Abstract
Hydroxyapatite (HAP) is a close synthetic analog of the bone mineral and is often considered as a material for bone graft substitutes and tissue engineering scaffolds. Despite its attractive bioactive properties low-fracture toughness limits the use of HAP ceramics to a number of non-load-bearing applications. To obtain a more adequate mechanical behavior, HAP is often combined with polymers based on lactic and glycolic acids or polycaprolactone using hot pressing. In such composite materials, the compatibility and bonding strength of HAP–polymer interfaces are critical parameters that must be controlled and improved. This may be achieved, for example, by covalent immobilization of organic moieties on the ceramic particles surface. In this work, the surface of calcium-deficient hydroxyapatite (CDHAP) was modified by reaction with hexamethylene diisocyanate (HDI) in a non-aqueous suspension. Composites of CDHAP–HDI with polylactide (PLA) were high pressure consolidated at room temperature at 2.5 GPa yielding up to 90% theoretical density. The effects of total organic fraction and modification extent on compression strength were studied. Materials with high extent of modification and high organic content exhibited compressive strength of ~295 MPa, much higher than reported in other studies. These materials are suitable candidates for load bearing orthopedic applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The requirement for new bone to replace or restore the function of lost or damaged one is a major clinical and socioeconomic need. To facilitate bone regeneration under sub-optimal conditions, bone supplementation is often needed. This is usually accomplished with autologous bone which, however, is not always available in sufficient amounts and whose harvesting imposes health concerns, e.g., donor site morbidity [1–3]. Bone allografts are inferior to autologous bone and have known risks of bacterial contamination, viral transmission, and immunogenicity [1, 3–5]. Due to the problems of bone grafting, there is an increased demand for synthetic bone graft substitutes. A bone graft substitute is a porous three-dimensional structure that acts as a scaffold for the ingrowth of capillaries and new bone tissue. In order to support bone ingrowth, the scaffold should be osteoconductive and have adequate mechanical properties to provide initial stability. Ideally, the scaffold should also be bioresorbable and be replaced, over time, by a new bone. The properties of a porous scaffold are controlled by the scaffold geometry (pore size and interconnectivity) and by the material it is made of. Choice of material will determine the cell–material interaction characteristics as well as the range of mechanical properties that can be achieved. The bulk material will set the maximum mechanical properties that can be achieved [1].
Bioresorbable materials that are considered for the fabrication of bone graft substitutes include calcium phosphate (CaP) ceramics, polymers, and composites thereof. Resorbable CaP ceramics such as β-tricalcium phosphate (β-TCP) and calcium-deficient hydroxyapatite (CDHAP) bear close resemblance to the bone mineral, however, they are intrinsically brittle (especially when porous) and thus unsuitable for use in load-bearing sites [6–9]. Degradable polymers such as lactic and glycolic acid polymers (PLA and PGA), polycaprolactone (PCL) and their copolymers lack osteoconductivity and degrade to acidic products that can cause late inflammation and osteolysis in bone contact [10, 11]. In addition, porous scaffolds made of these polymers do not have sufficient mechanical integrity to be implanted into load-bearing positions.
Combining bioresorbable polymers and CaP ceramics into composite materials offers the possibility of creating osteoconductive implants with improved mechanical characteristics [12]. In such composites, the alkaline resorption products of CaP can buffer the acidic degradation products of the α-hydroxyester.
The common feature of most proposed CaP–polymer composites is the low volume fraction (≤40 vol%) of the ceramic phase. The mechanical properties of such composites are close to those of the polymer matrix, with the highest compressive strength of 107–115 MPa reported for HAP–PLA composites with 9–28 vol% HAP forged at 103 °C [13]. Increasing CaP fraction will supposedly yield stronger and tougher composite materials where the ceramic skeleton will provide structural consistency while the polymeric phase will act as “cement” and enhance ductility. HAP–PLA composites with a relatively high (59 vol%) HAP volume fraction hot pressed at 194 °C and 98 MPa for 1 h were reported to have the compressive strength of 140 MPa [14–16]. No dramatic improvement over the composites with low ceramic volume fractions was achieved, possibly due to inhomogeneous phase distribution. The use of very fine nanometric ceramic powders and improved mixing techniques may yield much more uniform nanostructured materials similar to the high-toughness natural ceramic–organic composites such as nacre [17] and bone.
