Abstract
The most important requirements for surface water management include the availability of rapid and efficient specific adsorbents. This work presents synthesis, characterization and performance analysis of a novel silica aerogel for oil decontamination from surface water. Hydrophobic silica aerogel powders were synthesized by a rapid co-precursor method applying ambient pressure. An inexpensive aqueous sodium silicate solution was used as starting material, with hexamethyldisilazane (HMDS), tetraethyl orthosilicate and trimethylchlorosilane as organic co-precursors. The prepared silica aerogels were characterized by nitrogen adsorption/desorption technique (BET), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and contact angle measurements. Silica aerogel samples prepared using HMDS exhibited the highest BET surface area of about 936 m2/g, pore volume (1.715 cm3/g) and contact angle approaching 106°. The aerogels were then tested for oil adsorbing capacity in oil salt–water mixtures for oil spill clean-up applications. Preliminary oil adsorption experiments have shown that a maximum oil removal of 96 and 90 % using silica aerogel prepared from HMDS from saline and non-saline oily waste waters, respectively. Moreover, the minimization of the costly precursors permits a low-cost large scale production and economic oil spill clean-up applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Oil spills represent huge risk to coastal sea and river environments and ecosystems. They are mainly created by accidental episodes of super oil tankers, oil drilling, food and chemical industries, as well as, natural events. Therefore, the resulting environmental misfortunes necessitate cost effective clean-up systems [1]. Different materials have been used for oil remediation such as dispersants, adsorbents, solidifiers and booms [2–5]. Adsorbents are attractive materials for oil spill clean-up because of the possibility of efficient collection and oil removal from the oil spill site, sludge free cleaning operation and simplicity of application [6, 7].
Hydrophobic silica aerogels possess high porosities [~98 %], high surface area [700–1600 m2/g] and low density [6, 8]. These properties enable different types of hydrophobic aerogels to be applied for the sorption of oil spills [7, 9], dispersed oil [10], and emulsified oil using a surfactant as a stabilizer [11]. A hydrophobic aerogel containing CF3(CH2)2 surface groups was used to remove crude oil from a 3 % salt water and Prudhoe Bay crude oil mixture [7, 9]. The prepared aerogels have fully separated the oil from water for an oil to aerogel ratio up to 3.5. Rao et al. [12] used a super hydrophobic aerogel prepared by the sol–gel process using methyltrimethoxysilane (MTMS) as a precursor. This aerogel was used to adsorb diesel oils and organic liquids and has revealed a high uptake capacity range (9.83–20.64 g/g). Parale et al. [6], synthesized hydrophobic silica aerogel starting from TEOS and TMCS as silica precursors with ammonium fluoride. The uptake of the prepared silica aerogel was about 14.6 g engine oil/g aerogel. On the other hand, Wang et al. [13], studied the purchased Cabot nano-gel 301 and 302 for the removal of different types of oil from water. Vegetable, motor and crude oils uptake values were in the range of 11.7–15.1 g oil/g aerogel [13].
Common silica precursors applied are either silicon alkoxides (such as tetraethoxysilane or tetramethoxysilane) [14–16] or organically modified alkoxides (such as methyltrimethoxysilane or trimethylethoxysilane) [17, 18]. On the other hand, supercritical drying represents the most common silica aerogel drying technique used, even though it has limitations in terms of cost and safety [19]. The commercialization of these aerogels for oil spill clean-up faces difficulties because of the high cost of silica precursors and the complicated drying methodology. Thus, industrial commercialization of hydrophobic hybrid aerogels demands the use of low-cost precursors in combination with rapid and inexpensive drying methods. Accordingly, sodium silicate has been considered as a suitable silica precursor and ambient pressure drying as an effective substitute for solvent extraction [20–28].
In the present work, focus has been placed on the development of silica aerogel synthesis route using sodium silicate along with different organic modifiers via ambient pressure drying aiming short processing time, lower production costs and ease of production for oil spill clean-up applications. This, in turn, may permit large scale hydrophobic silica aerogel production. In addition, preliminary performance evaluation regarding oil adsorption using the synthesized silica aerogel, has been studied.
