Abstract
The experimental work has been carried out to study the absorption–desorption properties of the hydrophobic silica aerogel for the application of oil spill cleanup. Aerogels were synthesized by sol–gel route prior to ambient pressure drying, by keeping TEOS:MeOH:Acidic H2O:NH4F:NH4OH:HMDZ molar ratio constant at 1:16.5:0.81:0.62:0.63:0.41, respectively. The absorption of organic liquids by as-prepared aerogels has been carried out by adding the aerogel samples in organic liquid, viz. three alkanes: hexane, heptane, octane; the aromatic compounds: benzene, toluene, xylene; the alcohols: methanol, ethanol, propanol, and the three oils: petrol, diesel, and kerosene until it is totally wetted. It was observed that surface-modified TEOS-based aerogel absorbed the organic liquids and oils by nearly 12 times its own mass. After absorption, desorption time of liquids from the aerogel at various temperatures, i.e., at 30, 60, 80, and 100 °C was measured. The 50% volume shrinkage of the aerogel in case of oils and 20% in case of organic liquid was observed, after total desorption of liquids. No significant change in hydrophobicity of the aerogel was observed and it can be reused two times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a growing worldwide concern about the urgent need to control accidental and conscious release of oil during transportation, storage, and safe disposal of oil and other organic liquids effectively. A wide range of materials for oil remediation has actually been employed such as dispersants, absorbents, solidifiers, and skimmers [1–7]. Absorbent materials are attractive for some applications because of possibility of collection and complete removal of the oil from the oil spill site. Some good properties of the absorbents such as hydrophobicity and oleophilicity are primary determinants of successful absorbents; other important factors include the amount of oil/organic liquid absorbed per unit mass of absorbent, retention over time, the recovery of oil/organic liquid from the absorbents, and the reusability of the absorbent [4]. Aerogels are highly porous sol–gel-derived materials. Their properties, which are extremely fascinating as well as very promising for a large number of applications, are due to their nanostructured and porosity. Because of large pore volume of aerogels, a small weight of these solids can confine a large volume of liquid. Due to these properties aerogels can be used as good absorbents for oil spill clean up. In connection to this, the results have been reported by our group using MTMS precursor with supercritical drying method [8]. Though the results obtained are well, the commercialization of these aerogels for oil spill clean-up application was found difficult because of high cost precursor and drying methodology. Therefore, comparatively low cost precursor (TEOS) and simple ambient pressure drying method, have been adopted.
In the present study absorption–desorption of organic liquids from the low density, superhydrophobic aerogels, and usability of the aerogels as effective and efficient absorbents of the oils and organic liquids are reported. The superhydrophobic silica aerogels were synthesized using tetraethoxysilane (TEOS) as low cost precursor, by the two-step sol–gel process dried at ambient pressure. The superhydrophobic silica aerogels were found to be absorbers of oils and other organic liquids. The aerogels showed a high uptake capacity of the organic liquids and the aerogels could be re-used as absorbents. In all, 12 organic liquids—three alkanes, three aromatic compounds, and three alcohols, and three oils have been used to study the absorbing capacity and desorption time of the liquids from the superhydrophobic aerogels.
Experimental procedures
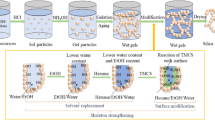
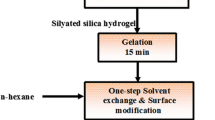
Preparation of the superhydrophobic aerogels
In two-step sol–gel process tetraethoxysilane (TEOS) was diluted in methanol (MeOH) solvent and was partially hydrolyzed with water under acidic conditions with oxalic acid (0.001 M). The sol was stirred for 1 h and kept for hydrolysis for 12 h. In the second step, the condensation of these hydrolyzed species was carried out by adding NH4F drop by drop in the sol initially, followed by the drop wise addition of NH4OH with constant stirring at room temperature, the gel was set within 5 min. Molar ratio of TEOS:MeOH:oxalic acid (0.001 M):NH4F:NH4OH:HMDZ was maintained at 1:16.5:0.81:0.62:0.63:0.41, respectively. The gels were then aged for 1 h for strengthening the gel network. The solvent exchange was carried out with hexane for 24 h. Surface chemical modification to convert hydrophilic property of the aerogel into hydrophobic was done with 5% hexamethyldisilazane (HMDZ) in hexane for 24 h and lastly the unreacted HMDZ was removed by washing the gel with hexane for 24 h. Then gels were dried at ambient pressure and various temperatures at 50, 150, and 200 °C for 1, 2, and 1 h, respectively. The good quality aerogel in terms of low density (0.065 g/cm3), superhydrophobicity (1530), high porosity (97%), and high pore volume (15 cm3/g) was obtained.