Weak (or a lack of) interfacial bonding between the ceramic and polymer constituents may be another factor responsible for the low-mechanical properties of composite materials. The compatibility and bonding strength of CaP–polymer interfaces can be improved by covalent immobilization of organic moieties on the ceramic particles surface. Various approaches may be employed, such as grafting with silanes [18] and organophosphonic acids [19, 20]. Surface hydroxyl groups of HAP can be utilized for initiation of radical ring opening polymerization of lactide, glycolide, or caprolactone to give a polymeric chain covalently attached to apatite particle surface at one end. For example, grafting of PLA on the surface of HAP particles (gHAP) resulted in a significant improvement in the tensile strength of PLA–HAP composites with 4–14.3% vol% gHAP [21]. Furthermore, OH− groups may be exploited to attach alcohols [22] and isocyanates [23, 24] to HAP particle surface through ester and urethane formation, respectively.
Ultimately, a consolidation step is required to transform CaP–polymer composite powders into useful bulk materials. Most currently used industrial or laboratory fabrication routes employ high temperature densification techniques, such as hot press or forging [13–16]. One drawback of the high-consolidation temperature is the inability to incorporate active ingredients (drugs, growth factors) that may assist in bone regeneration. Using higher pressures could allow one to achieve high density at near-room temperatures, which are less likely to be damaging for biomolecules. We have previously reported the fabrication of strong CaP–PLA and CaP–PCL nanocomposites with high ceramic volume fractions (>70 vol%) by cold sintering/high pressure consolidation [25, 26] of the corresponding composite nanopowders [27, 28].
In the present work, the above principles are applied to the processing of strong CDHAP–PLA nanocomposites. The effect of chemical modification of ceramic–polymer interfaces on the composite properties is reported.
Experimental
Calcium-deficient hydroxyapatite, (CDHAP) Ca10−x (HPO4) x (PO4)6−x (OH)2−x with Ca/P ratio ~1.5 was prepared by microwave accelerated wet method [29] according to the following reaction:
Phosphoric acid (H3PO4) was added to a 0.5 M solution of calcium nitrate tetrahydrate (Ca(NO3)·4H2O) at 1.5 Ca/P ratio. After stirring the solution for 30 min, large excess of NH4OH was added and stirred for additional 30 min. The obtained precipitate was filtered, washed with DI water and heated for 15 min in a microwave oven (750 W power). The resulting powder was vacuum dried at 120 °C for 48 h and was characterized by XRD (Philips PW 3710, CuKα), FTIR (Bruker Equinox 55), SEM (Leo Gemini 982), TEM (Titan 80-300 FEG-S/TEM), and BET surface area measurement (Nova3000-Quntachrome).
The surface of CDHAP was covalently modified with hexamethylene diisocyanate (HDI) according to a method reported in [23]. Dried CDHAP powder was suspended in anhydrous DMF under nitrogen atmosphere and heated to 65 ± 2 °C. Dibutyltin dilaurate (DBTU) catalyst was used at 0.1 wt% concentration and the desired amount of HDI was added dropwise to the suspension. After 4 h, the reaction was stopped with excess ethanol. The obtained CDHAP–HDI powders were centrifuged, washed three times with chloroform, and vacuum dried. FTIR and TGA (Mettler Toledo, heating rate 10 °C/min up to 500 °C) were used for powder characterization. Weight loss (in TGA) of HDI-modified powders relative to the pure CDHAP powder (recalculated on volumetric basis) was taken as modification extent. The powders were designated as CDHAP–xxH, where xx stands for the modification extent (volume fraction of HDI).
