Abstract
In this article, the influence of curing processing on the final ceramic yield of a highly branched polycarbosilane is discussed. Effect of pyrolysis conditions on the ceramic residue is also investigated. The results show that post-treatment can highly increase the crosslinking degree and pyrolysis yield, but has little influence on the final ceramic yield. The heating rate between 170 and 200 °C shows a little effect on the ceramic residue. Adding the catalyst into polymer, pyrolysizing with the gas pressure and improving the heating rate during the pyrolysis process can efficiently increase the final ceramic yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Silicon-based ceramics have attracted great interest for high-temperature applications due to their high mechanical strength, hardness, and corrosion resistance at elevated temperatures [1]. The technique to fabricate such ceramics from polymer precursors possesses many unique advantages over the powder-based ceramic processing such as fabricating unconventional structures and getting ceramics at relatively low temperatures [2, 3]. The ceramics obtained by such a technique have high thermal stability [4], thermal mechanical properties [5, 6], and oxidation resistance both in dry and wet environments [7–10]. These unique properties of polymer-derived ceramics render them as potential materials for high-temperature structural applications.
In the past decades, many research studies were reported concerning pre-ceramic precursors. Several researchers have set forth a series of empirical rules for designing a proper ceramic precursor [11–13]. They deduced that a useful polymer precursor should be liquid, fusible, and/or soluble for processing, and should possess high-molecular-weight, presence of latent reactivity, and cages or ring structure for high ceramic yield. In other words, the general requirement for the polymer precursor is a high ceramic yield and good processing characteristics.
The ceramic yield of polymer precursors strongly depends on their chemistry, backbone structure, the functionalities, and the degree of crosslinking. The crosslinked polysilazanes can give a ceramic yield of 80 wt% or higher, whereas the uncross-linked silazanes give only 20% ceramic residue [14]. The branched precursor had a much higher ceramic yield than that of the unbranched one due to its lower evaporation of oligomers [15]. The functionality such as vinyl group will also allow a high ceramic yield of the polymer [16]. Moreover, the pyrolysis conditions also greatly affect the final ceramic residue [17].
Polycarbosilanes have attracted great interest because they are important precursors to synthesize SiC-based ceramics. In our previous studies [18, 19], the ceramic yield of a new polycarbosilane is discussed based on its structure, including vinyl functionality, branched structure, as well as polymer molecular weight. For the highly branched polycarbosilane with certain structure and molecular weight, optimizing the curing and pyrolysis processing may be an efficient way to improve its final ceramic yield. Thus, in this study, a systematic investigation on the effect of curing procedure has been carried out to maximize the conversion of polycarbosilane to ceramic. The influence of the pyrolysis conditions is also studied to optimize the ceramic yield of this polymer.
Experimental procedure
A liquid polycarbosilane with highly branched structure (HBPCS) was synthesized in the Laboratory of Advanced Materials at Xiamen University. Briefly, HBPCS was synthesized by Grignard coupling of chloromethylmethyldichlorosilane (Cl2MeSiCH2Cl), (chloromethyl)trichlorosilane (Cl3SiCH2Cl), and allyl chloride (ClCH2CH=CH2), followed by the reduction with lithium aluminum hydride (LiAlH4) [20]. The characterizations of the HBPCS structure and polymer–ceramic conversion behaviors were reported in the previous studies [18, 19].
The polymer was crosslinked at 170 °C for 6 h with argon gas protection. In order to estimate the degree of crosslinking, the residues of the crosslinked polymers were examined by Sohex extractor with xylene as solvent. Pyrolysis at 900 °C of the cured samples with or without pressure was then carried out to determine the pyrolysis yield. Chloroplatinic acid (H2PtCl6 · 6H2O, 0.2 wt%, Shandong Boyuan Chemical co., ltd, China) was also introduced to investigate its influence on the ceramic residue of HBPCS. The thermal analysis for the crosslinked sample was conducted by thermal gravimetric analysis-differential scanning calorimetry (TGA-DSC) (Netzsch STA 409C) in argon gas with a ramping rate of 10 °C/min. Additionally, the pyrolysis yield and final ceramic yield were defined as follows:
Results and discussion
Influence of heat treatment on the ceramic yield of polymers
The number-average molecular weight (Mn) and polydispersity index of the highly branched polycarbosilane used in this investigation were 756 and 3.06, respectively. The content of vinyl functionalities calculated from 1H-NMR was about 3%. To investigate the effects of post-treatment on the final ceramic yield, the crosslinked products were heat treated between 200 and 400 °C for 2 h prior to pyrolysis. As we know, the crosslinked network of the HBPCS is insoluble in the solvents. Thus, the degree of crosslinking for the polymer can be determined by testing its gel content.
