Abstract
Nanosilica/poly(methyl methacrylate) (PMMA) composites are used to improve the mechanical properties of neat PMMA polymer. In order to obtain superior mechanical properties, it is essential to achieve good bonding between the SiO2 nanoparticles and the PMMA matrix, which is typically achieved by coating silica nanoparticles with silane coupling agents. In this study, conventional and supercritical coating methods were investigated together with their influence on the mechanical properties of the obtained nanosilica/PMMA composites. The results indicate advantageous properties of nanosilica modified in the supercritical phase of carbon dioxide and ethanol in terms of particle size distribution, amount of coated silane, and dispersion in the PMMA matrix. Careful dispersion of the starting silica nanoparticles in ethanol at low temperatures in order to obtain a nanosilica sol plays an important role in deagglomeration, dispersion, and the coating process. The resulting nanosilica/PMMA composite containing nanoparticles obtained by supercritical processing of the nanosilica sol showed an increase in hardness by 44.6% and elastic modulus by 25.7% relative to neat PMMA, as determined using the nanoindentation technique. The dynamic mechanical analysis reveals that addition of nanoparticles as nanosilica sol and nanosilica gel enhances composite storage modulus by about 54.3 and 46.5% at 40 °C. At the same temperature, incorporation of modified silica nanoparticles with conventional method leads to an increase of 15.9% for the storage modulus, probably due to a large silica particle size and lower silane content in this sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
NanoSiO2 particles display a range of specific properties, which contribute to their wide use and potential novel applications in the following areas: composite materials, biomaterials, sensor materials, and coatings. In recent years, nanoSiO2 particles have attracted much attention for potential application in polymer nanocomposites [1–3]. Considering the low surface energy and hydrophilic properties of nanoSiO2 particles, it is necessary to modify the surface of the nanoparticles in order to avoid their agglomeration and to facilitate interfacial adhesion between the nanoSiO2 particles and the hydrophobic polymer matrix [4, 5]. A silane coupling agent, as a conventional surface agent, has been considered as a popular chemical agent to chemically or physically modify nanoSiO2 particles to achieve better filler dispersion and to promote compatibility with matrices [2, 6]. Considering the two silanization mechanisms reported by Liu et al. [7], silanization processes are usually conducted in a water-containing organic medium or in an anhydrous environment. In the former environment, a lot of organic solvent, such as methanol, ethanol, acetone, and other organic solvents, are used [8–11] and the treatment time takes up to several hours, while in the latter environment, the processing temperature is usually higher and the presence of nitrogen is required [12, 13].
Recently, supercritical carbon dioxide (SCCO2) has received a great deal of attention and is currently being employed as an environmentally friendly solvent in the complex surface treatment of nanoparticles in order to achieve enhanced deagglomeration due to the specific physical properties of SCCO2. The low viscosity and the absence of surface tension in supercritical fluids allow the complete wetting of substrates with intricate geometries, including the internal surface of agglomerates. Cao et al. [14] used liquid CO2 and SCCO2 as the solvent for the surface treatment of silica substrates and silica gels for microelectronic applications. Loste et al. [15] used SCCO2 for coating hydroxyapatite and titania particles with silane coupling agents in order to obtain orthopedic implant materials. The hydrophilic surface of nanoSiO2 particles was converted into a hydrophobic one by modification with SCCO2 and a titanate coupling agent [16].
The nanoindentation technique has been proved to be a useful tool for the determination of the nanomechanical properties of a range of materials, such as polymers [17], fine grained ceramics [18], metal-based composites [19], and nanocomposites in the form of coatings [20]. Lin and Horkay [21] presented a survey of the progress made in the application of nanoindentation to characterize the local mechanical properties of polymer gels and biological tissues. Hard-tissue replacement biocomposites were characterized by nanoindentation and modulus mapping was reported by Uskokovic et al. [22]. Recently, a number of nanoindentation studies were performed on layered silicate-reinforced polymer nanocomposites, in which the fillers had high aspect ratios, for example by Shen et al. [23], Lam and Lau [24], and Treece and Oberhauser [25].