Composites with polylactide (NatureWorks® PLA 3001D resin, Mw ~100 kDa) were prepared by solvent evaporation method. First, predetermined amounts of PLA were dissolved in chloroform to give the composite a total organic fraction of 10, 20, 30, or 40 vol% (for example, for CDHAP–10H only 20% PLA is needed to give 30 vol% total organics). Next, CDHAP or CDHAP–HDI powders were suspended in the solution and mixed for 30 min. To obtain fine CDHAP/PLA or CDHAP–xxH/PLA mixtures the polymer was precipitated with excess ethanol and the solvent was evaporated under constant mechanical stirring. The composite powders were designated CDHAP–xxH–yy, where yy stands for the total organic content.
The obtained composite powders were high pressure consolidated (cold sintered) [25, 26] at room temperature and pressure of 2.5 GPa (~30 s under pressure) to yield 10.8 mm diameter and ~4 mm high disks. The dense specimens were tested in compression in an Instron testing machine, Model 1195, at the crosshead speed of 50 μm/min (corresponding to the strain rate \( \dot{\varepsilon } \approx 10^{ - 4} {\text{ s}}^{-1} \)). Fracture surfaces were studied by SEM.
Results and discussion
CDHAP preparation
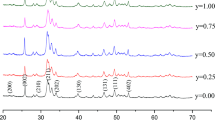
Figure 1a shows XRD pattern of the synthesized CaP powder. All the peaks correspond to hydroxyapatite (HAP), with distinctive broadening characteristic for nanocrystalline materials. FTIR spectrum (Fig. 2a) is also typical for HAP [29]. The presence of the P–O–H band at 873 cm−1 corresponding to bivalent phosphate ion, HPO4 2−, suggests that the HAP formed is a Ca-deficient compound, Ca10−x (HPO4) x (PO4)6−x (OH)2−x (CDHAP). CDHAP is indistinguishable from stoichiometric HAP by XRD analysis, however, unlike stoichiometric HAP, it will decompose upon heating to 750 °C to β-tricalcium phosphate, Ca3(PO4)2 (β-TCP), and stoichiometric HAP according to reaction (2) [30]:
Following a 2-h anneal at 750 °C, the only phase detected by XRD in our synthesized powder was β-TCP (Fig. 1b). Full conversion into β-TCP is also supported by FTIR analysis: the bands of structural OH− (at 635 and 3569 cm−1) and HPO4 2− (at 873 cm−1) characteristic of HAP disappear from the spectrum of the annealed powder (Fig. 2c). These results confirm that the powder synthesized is indeed a Ca-deficient HAP with x close to unity: Ca9(HPO4)(PO4)5(OH). The Ca/P ratio of such CDHAP is ~1.5, and its solubility is comparable to that of β-TCP [31]. HRSEM and TEM micrographs (Fig. 3) demonstrate the needle-like morphology of the obtained CDHAP particles and their nanoscale dimensions (≃15 nm diameter and 50–150 nm length).
The specific surface area (SSA) of the synthesized CDHAP powder, as determined by BET, was ~95 m2/g. For needle-like (cylindrical) powder morphology, the relation between particle’s dimensions and the SSA (m2/g) is given by [32]:
where L and d are particle length and diameter in nm, respectively, and ρ is the density (3.156 g/cm3 for CDHAP). Based on the above equation, the surface area of 95 m2/g corresponds to a very fine nanoscale powder with the particle diameter of ~15 nm, which is in a good agreement with the micrographs in Fig. 3.
Surface modification with HDI
The surface hydroxyl groups of HAP can react with isocyanates to form a covalent urethane bond as schematically shown in Scheme 1.