Method of characterizations and absorption–desorption study
The packing density of the aerogel was measured by mass-to-volume ratio of the aerogel where the mass was measured with microbalance of 10−5 g accuracy and volume of the aerogel was measured by filling aerogel granules in measuring cylinder of known volume. The percentage of volume shrinkage (Vs%), porosity, pore volume was determined using the formulae, which have been reported elsewhere [9]. The contact angle (θ) was measured with the contact angle meter (Rame-Hart, Model 500 F-1, USA). It was found that the aerogel sample was superhydrophobic with a contact angle of 153°. The surface chemical modification of the aerogels was studied using Fourier Transform Infrared Spectroscopy (Perkin Elmer Instruments, Spectrum one, USA), which gave the information about various chemical bonds such as O–H, C–H, and Si–O–Si.
The surface-modified silica aerogel was kept in an organic solvent until it was completely wetted by the organic liquid. It was then removed and maintained at various temperatures, such as room temperature (30 °C), 60, 80, and 100 °C in an oven (Termaks Company, Norway). The absorption intensity of the aerogel sample was studied by weighing the aerogel sample in a microbalance (10−5 gm accuracy) before absorption, immediately after absorption, and after total evaporation of the liquid from the sample. Desorption time was observed both on a butter paper and filter paper, and at different temperatures.
Results and discussion
Hydrolysis and condensation occurred as per the following reactions:
-
Hydrolysis:
$$ {\text{Si}}\left( {{\text{OC}}_{2} {\text{H}}_{5} } \right)_{4} + 4{\text{H}}_{2} {\text{O}}\xrightarrow{{{\text{C}}_{2} {\text{H}}_{2} {\text{O}}_{4} }}{\text{Si}}\left( {\text{OH}} \right)_{4} + 4{\text{C}}_{2} {\text{H}}_{5} {\text{OH}} $$(1) -
Condensation:
$$ {\text{Si}}\left( {\text{OH}} \right)_{4} + \left( {{\text{OC}}_{2} {\text{H}}_{5} } \right)_{4} {\text{Si}}\xrightarrow{{{\text{NH}}_{4} {\text{F}} + {\text{NH}}_{4} {\text{OH}}}}\left( {\text{OH}} \right)_{3} {\text{Si}}{-}{\text{O}}{-}{\text{Si}}\left( {{\text{OC}}_{2} {\text{H}}_{5} } \right)_{3} + {\text{C}}_{2} {\text{H}}_{5} {\text{OH}} $$(2) -
Silylation:
The surface chemical modification process with Hexamethyldisilazane (HMDZ) in hexane was carried out. The HMDZ provides the non-polar groups for the surface chemical modification of the gel through the –Si–(CH3)3 by the replacement of H from Si–OH group by Si–R groups, where R = methyl groups according to the following reactions:

Hydrophobic silica aerogels end capped with –(CH3)3 groups in their structure shift their absorption affinity away from polar molecules (like water) toward non-polar substances (like organic solvents, i.e., they are highly organophillic).
Absorption intensity of organic liquids by the superhydrophobic aerogels
The absorption intensity of the aerogel sample was quantified in terms of the mass of the organic liquid absorbed by unit mass (1 g) of the aerogel sample. For this study, all the 12 organic solvents, viz., three alkanes, three aromatic compounds, three alcohols, and three oils were used. The alkanes used were: hexane, heptane, octane; the aromatic compounds were: benzene, toluene, xylene; the alcohols were: methanol, ethanol, propanol; and the three oils used were: petrol, diesel, and kerosene.
Figure 1 gives absorption intensity of aerogel sample for various liquids. From the figure, it was observed that the aerogel absorbed nearly the same amount (~12 g) of the solvents, benzene, toluene and xylene. Among the oils, the mass of petrol absorbed was minimum (10.09 g) and that of diesel was maximum (11.02 g). Among the alkanes, the mass of hexane absorbed was minimum (6.42 g), while that of octane absorbed was maximum (8.68 g), and among the alcohols, the mass of methanol absorbed was minimum (~10 g) and that of propanol was maximum (~11 g).