Therefore, the nanomechanical characterization of composites filled with silane-treated SiO2 within a poly(methyl methacrylate) (PMMA) matrix is of research interest. In this study, the surface of nanoSiO2 particles was modified with a silane coupling agent in a supercritical ethanol–CO2 mixture. The modification state, structure, and size of the modified particles were analyzed and the hardness and reduced elastic modulus of PMMA nanocomposites filled with silanized nanosilica were measured and compared with values obtained for neat PMMA polymer. Additionally, nanoindentation results are compared to properties obtained by dynamic mechanical analysis (DMA).
Materials and experimental procedures
Materials
Silica nanoparticles (SiO2 powder with an average particle diameter of about 7 nm and a specific surface of 380 ± 30 m2/g, Degussa-Hüls AG, Aerosil 380) were used as-received. The silica powder was heated under vacuum at 140 °C for 4 h to remove adsorbed compounds and then cooled to room temperature. The organosilane γ-methacryloxypropyltrimethoxysilane (Dynasylan® MEMO, Degussa Hüls), with molecular formula C10H20O5Si, was used as the coupling agent for modification of the nanosilica surface.
Conventional method of silanization
The silane coupling agent used was γ-methacryloylpropyltrimethoxysilane, which contained a carbon–carbon double bond believed to be suitable for subsequent incorporation/polymerization into PMMA polymer. The standard procedure involved dissolving this liquid into a mixture of ethanol/water (w/w, 95/5) under stirring to produce a pH value of the solution containing acetic acid within the range of 3–4. Then, the silica nanoparticles were added under vigorous stirring into the MEMO prehydrolysis solution and the mixture was stirred for 30 min at 1,250 rpm. After heating for 2 h at 80 °C in an ultrasonic bath, the silica nanoparticles were filtered and washed five times using 2-propanol and ethanol, in order to remove physically adsorbed MEMO molecules. The resulting silanized nanoparticles were carefully dried at 25 °C under vacuum, subsequently dried under atmospheric pressure at 80 °C for 12 h, and then kept in a desiccator for future measurements [26, 27].
Silanization under supercritical conditions
The experimental set-up used to perform silanization of the silica particles under supercritical conditions (Autoclave Engineers Supercritical Extraction Screening System, schematically shown in Fig. 1) consists of: a CO2 cylinder, an inlet CO2 cooler, a high-pressure pump, and a high-pressure vessel (capacity 300 mL).
Aerosil 380 silica particles were dispersed in absolute ethanol (10 wt%) under magnetic stirring for 15 min and ultrasonically treated for 30 min at room temperature. In case when the solution was maintained at 40 °C during ultrasonic treatment, the resulting particle solution was in the form of a thyxotropic gel (in the further text designated as SC gel). Another sample was ultrasonically treated at 10 °C, and the obtained particle solution was in the form of a colloidal sol (in the further test designated as SC sol). The MEMO silane coupling agent was added dropwise to the silica SC gel and the SC sol. After addition of the coating agent, the high-pressure vessel was sealed, and CO2 was pumped into the system. As pressure and temperature were elevated, the content of the vessel was continuously stirred. When the desired pressure and temperature were attained, the vessel content was stirred for 1 h in order to perform silanization in the supercritical phase. Particles in SC gel were treated at 40 °C under a pressure of 200 bar, while the silica particles in the SC sol were treated at 40 °C under a pressure of 120 bar. In both cases, the following quantities were used: 45.0 g of ethanol, 5.0 g of silica particles, and 6.05 g of MEMO silane. The remaining volume of the vessel (300 mL) was initially filled with carbon dioxide. Due to the composition of the mixture in the high pressure vessel, a single supercritical phase was obtained under both operating pressures [28]. After 1 h of coating with MEMO silane in the supercritical mixture of carbon dioxide and ethanol, the outlet valve was opened, thereby providing a constant flow of CO2 for 2 h and a clean-up of the coated particles.