A typical FTIR spectrum of the synthesized CDHAP powder treated with hexamethylene diisocyanate (CDHAP-HDI) (Fig. 2b) contains new peaks in addition to those of CDHAP: 1574 cm−1 and 1628 cm−1 (amide bands); 2857 cm−1 and 2934 cm−1 (–CH2– bands); 3340 cm−1 (–NH– band). This shows formation of covalent urethane bonds during the modification reaction. The details of such attachment, however, require further exploration. In earlier solid state 1H-NMR [23, 24] and 13C- and 31P-NMR [33] studies, two different attachment paths were suggested: one through covalent bonding between isocyanate and structural hydroxyl groups, and the other through the formation of PO–C(O)NH– bonds between isocyanate and P–O–H moieties of HPO4 2− groups.
Figure 4 shows the extent of modification as a function of initial feed conditions. It can be seen that HDI immobilization efficiency is high and can reach up to 40 vol%. This could be due to the high surface area of the ceramic powder, as well as due to the formation of longer organic chains by side reactions of HDI. It is known that isocyanates undergo dimerization reactions via formation of uretdiones, or, in the presence of water traces, via formation of ureas. Branching reaction through allophanate formation have also been reported [23]. For further preparation of CDHAP–PLA composites, powders with modification extent of up to 10 vol% were used (CDHAP–7H and CDHAP–10H).
Mechanical properties
The density of cold sintered CDHAP–polymer specimens was 82–90% of the theoretical depending on the total amount of organics and extent of modification. The highest density of 90% was obtained for the material with 40 vol% total organics and 10 vol% modification (CDHAP–10H-40). Typical stress–strain curves of specimens tested in compression are presented in Fig. 5. It can be seen that both strength and ductility increase with increasing total organic content. At 40 vol% organics, the plastic strain reaches ≃2% which is comparable with the plastic strain of pure PLA at room temperature [13]. Even without HDI modification, cold sintered CDHAP–40 vol% PLA nanocomposites have a high compression strength, σc, of 225 MPa. This is 1.5 times stronger than the hot pressed (194 °C, 98 MPa) composites having a practically identical composition (HAP–41 vol% PLA, σc = 140 MPa) [14–16]. The high strength values obtained in our work may be the result of a more homogeneous phase distribution achieved through the use of the much finer CaP nanopowder (SSA = 95 m2/g in our work vs. SSA = 5 m2/g in [15]). The presence of very fine (≃200 nm thick) polymer nanofibers on the composite fracture surface, Fig. 6, suggests the uniform distribution of the PLA component.
As shown in Fig. 7, the modification of CDHAP with HDI further increased the compressive strength of CDHAP-based composites with organic content ≥20 vol%. The improvement of mechanical properties is especially obvious for the composites containing 40 vol% organics. Compressive strengths of ~265 and ~295 MPa were obtained for 7 and 10% modification, respectively, which constitutes 15 and 25% improvement compared to the unmodified CDHAP–40 vol% PLA composite. This improvement is believed to be due to the better bonding integrity between the HDI-modified CDHAP surface and PLA molecules.
Conclusions
The main thrust of the present study was to develop new approaches to the processing of bioresorbable CaP–polymer composites that will result in bone-healing devices with improved strength and toughness. Our main efforts were directed at improving the bonding between the ceramic and polymer phases via chemical coupling of an intermediate organic agent. A nanometric powder of calcium-deficient hydroxyapatite (CDHAP) with Ca/P ratio close to 1.5 was successfully modified by covalent attachment of 7–10 vol% HDI to CDHAP surface hydroxyl groups. The attached HDI acted as a coupling agent for the PLA polymer. High pressure consolidation (at room temperature and 2.5 GPa) of CDHAP/PLA and (CDHAP–HDI)/PLA powders with relatively small amounts of added PLA yielded dense nanocomposites with compressive strength and ductility increasing as the total organic content increased from 10 to 40 vol%. The room temperature processing in principle provides the possibility of incorporating biomolecules (drugs, growth factors) without damaging their biological activity. A high compressive strength of almost 300 MPa was obtained for (CDHAP–HDI)/PLA composites containing 40 vol% organic phase which constitutes an ~25% improvement over the corresponding CDHAP–40 vol% PLA material without the HDI interface modification (~225 MPa). These composites are suitable candidates for load-bearing orthopedic applications and are currently being studied as a material for porous bone ingrowth scaffolds.