As TEOS-based silica aerogel surface modified with hexamethyldisilazane (HMDZ) changes its hydrophilic properties into hydrophobic, the surface energy of the aerogel sample becomes lower. The mass of the organic liquid absorbed by an aerogel depends upon the surface tension (γ) of the corresponding liquid. As aerogel sample has low surface energy (superhydrophobic θ > 150°) it does not wet by water. But the organic liquids wet the surface and also get absorbed by the aerogels. This is due to the fact that the organic liquids are less polarizable than the solid aerogel. The organic liquids have very low surface tension values and therefore, they wet the aerogel surface completely and are easily absorbed. Since aerogels are highly porous material and absorption is due to the capillary action.
The mass (m) of a liquid that rises into the capillaries (aerogel pores) is given by the formula [10]:
For the liquids that completely wet the surface, the contact angle θ is zero and for such surfaces:
or
or
where r is the radius of the aerogel pores, V is the volume of the liquid absorbed, ρ is the density of the liquid, and k = g/2πr is a constant for the given aerogel sample. Therefore, it follows from Eq. 6 that the mass of the liquid absorbed increases linearly with an increase in the surface tension of the liquid as shown in Fig. 2. Therefore, it was observed that the mass of hexane (γ = 18.4 mN/m) absorbed was minimum and benzene (γ = 29 mN/m) was maximum.
Desorption time of liquid from the aerogel sample at various temperatures
The time taken by the liquid for complete evaporation from the aerogel pores starting from the moment of absorption is called desorption time. To study the effect of temperature on desorption time of the organic liquid from the aerogel, the aerogel samples were immersed in organic liquid until they are totally wet by liquid. These gels were then maintained at various temperatures at 30, 60, 80, and 100 °C and desorption time was measured (i.e., total evaporation of organic liquids from the aerogel sample) after immediate absorption of the liquid.
Surface tension of the liquid decreased with rise in temperature. When the difference of the temperature is small, the decrease of surface tension varies almost linearly with temperature.
where γ0 and γt are surface tension at 0 and t °C, respectively, α is the temperature co-efficient of surface tension for the liquid. It was observed that with an increase in temperature, desorption time decreased significantly. Table 1 shows desorption time for the liquids at various temperatures. From the table, desorption time for propanol, for example, reduced from 150 min at 30 °C to 10 min at 100 °C. Similarly, for benzene, it reduced from 60 min at 30 °C to 5 min at 100 °C. Figure 3 shows change in desorption time of the propanol at various temperature. It was also, observed that desorption time increases from shorter chain (hexane) to the longer chain (octane) organic liquids. This is due to the fact that the evaporation of liquids takes place in two stages. In the first stage, molecules of liquid are brought from the interior up to the surface, overcoming the surface tension effect. During the second stage, they vaporize from the surface film depending on the vapor pressure of the liquid. Therefore, lesser the surface tension, easier for the molecules to come on to the surface and hence faster would be the evaporation. Figure 4 shows various stages of absorption and desorption of hexane from the aerogel, at room temperature. Also, the vapor pressure decreases with the increase in the chain length and the molecular weight of the organic liquid. The vapor pressure (226 mmHg at 30 °C) for hexane is very high which led to faster evaporation and that for octane, is very less (24.05 mmHg at 30 °C), and hence desorption time is more. Therefore, with increase in temperature the surface tension of the liquid decreases and it is easy for liquid molecules to come out of the aerogel pores at higher temperatures due to higher temperature gradient.