The modified nanosilica particles were finally dried at 25 °C under vacuum for 4 h and then dried in an oven under atmospheric pressure at 110 °C for 2 h.
Preparation of nanocomposites
Poly(methyl methacrylate) nanocomposite samples containing modified silica nanoparticles were prepared at a constant silica particle concentration of 3 wt% using an in situ polymerization method. Silanized silica was added to MMA monomer and N,N-dimethyl-p-toluidine was used as the initiator. The mixture was sonicated for 30 min. PMMA powder were dispersed in the mixture, also containing dibenzoyl peroxide (DBPO) catalyst (Mecaprex KM, PRESI). The composites were cured at room temperature for 48 h and then at 60 °C for 6 h. After the heat curing, the samples were reheated at 110 °C for 2 h to eliminate residual stresses.
Characterization
The FTIR spectra were obtained in the transmission mode between 400 and 4,000 cm−1 using a BOMEM instrument (Hartmann & Braun, MB-series). Scanning electron microscopy (SEM) was performed using a FIB DB235 focused ion beam scanning microscope operating at 5 kV on carbon-coated samples. A thermal gravimetric (TG) analysis (TA Instruments SDT Q600) was used to measure the content of surface silica before and after surface treatment. The silica powder was heated from room temperature to T1 = 120 °C at 10 °C/min, held at this temperature for 10 min, and then heated at 20 °C/min to T2 = 800 °C and held at this temperature for 10 min, under a nitrogen gas flow of 100 mL/min. The samples of PMMA and composites (~10 mg) were tested under nitrogen purge, with a flow rate of 60 mL/min. The samples were ramped from room temperature to 600 °C at a heating rate of 10 °C/min. The average particle size and particle size distribution were determined using a laser particle size analyzer (PSA) Mastersizer 2000 (Micro Precision Hydro 2000 μP sample dispersion unit, Malvern Instruments Ltd.), which covers the particle size range of 0.02–2000 μm. For PSA measurements, the powders were dispersed in methanol, and treated in an ultrasonic bath for 15 min (low-intensity ultrasound, at a frequency of 40 kHz and power of 50 W). A DMA (TA Instruments Q800) was used to determine the dynamic mechanical properties of the different samples. The experiment was done in single-cantilever mode using a ramping rate of 3 °C/min from room temperature to 170 °C, with an oscillation frequency of 1 Hz.
The nanoindentation experiments on PMMA and the composites were realized using a Nano Indentation Tester—NHT (CSM Instruments SA, Peseux) equipped with an atomic force microscope (AFM). The frequently used power law method, developed by Oliver and Pharr [29], involves the extrapolation of a tangent to the top of the unloading curve to determine the depth (a combination of elastic and plastic displacement) over which the indenter is in contact with the specimen at the maximum load. The following test parameters were used for all samples: indenter type: Berkovich; approach speed: 4,000 nm/min; loading type: linear; loading rate: 2 mN/min (10 mN for the AFM images); maximum load: 4 mN; pause at the maximum load: 15 s; unloading rate: 4 mN/min. A Poisson’s ratio of 0.36 for the elastic modulus calculation was assumed for all samples.