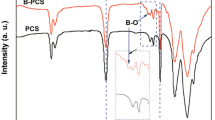
Figure 1 shows the crosslinked residues of the heat-treated HBPCS examined by soxhlet extraction. Compared with the gel content of untreated sample (17.7%), the residues of the heat-treated polymers are highly increased. Its also can be seen that the gel content increases with the elevating-treated temperature. Moreover, when the treated temperature is higher than 250 °C, the gel content changes little. IR integration ratios of Si–H/Si–CH3 decrease from an initial 7.94 to 5.71 as shown in Fig. 2, indicating that a portion of the Si–H groups are continually consumed by the crosslinking reactions with increasing temperatures. It is believed that hydrosilylation and dehydrocoupling reactions occurred at the lower temperature during the crosslinking process [19]. With temperatures increase, the abovementioned reactions together with vinyl group polymerization are taken place much more thoroughly to form the highly interlocked backbones. The low-weight molecules that further crosslinked with raised temperatures will not escape out during the pyrolysis process. This is why the higher pyrolysis yield was obtained when the polymer was heat treated at higher temperatures (Fig. 3b).
However, whatever the heat-treated temperature is, the final conversion percentage from the liquid polymer to the derived ceramic changes little (Fig. 4b). The result reveals that the weight loss during the ramping temperature process is also an important factor to have a high ceramic yield. The thermal analysis indicates that there is an exothermic peak at about 230 °C (Fig. 5), which arises from the hydrosilylation and dehydrocoupling reactions. Meanwhile, the weight change in this stage (Fig. 5) is small. It is mainly due to the evaporation of hydrogen gas and small oligomers at this temperature. The CH2=CH– functionality will evolve in the crosslinking reaction with the increasing temperature. The exothermic peak at about 380 °C should account for such reactions. Gaseous products such as CH4 and H2 will evaporate out, contributing to the weight loss in this stage. The low-weight molecules will partially escape out at high temperatures, which should also account for the weight loss in this stage. The higher the post-treated temperature is, the more weight loss during the raising temperature process will be found. Thus, the final ceramic yield is increased little though the pyrolysis yield from the cured sample to the ceramic residue is higher. In conclusion, there are two important factors to corporately influence the final ceramic yield. One is the degree of crosslinking, and the other is the weight loss during the ramping temperature process. In order to gain the high ceramic yield, raising the curing temperature to have the highly crosslinked structure of the polymer is an effective way. Meanwhile, the elevating of the curing temperature can also result in more weight loss, which is adverse to the improvement of the ceramic yield.
Influence of curing time on the ceramic yield of the polymers
A three-stage weight loss is observed for the cured sample of HBPCS from the thermal analysis (Fig. 5). The first weight loss that occurred between 150 and 290 °C is caused by the distillation of light oligomers, the second one between 290 and 490 °C is associated with hydrosilylation and dehydrocoupling reactions, and the third one above 490 °C was caused mainly by evolution of methane and hydrogen gases. Choong Kwet Yive et al. [21] found that the first weight loss step is most important. Thus, the most significant effects for raising ceramic yields should be concentrated on preventing the evolution of Si-containing oligomeric products at lower temperatures.
Compared with aforementioned HBPCS, the vinyl content of the polymer used in this part was increased from 3 to 9%. Likewise, the crosslinking process of HBPCS was conducted at 170 °C for 6 h under argon protection. The cured products were then heated at 200 °C for 2 h before pyrolysis. Differently, the ramping time from 170 to 200 °C was varied from 20 to 4320 min to reduce the weight loss. The final ceramic conversions as shown in Tables 1 and 2 are almost the same although the ramping temperature times are highly changed. Similarly, the gel content and pyrolysis yield increase a little with the enlarged curing time. It is suggested that the lower ramping rate from 170 to 200 °C has little effect on the degree of crosslinking. The weight loss during such treatments is deduced to almost the same, revealing that the heating rate during the post-treatment process has not obvious effect on reducing the weight change.
Similar trends for the change of final ceramic yield (seen in Tables 1 and 2) are also found when the chloroplatinic acid is introduced into HBPCS. However, the conversion percentages of ceramic to polymer treated with different ramping rates between 170 and 200 °C are improved by about 3%, when comparing with the ones without catalyst. The addition of chloroplatinic acid promotes the hydrosilylation reactions and thus enhances the degree of crosslinking [22]. Higher content of crosslinked residue will be obtained and results in the increased ceramic yield.
Influence of pyrolysis conditions on the ceramic yield of the polymers
Besides the precursor chemistry and pyrolysis temperatures, the ceramic yield is also affected by pyrolysis conditions including heating rate and pyrolysis pressure. It is obviously seen that the pyrolysis yield (Fig. 3a) and final ceramic yield (Fig. 4a) of the cured samples are increased by 5–8% when the conversion process was carried out with a pressure of 0.1 MPa. As aforementioned, the weight loss during pyrolysis is caused predominately by the evolution of oligomers at lower temperatures and by-product gases at higher temperatures. Under the high gas pressure, the oligomeric products are much more difficult to evaporate from the system and will take part in the further crosslinking reactions to form the deep interlocked backbones. The degree of crosslinking at low temperature is thus enhanced. Meanwhile, high pressure during the pyrolysis process partially avoids the release of volatile components and permits densification of the material, thus favoring formation of the final ceramic [23].