2 Materials and methods
2.1 Synthesis of silica aerogel powders
The hydrogels were directly prepared from sodium silicate without prior ion exchange and employed a co-precursor method (i.e. addition of the silylating agent directly to the sodium silicate) for the organic modification of hydrogels in the aqueous phase. Sodium silicate (NSF) was dissolved in distilled water (DW) so as to obtain 4.35 and 20 wt% of silica in the starting silicate solution (SS). The silylated hydrogels were then prepared by a co-precursor method where in the nitric acid HNO3 (Adwia), HMDS (C6H19NSi2) (Sigma Aldrich), TMCS (C3H9ClSi) (Sigma Aldrich) and TEOS (C8H20O4Si) (Acros) were added to the sodium silicate solution under constant stirring. Each of the silica precursors has been used alone and combined with the others. The volume ratios of SS: HNO3: silica precursor were 100: (8.6–9.2): 12–12.8. The pH was adjusted by using different nitric acid ratios (8.6 and 8.8). Each silica precursor was used alone or in combination with another precursor, keeping the volume ratio of HMDS: other precursors fixed at 2:1. The gel then undergoes gelation for about 15 min at 27 °C. Table 1 represents synthesis conditions for different prepared silica aerogel samples. It is worth mentioning that the pH of the SS solution was about 13. The volume ratios SS: HNO3: silica precursor were 100: (8.6–8.8): 12, except for sample 7 which starts with higher HNO3 ratio to accommodate for the higher pH obtained for the SS solution. The ratios in the latter case were 100: 9.2: 12.84.
The silylated hydrogels were then immersed in 200 mL n-hexane for one-step solvent exchange and sodium ion removal which takes about 3 h. The aspect of this process is that all the three steps such as sodium ion removal, solvent exchange and surface modification were accomplished simultaneously in one step. Filtration and drying was then accomplished. Drying at ambient pressure was adopted in two steps: first at 170 °C for 20 min and second at 200 °C for 20 min to obtain the aerogel powder. Figure 1 highlights the steps during the silica aerogel synthesis.
According to this procedure, the processing time of the aerogel powders could be reduced to about 5 h which makes this route very promising for the large scale production of powdered aerogels.
2.2 Characterization and analysis
Different characterization techniques were used to identify the structural and morphological properties of the prepared silica aerogel samples. The surface chemical modification of the aerogel powders was confirmed by means of Fourier transform infrared spectroscopy (FTIR) using FT/IR-6100 type A Jasco Japan TGS detector, resolution 4 cm−1, scanning speed: Auto (2 mm/s). The contact angle measurements were done to quantify the degree of hydrophobicity using a contact angle meter, Drop master DM-701, Japan. The textural properties of the aerogel powders were investigated by the standard N2 gas adsorption method using a surface area analyzer (Quantachrome, NOVA automated gas sorption instrument, version 1.72). The specific surface area was calculated using the standard Brunauer Emmett and Teller (BET) method. The cumulative pore volume was calculated from the N2 adsorption/desorption profiles. The average pore diameters and pore size distributions were estimated by the Barrette Joynere Halenda (BJH) method. The surface morphology and the microstructure of the aerogel powders were probed by a Field Emission Scanning Electron Microscope (SEM) (JEOL: JXA-840A).
2.3 Oil spills clean-up (oil adsorption)
Adsorption experiments for synthetic oily wastewater were undertaken using vegetable oil at different concentrations. All the synthesized silica aerogel samples were tested for oil adsorption from synthetic oily wastewater with and without sodium chloride which was used for salting out. A 100 ml of distilled water (DW) (containing 3 wt% NaCl), oil and 0.9 wt% silica aerogel (<75 microns) was placed in a 250 ml conical flasks. The oil/silica aerogel (O/SA) weight ratio was kept at 3.5. The flasks were sealed and then shaken in a water bath shaker (Julabo, SW-20C) at 150 rpm at 27 °C. Upon reaching equilibrium (3 h) [13], the flasks were then left to settle. The next day, all the samples were withdrawn and analyzed for oil and grease test according to APHA. 2012 [29].
3 Results and discussion
The results section is divided into two main sections, the first one represents the characteristics of the prepared silica aerogels, while the other section shows the aerogel oil adsorption capacity. It is worth mentioning that all the silica precursors used have formed a gel except for sample (6), which was prepared using TEOS alone under the studied conditions where no gel was formed. This may be attributed to the short gelation time applied.
As a measure of the reaction total yield, a mass percentage of the final product in relation to the used silica precursors was considered. It was estimated for each sample by rationing the final weight of the dry hybrid silica powder versus the total weight of waterglass and silyating agents as the initial co-precursors used, as shown in Table 2. It is clear that the product yield ranges from 12.9 to 46.7 % using the different precursors.
