se4_et des solutions solides Cr3_SetS4 − t. Solid State Commun. 30, 253–257 (1979)
Winterberger, M., André, G., Hammann, J.: Composition and temperature dependent magnetic structures of monoclinic chromium selenides Cr3 ± xSe4, x ≤ 0.2. J. Magn. Magn. Mater. 147, 167–176 (1995)
Dijkstra, J., van Bruggen, C. F., Haas, C., deGroot, R. A.: Effect of anion substitution onto structural and magnetic properties of chromium. J. Phys. Condens. Matter. 1, 9163–9174 (1989)
Akaltun, Y., Yıldırım, Y.A., Ateş, A., Yıldırım, M.: The relationship between refractive index-energy gap and the film thickness effect on the characteristic parameters of CdSe thin films. Opt. Commun. 284, 2307–2311 (2011)
Benramdane, N., Murad, W.A., Misho, R.H., Ziane, M., Kebbab, Z.: A chemical method for the preparation of thin films of CdO and ZnO, Mater. Chem. Phys. 48, 119–123 (1997)
Pejova, B., Grozdanov, I., Tanusevski, I.: Optical and thermal band gap energy of chemically deposited bismuth(III) selenide thin films, Mater. Chem. Phys. 83, 245–249 (2004)
Kasap, S., Capper, P.: Springer handbook of electronic and photonic materials, pp. 48–51. Springer, Würzburg (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
kariper, I.a. Synthesis and characterization of CrSe thin film produced via chemical bath deposition. Opt Rev 24, 139–146 (2017). https://doi.org/10.1007/s10043-017-0307-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10043-017-0307-1