Results and discussion
The processing methods and process parameters of the investigated raw and silanized nanoparticles are shown in Table 1, together with the percentage of chemisorbed MEMO silane as determined by TG analysis and from the theoretical amount of MEMO silane required for a monolayer coverage. As can be seen from the percentage of chemisorbed silane, the supercritical coating methods (SC gel and SC sol) were more efficient than the conventional coating method. The TG and derivative thermal gravimetric (DTG) traces of the silica particles and modified silica particles are shown in Fig. 2a, b, respectively. The weight loss up to 150 °C of the unmodified silica particles can be attributed to removal of water adsorbed on the surface and the further weight loss above 150 °C was due to dehydration of the surface silanol groups [30]. The weight loss of the silica particles modified using the conventional method was much lower than those for the particles modified by the SC gel and sol methods. In order to estimate the percentage of surface coverage of the modified samples, it was assumed that the MEMO silane molecules were oriented parallel to the silica surface. If the MEMO silane molecules are oriented parallel to the surface, which can be induced by hydrogen bonding between an MEMO-carbonyl and a hydroxyl group of the oxide, only 3.0 μmol/m2 is required for monolayer coverage [31]. Based on the data of the weight loss (1.1 μmol/m2 for the conventionally modified particles, 2.9 μmol/m2 for the SC sol, and 3.4 μmol/m2 for the SC gel) and the quantity required for monolayer coverage, the percentages of surface coverage were calculated [32] (Table 1). The DTG traces shown in Fig. 2b reveal that the MEMO silane molecules were covalently bonded to the nanoSiO2 surface. This can be seen from the temperature range corresponding to the peaks of DTG traces. These data lead to the conclusion that the SC sol modified particles were more stable than the SC gel modified ones (slightly higher temperature of the DTG peak maximum for the SC sol modified particles). This can be explained by the excess coverage of nanoSiO2 for SC gel modified particles, which resulted in a lower thermal stability. It is also interesting to note that the weight loss of the conventionally modified nanoSiO2 proceeded without a distinctive peak on the DTG trace, which is probably due to the low coverage, only 37%, of the particles with MEMO silane (decreased presence of the less thermally stable organic phase also resulted in a slight shift of the DTG peak to higher temperatures).
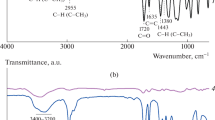
An appropriate modification of silica particles by MEMO silane assumes that the hydrolyzed silane is expected to lose its alkoxy groups and to chemically react with the hydroxyl groups of the particle surface. The FTIR spectra of the silica nanoparticles before and after the modification by the MEMO coupling agent are shown in Fig. 3. Bands characteristic of silica, such as the stretching vibration at 1,099 cm−1, the bending vibration at 455 cm−1 and the bands at 800 and 561 cm−1, can be seen in the spectra. The spectrum of the unmodified silica particles is also characterized by the presence of a band at 3,450 cm−1 and a band/shoulder at 950 cm−1, characteristic for the presence of hydroxyl groups. The presence of adsorbed water was confirmed by the Si–H2O flexion at 1,635 cm−1. The spectra of the modified silica nanoparticles exhibit new bands at 1,723 cm−1 (SC gel), 1,703 cm−1 (SC sol), and 1,700 cm−1 (conventionally modified), characteristic for carbonyl groups C=O, thereby indicating the presence of the silane coupling agent on the surface of the particle. The carbonyl band exhibited a slight shift from the theoretical value (SC sol and conventionally modified sample), which indicates that hydrogen bonds formed between the surface silanol groups and the carbonyl groups of the attached silane molecules [31]. This shows that silane covalently bonded to the surface of the silica nanoparticles existed in several forms. Hydrogen bonding was present in the SC sol and conventionally modified samples, indicating parallel orientation of the bonded molecules relative to the surface of the silica. In the case of the SC gel modified particles, the silane molecules were tightly packed and the attached molecules were almost perpendicular to the surface of the nanoparticles (there was no hydrogen bonding with the silica surface) [7]. The reduced amount of silane coupling agent and, therefore, the reduced particle surface coverage for the conventionally modified sample is indicated by the increased intensity of the bands at around 3,400 cm−1 (OH groups). It is interesting to observe the bands at 3,750 cm−1 in the spectra of the SC gel and SC sol modified particles, which indicates the existence of residual silanol groups on the silica surface.