3.1 Characteristics of the prepared silica aerogel samples
3.1.1 Simultaneous surface modification and solvent exchange
The surface modification in aqueous phase essentially led to the displacement of pore water from the hydrogel. During this process the hydrogel was immersed in a water immiscible solvent i.e. n-hexane. As a consequence of water displacement, n-hexane entered in the pore and eventually the hydrogel was transformed into a silylated organo-gel and thus accomplished the solvent exchange. The solvent exchange is considered a one-step process which is driven by the displacement of pore water from the gel. The amount of the displaced pore water strongly depended on the HNO3/SS and the modifying agent/SS volume ratios. The increase in the HNO3/SS volume ratio increases the degree of surface modification and thereby the amount of displaced pore water. Further increase in the HNO3/SS volume ratio results in the formation of an acidic silica sol which may have led to improper water displacement. Further, the sodium ions present in the hydrogel were removed with the displaced pore water during the one-step solvent exchange process [20, 21, 25].
The grafting of –Si–(CH3)3 groups to silica surface was confirmed by means of FTIR as shown in Fig. 2. FTIR spectrum of the studied hydrophobic silica aerogels show characteristic bands at 1254, 1400 and 2960 cm−1 assigned to the IR adsorption by Si–CH3 groups present in the aerogel powder which confirms the surface modification [20, 21]. On the other hand, the peaks around 2900, 1260 and 850 cm−1 were assigned to the Si–CH3 terminal, which originates from the TMCS-modified silica surface [30]. The broad adsorption band in the region 3450–3200 cm−1 and band at 1638 cm−1, respectively are due to the adsorbed water and surface silanol groups. The silica aerogel exhibits bands in the 1250–1050 cm−1 region and 800 cm−1 which are attributed to the Si–O–Si asymmetric and symmetric stretching vibrations of the silica network respectively [31]. In general there is a great similarity in the band frequencies positions in all studied samples.
3.1.2 Hydrophobicity of the synthesized silica aerogel samples
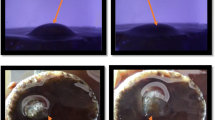
The hydrophobicity of these hybrid powders is confirmed by the contact angles of water on silica aerogel covered surfaces. Figure 3 shows images of a water droplet placed on the surface of glass substrate coated with the hydrophobic aerogel powder. The values of measured contact angles for each of the prepared aerogel samples were illustrated in Fig. 3. The drop maintained spheroid shape with contact angle of 103–109, with 5.8 % variation. The hydrophobic property of the aerogel powder is due to the organic modification of the hydrogels by HMDS, TMCS and TEOS, respectively. The attachment of these hydrolytically stable groups to the silica surface leads to the lowering of surface energy and thus results in the formation of the hydrophobic surface [21].
3.1.3 Textural properties of the synthesized silica aerogel samples
The textural properties of the aerogel powders were further investigated by the nitrogen adsorption–desorption and pore size distribution (PSD) analyses. The nitrogen adsorption–desorption isotherms obtained at 77 K are shown in Fig. 4. The physisorption isotherm obtained for samples (1–5) exhibited hysteresis loops which are the characteristic feature of the mesoporous materials (type IV isotherm) [32, 33]. The desorption cycle of the isotherm showed a hysteresis loop which is attributed tothe capillary condensation occurring in the mesopores [34]. The shape of hysteresis loops is identified with the specific pore shape. The samples (1–5) exhibited H2 type of hysteresis loop and corresponds to the pores with ink bottle shape which [21, 35, 36]. As for sample (7); the shape of the hysteresis loop was intermediate of types H2 and H3 [21].
In addition, Fig. 5 depicts the pore size distribution obtained for samples (1–5 and 7). The pore size distributions were multimodal with the peak pore diameters at 19, 27 and 42 nm. This implies that the aerogel powder consists of interpenetrating mesopores, with the highest peak at 27 nm. The mesoporosiry (pores below 50 nm) is the result of surface silylation of the wet gel [21, 26]. The textural properties of the aerogel powders synthesized samples have been summarized in Table 2. The values obtained for the specific surface areas of these aerogel powders (345–936 m2/g) are in the range obtained by other authors that used sodium silicate with equivalent silica content as co-precursor [20, 21]. It is clear that sample (7), prepared using higher silica ratio and HMDS, exhibits the highest BET surface area (936 m2/g) and pore volume (1.715 cc/g). Inclusion of TMCS along with HMDS in sample (4) has increased the BET surface area by about 15 %. On the other hand, inclusion of TEOS and using the TMCS alone has decreased the aerogel surface area.