The extent of volatile oligomer distillation also depends on the heating rate. Bahloul et al. [17] observed that the heating rate does not (or very little) interfere in the decomposition process of the polymer. It highly influences the evaporation of the low molecules. The higher the heating rate, the more important is the first weight loss. They found that the weight change of ceramic residue for vinylsilazanes increases from 15 to 30 wt% when heating rate is changed from 1 to 60 °C/min. However, in our investigation, the final ceramic yield of HBPCS with a conversion heating rate of 4 °C/min is higher (Table 2) than that pyrolysized at a speed of 1 °C/min (Table 1). According to TGA results (Fig. 5), the weight loss mainly happens in the temperature range of 300 to 500 °C. In this stage, the polymerization of C=C, hydrosilylation, and dehydrocoupling reactions occur to increase the degree of crosslinking, accompanying the process of the depolymerization, and evaporation of low molecular weight molecules, i.e., there is a competition between crosslinking and escape of the oligomers. When the heating rate is relatively higher, the evaporation of low-molecular-weight oligomers occurs lower than crosslinking reactions. Sufficient crosslinking at the beginning of this period is critical to reduce the weight loss and to get a higher final ceramic yield.
Conclusion
In conclusion, the effects of curing processing and pyrolysis conditions on the final ceramic yield of a highly branched polycarbosilane are discussed. With increasing in post-treated temperature, the crosslinking degree and the pyrolysis yield is highly improved though the ratio of final ceramic to original polymer changes little. The ramping time from 170 to 200 °C shows a little effect on the final ceramic residue. The addition of catalyst can promote the degree of crosslinking to have high ceramic yield. Pyrolysized under the gas pressure and improving the heating rate, which will reduce the weight loss and accelerate the crosslinking degree, can efficiently increase the final ceramic residue.
References
Goto Y, Thomas G (1995) J Mater Sci 30:2194. doi:https://doi.org/10.1007/BF01184561
Yajima S, Hasegawa Y, Okamura K et al (1978) Nature 273:525
Riedel R, Passing G, SchoÈnfelder H et al (1992) Nature 355:714
Riedel R, Kienzle A, Dressler W et al (1996) Nature 382:796
An L, Riedel R, Konetachny C et al (1998) J Am Ceram Soc 81:1349
Riedel R, Ruwisch LM, An L et al (1998) J Am Ceram Soc 81:3341
Wang Y, Fan Y, Zhang L et al (2005) J Am Ceram Soc 88:3075
Wang Y, Fan Y, Zhang L et al (2006) Scr Mater 55:295
Wang Y, Fei W, An L et al (2006) J Am Ceram Soc 89:1079
Wang Y, Fei W, Fan Y et al (2006) J Mater Res 21:1625
Gmelin L (1989) Gmelin handbook of inorganic chemistry, 8th edn. Silicon Supplier, Berlin; Springer-Verlag, New York
Birot M, Pillot JP, Dunogues J (1995) Chem Rev 95:1443
Kroke E, Li Y, Konetschny C et al (2000) Mater Sci Eng R 26:97
Seyferth D, Wiseman GH et al (1984) J Am Ceram Soc 67:C132
Lücke J, Hacker J, Suttor D et al (1997) Appl Organomet Chem 11:181
Lavedrine A, Bahloul D, Goursat P et al (1991) J Eur Ceram Soc 8:221
Bahloul D, Pereira M, Goursat P et al (1993) J Am Ceram Soc 76:1156
Li HB, Zhang LT, Cheng LF et al (2008) J Mater Sci 43:2806. doi:https://doi.org/10.1007/s10853-008-2539-8
Li HB, Zhang LT, Cheng LF et al (2008) J Eur Ceram Soc 28:887
Huang TH, Yu ZJ, He XM et al (2007) Chin Chem Lett 18:754
Choong Kwet Yive NS, Corriu RJP, Leclerq D et al (1992) Chem Mater 4:141
Bischoff R, Cray SE (1999) Prog Polym Sci 24:185
Sandeep RS, Rishi R (2002) Acta Mater 50:4093
Acknowledgement
The project was supported by the Natural Science Foundation of Fujian Province of China (No. E0510002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Zhang, L., Cheng, L. et al. Effect of curing and pyrolysis processing on the ceramic yield of a highly branched polycarbosilane. J Mater Sci 44, 721–725 (2009). https://doi.org/10.1007/s10853-008-3176-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3176-y