The results of the particle size distribution measurements of unmodified and modified silica particles are given in Table 2 and shown in Fig. 4. The results indicate that deagglomeration and dispersion of the silica particles had occurred during the coating procedure, which is very important for a good dispersion of nanoparticles within composite materials and, consequently, for an improvement of the mechanical properties of composite materials. The extent of deagglomeration and dispersion was much higher in the case of the supercritical coating method, regardless of whether the starting material was in the form of a gel or a sol (SC gel and SC sol). The most successful deagglomeration of the silica particles was achieved by the SC sol method (the silica nanoparticles remained stable in ethanol over a wide temperature range, 24–304 °C at 12.3 MPa, which is in agreement with results previously published in the literature) [33].
The very good deagglomeration of the silica nanoparticles by application of the supercritical coating methods was further confirmed by the SEM images shown in Fig. 5. From these images, it is clearly visible that the conventional coating method yields significantly larger particles than both the supercritical coating methods. It is also very important to observe that the SC sol modified particles retained their initial particle size distribution after incorporation into PMMA.
Thermal decomposition of PMMA prepared by a conventional free radical method proceeds in three weight loss steps even though monomer is practically the sole degradation product [34]: the least stable step (at about 211 °C) is attributed to depolymerisation initiated by scission of weak head-to-head linkages in the main chain, the second step (at about 300 °C) results from depolymerisation initiated by scission of the β-bonds to chain end vinylidene groups, and the highest temperature step (about 390 °C) is attributed to depolymerisation initiated by random main chain scission, which is the dominant degradation pathway (Fig. 6). For PMMA composites with coated SiO2 nanoparticles, only two degradation steps from 300 to 390 °C can be found. That is, the addition of SiO2 nanoparticles eliminates the least stable step (211 °C), indicating that the addition of nanoSiO2 thermally stabilizes PMMA nanocomposites [2]. The increased thermal stability of the SiO2/PMMA composites is also demonstrated by the shift of the main degradation process toward higher temperatures.
Several nanoindentation tests were performed on each sample of neat PMMA and the composites with nanoSiO2 silanized by conventional and supercritical CO2 treatment using a CSM Nano Indentation Tester. The following test parameters were employed for all samples: approach speed: 4,000 nm/min, loading type: linear, loading rate: 2 mN/min (10 mN for the AFM images), pause at max load: 15 s and unloading rate: 4 mN/min. Figure 7 shows typical load–displacement curves of neat PMMA and its nanocomposites on two loading levels, which appear to be smooth without pop-ups or discontinuities. The difference in the mechanical response of neat PMMA and SC gel composite under the 10-mN indent load is visible. A hold time step at the peak load was used to minimize the creep effect, which might influence the shape of the unload curve and as a result might affect the values of the reduced modulus and hardness. AFM microphotographs of the indent imprints after the maximal imposed load of 20 mN for PMMA and an SC gel composite are displayed in Fig. 8. The absence of visible cracks in the vicinity of the indent imprint indicates that no cracks and fractures occurred during the indentation. It was noticed that the application of a sharp indenter was crucial for the determination parameters of the nanoscale material, such as elastic modulus and hardness, because of the accurate extraction of the contact area. Therefore, it can be concluded that the nanoindentation test was successfully applied in the present work to analyze the nanomechanical properties of the PMMA/silanized nanoSiO2 nanocomposites.
The hardness and elastic modulus of the nanocomposites are presented in Fig. 9a, b, respectively. The nanoindentation tests revealed that the hardness of the composites was significantly increased compared to that of neat PMMA. Precisely, for an indent load of 5 mN, 3 wt% of silica led to an increase in hardness by 14.6, 20.9, and 44.6% for conventional, SC gel, and SC sol treatment of the silica particles, respectively. When the higher indent load of 20 mN was imposed, the increase in composite hardness was shown to be 6.8, 9.9, and 27.2% for the conventional, SC gel, and SC sol treatment, respectively.