Variation of resulted BET values may be attributed to the fact that the samples with lower surface area shrunk more during the ambient pressure drying because of the less water displacement which implies lower content in mesopores corresponding to a denser network. On the other hand, the samples with higher surface area exhibit higher water displacement which is due to the high degree of surface modification. Such gels spring back during the ambient pressure drying preserving the aerogel properties such as high specific surface area and large pore volume [21].
3.1.4 Microstructure and surface morphology of the synthesized silica aerogel samples
Figure 6a–f represents the SEM images of the prepared silica aerogel samples under different conditions. It is very clear that the silica aerogel is multi porous nanomaterial with concatenate network structure [21, 25, 37] with dense aggregates of spheres. From the SEM images shown in Fig. 6, it is clear that sample (1) prepared using HMDS exhibits dense structure with porous particles, while sample (2) prepared using higher acid ratio seems dense with more porous structure and smaller particles. As for sample (3), prepared using HMDS and TEOS, it resembles a porous structure with observed bigger spherical particles. Sample (4), prepared using HMDS and TMCS, shows highly porous less dense structure with smaller particles. Finally, sample (5), prepared using only TMCS exhibited completely different structure than the previous ones. The structure seems less porous with rod and pyramid shapes, this may be attributed to the different features of TMCS. Sample (7) looks more dense than samples (1) and (2), this may be attributed to higher SiO2 wt% used in the synthesis process.
3.2 Oil spills clean-up (oil adsorption)
Adsorption results for oily synthetic wastewater were undertaken using vegetable oil. The oil measured density and viscosity were 0.9 g/cc and 51 cp at 25 °C, respectively. Figure 7 represents the comparative adsorption results of all the prepared silica aerogel samples for oil removal in water and saline water. Figure 7 shows that samples (1) and (2) exhibit the highest oil removal from synthetic oily wastewater. While, samples (2) and (7) exhibit the highest oil removal from, saline oily wastewater. Sample (7) prepared using higher SiO2 wt% exhibited almost the same removal as for samples (1) and (2) even though it has higher surface area, as mentioned in Table 2. Figure 7 presents the salting out effect of sodium chloride on the oil removal values. It seems that oil removal is a little bit higher in saline solutions for all samples, except for samples (4), (5) and (7) which have almost equal oil removal values for both saline and non-saline oil water samples. As for samples (3) and (4) possessing relatively lower oil removal than the other samples, which may be attributed to inclusion of TEOS (sample 3) and TMCS (sample 4) in the preparation with HMDS, which has resulted in lowering their performance than samples prepared using HMDS alone at the specified conditions.
It is worth mentioning that most of the reported literature reflects similar milestones without due consideration of the scaling up requirements. However, process economy has been touched [20, 21, 25], where a shortcut methodology has been presented which is considered appealing from the industrial stand point. With this in mind, a shortcut procedure has been addressed in this study after careful readjustment of process variables to balance performance and scaling up requirements. We could manage to obtain a relatively high yield approaching 46.5 % while maintaining reasonable hydrophobicity as required for acceptable performance. We carefully balanced reactant ratios, processing time and manipulating related process conditions (data not shown) to come up with an optimal configuration for industrial manufacture. In addition, different silica precursors (TMCS, TEOS) have been used alone or accompanied with HMDS which was used in literature via this unique preparation method aiming lower short processing time and possible large scale production.
3.3 Preliminary cost indicators
Preliminary cost indicators for the produced silica aerogel have been investigated. This has been achieved through selection of the best silica aerogel prepared sample, regarding mainly the oil removal, as well as, product yield. It is clear from Table 2 that sample (2) exhibited the highest oil removal (96–89 %) and relatively high product yield (36 %). The silica aerogel unit cost was then calculated based on the price of commercial starting materials (sodium silicate, HMDS, nitric acid, n-hexane and water) (www.alibaba.com) based on mass balance. The preliminary cost analysis revealed that the unit cost of the produced silica aerogel is in the range of 1.2–2.84 $/kg. Detailed process design and techno-economic assessment and sensitivity analysis are currently underway.