Desorption study was also carried out keeping the wetted gel on butter paper and ordinary filter paper at 30 °C. Table 2 shows desorption time of organic liquids from the aerogel kept on butter paper and filter paper. It was observed that the desorption rate enhanced as desorption time decreased when the aerogel was placed on the filter paper. This is due to the fact that on the filter paper, there is not only the evaporation but also the absorption of the liquid by the filter paper from the bottom surface of the aerogel. Thus, for the organic liquid clean-up purposes, the aerogels can be used on a filter paper so that they can be re-used almost immediately. Aerogel shrinks after immediate absorption of organic liquid and regains 80% of volume after desorption of liquid. From Fourier Transform Infrared Spectroscopy, it is observed that there is no significant change in the bond formation before and after desorption of the organic liquids. Figure 5a, b, c, and d shows the FTIR spectra of the as-produced aerogel, and of the aerogels after desorption of hexane, benzene, and methanol, respectively. The slight difference in the FTIR for methanol solvent desorption (Fig. 5d) is due to the fact that polar groups are present in the methanol solvent leading to a relatively lower intensity of the C–H absorption at around 3000 cm−1, and higher intensity of Si–OH absorption around 3500 cm−1[11]. We measured the contact angle of the liquid before absorption and after desorption of the organic liquid. It was observed that there is no significant change in hydrophobicity of the aerogel sample after desorption of the liquid. Figure 6 shows the FTIR spectra of the aerogel after the desorption of petrol which shows an increase in the intensity of the C–H bonds at 2950 and 1400 cm−1 as compared to that before absorption (Fig. 5a). This increase in the intensity of the C–H bonds might be due to the presence of latent petrol in the aerogel sample. As shown in Fig. 7, the lack of springback in such aerogel samples might be due to the surface tension induced microfracture of the ligaments. As a result, the aerogels shrink more and do not regain the original size after total desorption of the oil. Therefore, the TEOS-based hydrophobic silica aerogels show ~50% shrinkage after the total desorption of the oil.
Conclusions
TEOS-based silica aerogels were synthesized by two-step sol–gel process and used as effective and efficient absorbents to study the absorption–desorption of organic liquids for oil spill clean-up purpose. Nine organic liquids and three oils were studied. The following are the foremost findings of the present experimental studies:
-
1.
Surface-modified TEOS-based aerogel absorbed the organic liquids and oils by nearly 12 times its own mass.
-
2.
The mass of the organic liquids absorbed was found to depend upon the density and the surface tension of the organic liquid.
-
3.
Desorption time of the liquid was found to depend upon the temperature. The rate of desorption increased with an increase in the temperature.
-
4.
The aerogels were found to shrink and the shrinkage was nearly 20% for organic liquids and 50% for oils (petrol, diesel, and kerosene) after desorption of the liquids.
-
5.
After desorption of liquids, no significant change in hydrophobicity was observed and it could be re-used at least two times.
-
6.
The rate of desorption could be enhanced by placing the aerogel on an ordinary filter paper.
Thus, the TEOS-based superhydrophobic silica aerogels could be used as efficient absorbents for the purposes of storage, clean-up, transport, and safe disposal of organic liquids and oils.
References
Fingas M (1995) Chem Ind 24:1005
Arthur B (1962) The mainstream of physics. Addison Wesley Publishing Company Inc., Second Printing, p 124
Delaune RD, Lindau CW, Jugsujinda A (1999) Spill Sci Technol Bull 5:357
Teas Ch, Kalligeros S, Zanikos F, Stoumas S, Lois E, Anastopoulos G (2001) Desalination 140:259
Doerffer JW (1992) Oil spill response in the marine environment. Pergamon Press, Oxford
Lessard RR, Demarco G (2000) Spill Sci Technol Bull 6:59
Hering N, Schriber K, Riedel R, Lichtenberger O, Woltersodorf J (2001) Appl Organomet Chem 15:879
Rao AV, Hegade ND, Hirashima H (2007) J Colloid Interface Sci 305:124
Parvathy Rao A, Panjonk GM, Rao AV (2005) J Mater Sci 40:3481. doi:https://doi.org/10.1007/s10853-005-2853-3
Newman FH, Searle VHL (1957) The general properties of matter, 5th edn. Orient Longmans, London, p 353
Laczka M, Cholwa-Kowalska K, Kogut M (2001) J Non-Cryst Solids 287:10
Acknowledgement
The authors are grateful to the Department of Science and Technology (DST) New Delhi, Government of India, for the financial support for this work through a major research project on “Aerogels” (N0. SR/S2/CMP-67/2006). Also, assistance from UGC ASSIST and DRS-phase IInd is also acknowledged. One of the author Jyoti Gurav wishes to thank Brain Korea 21 for the award of post-doc fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gurav, J.L., Rao, A.V., Nadargi, D.Y. et al. Ambient pressure dried TEOS-based silica aerogels: good absorbents of organic liquids. J Mater Sci 45, 503–510 (2010). https://doi.org/10.1007/s10853-009-3968-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3968-8