The increase in the modulus of the composites clearly shows that the highest increase was achieved with reinforcement by nanosilica silanized using the SC sol method. For the lower imposed load of 5 mN, the conventional treatment and the SC gel procedure showed a modest increase of 5.2 and 9.3%, respectively, while the SC sol method led to an increase in the modulus of 25.7% compared to neat PMMA. For loads of 10 and 20 mN, the conventional and SC gel methods showed an increase in the elastic modulus by about 2.5 and 0.6% in comparison to pure polymer, respectively. The composite containing silica silanized using the SC sol method showed a significant increase in the elastic modulus of 20.9%. It is interesting to mention that the SC sol silanization procedure led to the highest increases in the elastic modulus when compared to composites with reinforcing particles silanized by the other employed methods, namely, the conventional and the SC gel procedure. The presented results suggest that silanization is beneficial to enhance dispersion, thereby enabling the formation of a composite with more consistent mechanical properties. The quality of the particle dispersion throughout the matrix is likely to optimize the interfacial area, promote the stress transfer efficiency and increase the mechanical properties. Moreover, the processing parameters of the supercritical coating procedure and the silane content contribute to the final mechanical properties of a composite more than the fraction of the reinforcement phase.
The larger indentation load, 20 mN, was applied in order to cover a larger area and thus collect in that way more representative composite data. Then again, when a larger load is imposed on a specimen surface, the indenter tip can displace the nanoparticles due to their flexibility and small dimensions. As a result, the indenter senses the resistance only from the surrounding matrix and the mechanical response of the composite is closer to that of the polymer. Since in this study, the larger load led to slightly higher values of the modulus and hardness, it is reasonable to hypothesize that under the imposed loads, the particles were firmly bonded to the matrix and contributed to the overall resistance to the indenter force. Thus, the modulus and hardness values could be adopted as absolute material properties and the method was shown to be reliable for the measurement of the mechanical properties of the nanocomposites investigated in this study.
Analysis of storage modulus and loss factor (tan δ) curves using DMA are useful in ascertaining the performance of a sample under the stress at a temperature range from 40 to 170 °C. The thermo-mechanical spectra of the composites reinforced with modified silica with different silanization methods and the pure PMMA are presented in Fig. 10. It was observed that the incorporation of modified fillers into PMMA matrix resulted in an increase in the storage modulus over the entire investigated temperature range. The composites containing modified silica by SC sol method showed a higher storage modulus than that of composites with modified silica by SC gel and conventional method. Figure 10a show that addition of 3 wt% modified nanoSiO2 particles with SC sol and SC gel method enhances storage modulus by about 54.3 and 46.5% at 40 °C. At the same temperature, incorporation of modified silica nanoparticles with conventional method leads to an increase of 15.9% for the storage modulus, probably due to a large silica particle size and lower silane content in this sample. Although the nanoindentation and DMA techniques yielded different absolute modulus values, the trend in each test was consistent. With the consideration of the nature of the sample and the testing approach, one would describe DMA examining properties of more localized but still bulk material, and nanoindentation examining properties in local scale [35].
Variation of tan δ with temperature of PMMA and different modified composites are compared in Fig. 10b. The increase in glass transition temperature (T g) may be attributed to better interfacial interaction in modified nanocomposites, which decreases the polymer chain mobility. The highest peak temperature for tan δ is observed for composite with silica nanoparticles modified by SC sol method. This indicates that the best interfacial interaction between matrix and modified particles most likely occurs in SC sol composites where the T g peak temperature is the highest (125 °C) [36].