4 Conclusions
Silica aerogel samples have been synthesized via ambient pressure drying with a novel procedure targeting short processing time, low production cost and ease of production. The prepared samples have been characterized using FTIR, BET, contact angle and SEM, where most of the samples exhibited nanoporous structure. Silica aerogel sample prepared using higher silica ratio and HMDS, exhibits the highest BET surface area of about 936 m2/g and pore volume (1.715 cm3/g). All the synthesized silica aerogel samples exhibited hydrophobic nature with the highest contact angle of 109°. Preliminary oil adsorption experiments have shown oil removal values up to 96 % from saline and non-saline oily waste waters using the silica aerogel sample prepared from sodium silicate and HMDS. The minimization of the costly precursors permits a low-cost large scale production and, moreover, the efficient and economic oil-spill clean-up applications. The preliminary cost indicators revealed that the unit cost of the produced silica aerogel is in the range of 1.2–2.84 $/kg.
References
A. Bayat, S.F. Aghamiri, A. Moheb, G.R. Vakili-Nezhaad, Oil spill cleanup from sea water by sorbent materials. Chem. Eng. Technol. 28, 1525–1528 (2005). doi:10.1002/ceat.200407083
M. Fingas, Oil spills and their cleanup. Chem. Ind. 24, 1005–1008 (1995)
R.D. Delaune, C.W. Lindau, A. Jugsujinda, Effectiveness of nochar solidifier polymer in removing oil from open water in coastal wetlands. Spill Sci. Technol. Bull. 5, 357–359 (1999). doi:10.1016/S1353-2561(99)00081-X
C.H. Teas, S. Kalligeros, F. Zanikos, S. Stoumas, E. Lois, G. Anastopoulos, Investigation of the effectiveness of absorbent materials in oil spills clean up. Desalination 140(3), 259–264 (2001). doi:10.1016/S0011-9164(01)00375-7
R.R. Lessard, G. Demarco, The significance of oil dispersants. Spill Sci. Technol. Bull. 6(1), 59–68 (2000). doi:10.1016/S1353-2561(99)00061-4
V.G. Parale, D.B. Mahadik, M.S. Kavale, A.V. Rao, P.B. Wagh, S.C. Gupta, Potential application of silica aerogel granules for cleanup of accidental spillage of various organic liquids. Soft Nanosci. Lett. 1, 97–104 (2011)
J.G. Reynolds, P.R. Coronado, L.W. Hrubesh, Hydrophobic aerogels for oil-spill clean up—intrinsic absorbing properties. Energy Sour. 23, 831–843 (2001)
J.L. Gurav, I.-K. Jung, H.-H. Park, E.S. Kang, D.Y. Nadargi, Review article silica aerogel: synthesis and applications. J. Nanomater. 2010, Article ID 409310
J.G. Reynolds, P.R. Coronado, Hydrophobic aerogels for oil-spill cleanup—synthesis and characterization. J. Non-Cryst. Solids 292, 127–137 (2001)
J.A. Quevedo, G. Patel, R. Pfeffer, Removal of oil from water by inverse fluidization of aerogels. Ind. Eng. Chem. Res. 48, 191–201 (2009)
D. Wang, T. Silbaugh, R. Pfeffer, Y.S. Lin, Removal of emulsified oil from water by inverse fluidization of hydrophobic aerogels. Powder Technol. 203, 298–309 (2010)
A.V. Rao, S.D. Bhagat, H. Hirashima, G.M. Pajonk, Synthesis of flexible silica aerogels using methyltrime–thoxysilane (MTMS) precursor. J. Colloids Interface Sci. 300(1), 279–285 (2006)
D. Wang, E. McLaughlin, R. Pfeffer, Y.S. Lin, Adsorption of oils from pure liquid and oil–water emulsion on hydrophobic silica aerogels. Sep. Purif. Technol. 99, 28–35 (2012)
A.V. Rao, M.M. Kulkarni, Hydrophobic properties of TMOS/TMES-based silica aerogels. Mater. Res. Bull. 37, 1667–1677 (2002)
A.V. Rao, R.R. Kalesh, Comparative studies of the physical and hydrophobic properties of TEOS based silica aerogel using different co-precursors. Sci. Technol. Adv. Mater. 4, 509–515 (2003)
S.D. Bhagat, Y.-H. Kim, Y.-S. Ahn, J.-G. Yeo, Textural properties of ambient pressure dried water-glass based silica aerogel beads: one day synthesis. Microporous Mesoporous Mater. 96, 237–244 (2006)
N.D. Hedge, H. Hirashima, A.V. Rao, Two step sol–gel processing of TEOS based hydrophobic silica aerogels using trimethylethoxysilane as a co-precursor. J. Porous Mater. 14, 165–171 (2007)