References
Ilan DI, Ladd AL (2003) Operat Tech Plast Reconstr Surg 9:151
St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, Albert T, Hillbard A (2003) Am J Orthop 32:18
Ducheyne P, Qiu Q (1999) Biomaterials 20:2287
Johnson KD, Frierson KE, Keller TS, Cook C, Scheinberg R, Zerwekh J, Meyers L, Sciadini MF (1996) J Orthop Res 14:351
Vaccaro AR, Cirello J (2002) Clin Orthop Relat Res 394:19
Yaszemski MJ, Payne PG, Hayes WC, Langer R, Mikos AG (1996) Biomaterials 17:175
Hench LL (1998) J Am Ceram Soc 81:1705
Metsger DS, Rieger MR, Foreman DW (1999) J Mater Sci Mater Med 10:9
De Long WG, Einhorn TA, Koval K, McKee M, Smith W, Sanders R, Watson T (2007) J Bone Joint Surg Am 89:649
Sung HJ, Meredith C, Johnson C, Galis ZS (2004) Biomaterials 25:5735
Liu C, Xia Z, Czernuszka JT (2007) Chem Eng Res Des 85:1051
Neumann M, Epple M (2006) Eur J Trauma 32:125
Shikinami Y, Okuno M (1999) Biomaterials 20:859
Ignjatović N, Delijić K, Vukčević M, Uskoković D (2001) Zeitschrift für Metallkunde 92:145
Ignjatović N, Tomic S, Dakic M, Miljkovic M, Plavsic M, Uskokovic D (1999) Biomaterials 20:809
Ignjatović N, Sutjovrujic E, Budinski-Simendic J, Krakovski I, Uskokovic D (2004) J Biomed Mater Res B 71B:284
Li X (2007) J Miner Met Mater Soc 59:71
Dupraz AMP, de Wijn JR, vd Meer SAT, de Groot K (1996) Biomed Mater Res 30:231
D’Andrea SC, Fadeev AY (2003) Langmuir 19:7904
Choi HW, Lee HJ, Kim KJ, Kim HM, Lee SC (2006) J Colloid Interface Sci 304:277
Hong Z, Zhang P, Liu A, Chen L, Chen X, Jing H (2007) Biomed Mater Res 81A:515
Borum-Nicholas L, Wilson OC Jr (2003) Biomaterials 24:3671
Liu Q, de Wijn JR, van Blitterswijk CA (1998) Biomed Mater Res 40:358
Liu Q, de Wijn JR, de Groot K, van Blitterswijk CA (1998) Biomaterials 19:1067
Gutmanas EY (1983) Powder Metall Int 15:129
Gutmanas EY (1998) Cold-sintering—high pressure consolidation. Powder metal technologies and applications. ASM International, Materials Park, OH
Bernstein M (2006) Msc Thesis, Technion
Makarov C, Gotman I, Gutmanas EY (2007) In: Proceedings of 21th European conference on biomaterials (ESB 2007), Brighton, UK
Siddharthan A, Seshadri SK, Sampath Kumar TS (2004) J Mater Sci Mater Med 15:1279
Ishikawa K, Ducheyne P, Radin S (1993) J Mater Sci Mater Med 4:165
Bohner M (2000) Injury 31:S-D37
Chen CW, Riman RE, TenHuisen KS, Brown K (2004) J Cryst Growth 270:615
Dong GC, Sun JS, Yao CH, Jiang JG, Huang CW, Lin FH (2001) Biomaterials 22:3179
Acknowledgement
The research was supported by U.S.-Israel Binational Science Foundation (BSF) through research grant No. 2004-293.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rakovsky, A., Gutmanas, E.Y. & Gotman, I. Ca-deficient hydroxyapatite/polylactide nanocomposites with chemically modified interfaces by high pressure consolidation at room temperature. J Mater Sci 45, 6339–6344 (2010). https://doi.org/10.1007/s10853-010-4543-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4543-z