The testing of the mechanical properties of the composite materials indicated that the PMMA composite containing SC sol type of modified nanoparticles exhibited advantageous properties, as determined by nanoindentation and DMA techniques. There are several reasons for this result. The smaller diameters of the SC coated particles (PSD data in Fig. 4 and Table 2) and their good dispersion in the PMMA matrix, as shown by the SEM images of the tested composite samples (Fig. 5), can explain the superior mechanical properties of composites obtained with the particles modified under supercritical conditions. Also, the surface coverage of the silica particles modified by the conventional method was insufficient, as indicated by TG analysis, thereby resulting in a high concentration of surface silanol groups (FTIR analysis in Fig. 3) and, consequently, poor bonding between the PMMA matrix and the modified silica particles. Furthermore, as evidenced by TG analysis and FTIR determination of particle surface groups, it can be said that the SC sol method results in the formation of a monomolecular layer of silane molecules, which form hydrogen bonds with the surface silanol groups, all resulting in an almost parallel orientation of the silane molecules relative to the surface of the silica particles. The SC gel method, however, yields a different type of surface orientation and bonding of the silane molecules. In this case, hydrogen bonding of the silane molecules is almost entirely absent; there is a high concentration of surface hydroxyl groups (FTIR analysis) and also silane in excess of a single molecular layer (TG analysis). All these results indicate an insufficient surface coverage and the existence of silane multilayers (through cross linking of the MEMO silane molecules) and a perpendicular orientation of the silane molecules, relative to the surface of the silica particle. This type of coating results in weaker bonding between the modified nanoparticles and the PMMA matrix and, therefore, leads to inferior mechanical properties of the finished composite material. An improved bonding between nanoparticles modified under supercritical conditions and PMMA was also confirmed by the increased thermal stability of the composites with this type of modified nanoparticles (TG and DTG results) [37]. The main steps of the coating process under supercritical conditions, together with the principal chemical bonding and structural features of the obtained composite material, are depicted in the simplified schematic representation shown in Fig. 11 [26, 38]. Coating under supercritical conditions seems to be a very successful method of obtaining fillers with advantageous properties, when performed by supercritical processing of nanoparticles carefully prepared to obtain a colloidal silica sol, which avoids agglomeration of the nanoparticles. The properties of supercritical phase, such as: low viscosity, high molecular diffusivity, and inherent acidity in the presence of water molecules, can enable the synthesis of nanosized reinforcing agents for composite materials. These properties of supercritical fluids allow a good dispersion of the reinforcing agent and surface modification in the form of a layer, which is close to the theoretical monolayer.
Conclusions
The supercritical coating method was used to modify the surface of silica nanoparticles with MEMO silane coupling agent. It was shown that the coating procedure in a supercritical mixture of carbon dioxide and ethanol is more efficient than the conventional procedure in terms of the amount of silane bonded to the surface, deagglomeration, and dispersion of the nanoparticles and propagation of the bonding between the coupling agent and the particle surface. Careful preparation of the starting sol at a low temperature plays an important role in this process, as it provides a way to suppress nanoparticle agglomeration. The monolayer of bonded MEMO silane and the parallel molecular orientation of the silane molecules relative to the surface of the silica resulted in improved mechanical properties of the obtained PMMA–silica composite material. This is most probably due to: the small particle diameters, the good dispersion, and distribution of the modified nanoparticles within the PMMA matrix and improved bonding between the modified nanoparticles and the PMMA matrix.