M. Nogami, S. Hotta, K. Kugimiya, H. Matsubara, Synthesis and characterization of transparent silica-based aerogels using methyltrimethoxysilane precursor. J. Sol–Gel. Sci. Technol. 56, 107–113 (2010)
G. Pajonk, E. Elaloui, R. Begag, M. Durant, B. Chevalier, J.L. Chevalier, P. Achard, Hydrolysis of an organosilicon compound, U.S. Patent 5,795,557, 18 Aug 1998
S.D. Bhagat, Y.-H. Kim, M.-J. Moon, Y.-S. Ahn, J.-G. Yeo, A cost-effective and fast synthesis of nanoporous SiO2 aerogel powders using water-glass via ambient pressure drying route. Solid State Sci. 9, 628 (2007)
S.D. Bhagat, Y.-H. Kim, K.-H. Suh, Y.-S. Ahn, J.-G. Yeo, J.-H. Han, Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater. 112, 504–509 (2008)
A.P. Rao, A.V. Rao, G.M. Pajonk, Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents. J. Appl. Surf. Sci. 253, 6032–6040 (2007)
S. Hwang, T. Kim, S. Hyun, Effect of surface modification conditions on the synthesis of mesoporous crack-free silica aerogel monoliths from water glass via ambient-drying. Microporous Mesoporous Mater. 130, 295–302 (2010)
U.K.H. Bangi, I. Jung, C. Park, S. Baek, H. Park, Optically transparent silica aerogels based on sodium silicate by a two step sol–gel process and ambient pressure drying. Solid State Sci. 18, 50–57 (2013)
M.F. Júlio, L.M. Ilharco, Super hydrophobic hybrid aerogel powders from water glass with distinctive applications. Microporous Mesoporous Mater. 199, 29–39 (2014)
S.D. Bhagat, K.-T. Park, Y.-H. Kim, J.-S. Kim, J.-H. Han, A continuous production process for silica aerogel powders based on sodium silicate by fluidized bed drying of wet–gel slurry. Solid State Sci. 10, 1113–1116 (2008)
S.W. Hwang, T.-Y. Kim, S.-H. Hyun, Optimization of instantaneous solvent exchange/surface modification process for ambient synthesis of monolithic silica aerogels. J. Colloid Interface Sci. 322, 224–230 (2008)
S.S. Kistler, Coherent expanded aerogels and jellies. Nature 127, 741 (1931)
APHA, Standard Methods for Water and Wastewater Examination, 22nd edn. (American Public Health Association, Washington, 2012)
S. Ki Hong, M.Y. Yoon, H.J. Hwang, Fabrication of spherical silica aerogel granules from water glass by ambient pressure drying. J. Am. Ceram. Soc. 94(10), 3198–3201 (2011)
F. Tadayon, S. Motahar, M. Hosseini, Application of Taguchi method for optimizing the adsorption of lead ions on nanocomposite silica aerogel activated carbon. Acad. Res. Int. 2(2), 42–48 (2012)
A.C. Pierre, E. Elaloui, G.M. Pajonk, Comparison of the structure and porous texture of alumina gels synthesized by different methods. Langmiur 14, 66–73 (1998)
W.C. Li, A.H. Lu, S.C. Guo, Control of mesoporous structure of aerogels derived from cresol–formaldehyde. J. Colloid interface Sci. 254, 153 (2002)
J.L. Mohanan, S.L. Brock, Influence of synthetic and processing parameters on the surface area, speciation, and particle formation in copper oxide/silica aerogel composites. Chem. Mater. 15, 2567–2576 (2003)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouqerol, T. Siemieniewska, Lewis base mediated efficient synthesis and solvation. Pure Appl. Chem. 57(4), 803 (1985)
Z. Shao, F. Luo, X. Cheng, Y. Zhang, Superhydrophobic sodium silicate based silica aerogel prepared by ambient pressure drying. Mater. Chem. Phys. 141, 570–575 (2013)
H.X. Shi, J. Cui, H. Shen, H. Wu, Preparation of silica aerogel and its adsorption performance to organic molecule. Adv. Mater. Sci. Eng. 2014, Article ID 850420
Acknowledgments
This work was financially supported by the National Research Centre (NRC) of Egypt, Tenth plan, under Grant Number 10070406.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorour, M.H., Hani, H.A., Al-Bazedi, G.A. et al. Hydrophobic silica aerogels for oil spills clean-up, synthesis, characterization and preliminary performance evaluation. J Porous Mater 23, 1401–1409 (2016). https://doi.org/10.1007/s10934-016-0200-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0200-5