References
Etienne S, Becker C, Ruch D, Grignard B, Cartigny G, Detrembleur C, Calberg C, Jerome R (2007) J Therm Anal Calorim 87:101
Garcia N, Corrales T, Guzman J, Tiemblo P (2007) Polym Degrad Stab 92:635
Huang YQ, Jiang SL, Wu LB, Hua YQ (2004) Polym Test 23:9
Jesionowski T, Krysztafkiewicz A (2001) Appl Surf Sci 172:18
Chen H, Zhou SX, Gu GX, Wu LM (2005) J Dispers Sci Technol 26:27
Mahaling RN, Kumar S, Rath T, Das CK (2007) J Elastomers Plastics 39:253
Liu Q, Ding J, Chambers DE, Debnath S, Wunder SL, Baran DR (2001) J Biomed Mater Res 57:384
Ven Hoven BAM, DeGee AJ, Werner A, Davidson CL (1996) Biomaterials 17:735
Daniels MW, Sefcik J, Francis LF, McCormic AV (1999) J Colloid Interface Sci 219:351
Hong SG, Lin JJ (1997) J Polym Sci B Polym Phys 35:2063
Wu YH, Jada S, Xu R (1998) J Mater Res 13:1204
Tsubokawa N, Shirai Y, Tsuchida H, Handa S (1994) J Polym Sci A Polym Chem 32:2327
Tsubokawa N, Ishida H (1992) Polym J 24:809
Cao C, Fadeev AY, McCarthy TJ (2001) Langmuir 17:757
Loste E, Fraile J, Fanovich MA, Woerlee GF, Domingo C (2004) Adv Mater 16:739
Wang ZW, Wang TJ, Wang ZW, Jin Y (2004) Powder Technol 139:148
Klapperich C, Komvopoulos K, Pruitt L (2001) ASME J Tribol 123:624
Nelea V, Morosanu C, Iliescu M, Mihailescu IN (2003) Surf Coat Technol 173:315
Morsi K, Patel VV, Moon KS, Garay JE (2008) J Mater Sci 43:4050. doi:https://doi.org/10.1007/s10853-007-2225-2
Olek M, Kempa K, Jurga S, Giersig M (2005) Langmuir 21:3146
Lin DC, Horkay F (2008) Soft Mater 4:669
Uskokovic PS, Tang CY, Tsui CP, Ignjatovic N, Uskokovic DP (2007) J Eur Ceram Soc 27:1559
Shen L, Phang IY, Chen L, Liu T, Zeng K (2004) Polymer 45:3341
Lam CK, Lau KT (2006) Compos Struct 75:553
Treece MA, Oberhauser JP (2007) J Appl Polym Sci 103:884
Wang Y, Li Y, Zhang R, Huang L, He W (2006) Polym Compos 27:282
Du M, Zheng Y (2007) Polym Compos 28:198
Secuianu C, Feroiu V, Geana D (2008) J Supercrit Fluids 47:109
Oliver WC, Pharr GM (1992) J Mater Res 7:1564
Wang ZW, Wang TJ, Wang ZW, Jin Y (2006) J Supercrit Fluids 37:125
Posthumus W, Magusin PCMM, Brokken-Zijp JCM, Tinnemans AHA, Vander-Linde R (2004) J Colloid Interface Sci 269:109
Stojanović D, Vuković G, Orlović A, Uskoković PS, Aleksić R, Bibić N, Dramićanin M (2007) Ind Eng Res 4:93
Ghosh SK, Deguchi S, Mukai S, Tsujii K (2007) J Phys Chem B 111:8169
Kashiwagi T, Inaba A, Brown JE, Hatada K, Kitayama T, Masuda E (1986) Macromolecules 19:2160
Ciprari D, Jacob K, Tannenbaum R (2006) Macromolecules 39:6565
Hu Y-H, Chen C-Y, Wang C-C (2004) Polym Degrad Stab 84:545
Liu YL, Hsu CY, Hsu KY (2005) Polymer 46:1851
Tai Y, Qian J, Zhang Y, Huang J (2008) Chem Eng J 141:354
Acknowledgements
The authors wish to acknowledge the financial support from the Ministry of Science and Technological Development Republic of Serbia through projects E!3524 and E!4040. In addition, the authors would like to thank CSM Instruments SA and Mr G. Favaro for providing the equipment for and Dr J. Nohava for assistance in the nanomechanical tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stojanovic, D., Orlovic, A., Markovic, S. et al. Nanosilica/PMMA composites obtained by the modification of silica nanoparticles in a supercritical carbon dioxide–ethanol mixture. J Mater Sci 44, 6223–6232 (2009). https://doi.org/10.1007/s10853-009-3842-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3842-8