Abstract
The hydrothermal autoclave experiments were conducted to simulate the interactions in the scCO2/water/rock minerals (quartz, biotite and granite) reaction systems using a Hastelloy C reaction cell at 100 °C. The dissolution characteristics of rock minerals and their surface texture alternation after hydrothermal treatment were examined by ICP-AES and SEM/EDX investigation, respectively. The results suggested that the hydrolysis of plagioclase phase should be mainly responsible for the elements dissolved from the Iidate granite samples. The dissolution was encouraged by the introduction of CO2 in the water/granite system, and generated an unknown aluminosilicate. No distinct chemical alternations occurred in the water-free scCO2/granite system, which indicated that rock minerals should be chemically stable in the water-free scCO2 fluids under the current mild experimental conditions. Both the highest concentration of Ca existing in the scCO2/vapor/granite system and the SEM observation results of calcite deposit, suggested that a meaningful CO2 minerals trapping process should be potential in the CO2-rich field during a short physicochemical interaction period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past several decades worldwide hydrocarbon consumption has lead to a substantial increase in emission of carbon dioxide (CO2) [1]. Many evidences suggest that the burning of fossil fuels has contributed to the accumulation of CO2 in the atmosphere. Global circulation models indicate that these increased CO2 accumulations are an important contribution to warmer temperature, increased rainfall, and raising sea level [2]. In an attempt to reduce or at least slow the increase of atmospheric CO2 concentration, thereby reducing the risk of global climate change, one important potential approach for C management is the capture of CO2 emissions and subsequent injection of CO2 into geologic media for sequestration of CO2. And the presence of natural CO2 reservoirs supports this idea [3]. The benefits of CO2 sequestration in the subsurface are great. CO2 injected into the subsurface can potentially be isolated from the atmosphere for long-term periods. It has been estimated that there is sufficient capacity in deep brine filled sedimentary rocks and depleted hydrocarbon reservoirs to store up to 1000 years worth of CO2 emissions. During geologic sequestration, three trapping mechanisms exist to retain CO2 in a saline aquifer: hydrodynamic, solubility, or mineral trapping [4]. Hydrodynamic trapping involves the storage of CO2 as supercritical fluid beneath a low permeability cap rock. Solubility trapping involves the dissolution of CO2 into a fluid phase, including both aqueous brines and oil. Mineral trapping involves CO2 rich reservoir brine reaction with the reservoir rocks to participate carbonate minerals [5]. Permanent sequestration of CO2 can be achieved by the third mechanism [6–8]. However, risks are present. CO2 can leak from the subsurface returning at least some of the stored CO2 to the atmosphere. Such leaks may be exacerbated by the dissolution of cap rock by acidic CO2-rich fluids resulting from CO2 injection [5]. In addition, precipitation of secondary minerals near the injection site may lead to lower permeability holding back further CO2 injection. Therefore, the risk from the interaction in the supercritical CO2/water/rock system needs to be considered carefully.
A few studies do examine reactions in scCO2-brine-aquifer system or the rock (granite or sandstone)/H2O/CO2 system under the reservoir temperature and pressure conditions [9–11]. Additionally, the reactive behaviour of supercritical CO2 (scCO2) under the relevant geologic conditions is largely unknown [11]. Our previous experiments conducted in a broad temperature range up to 350 °C to understand granite and sandstone reactions in hot water in the absence or presence of excess CO2, have been examined the significant effect of CO2 on the dissolution of rock minerals and the deposition of aluminosilicate and calcium-aluminosilicate secondary minerals [10, 12]. Our results suggest that the alteration of granite under CO2-saturated hydrothermal conditions has the potential to capture CO2 when it is injected at moderate temperatures (150–250 °C) into granite-hosted rock masses.
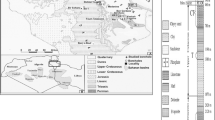
Many researchers refer to the injection of CO2 into modeling aquifer at least 800 m deep where CO2 is in supercritical state because of its critical point lying at 31 °C and 7.38 MPa [13]. Nevertheless, an excessive sequestration deep should not be welcomed by energy developers from the economical viewpoint in view of cost for CO2 injection, and depth and relative temperature for a suitable aquifer should be in the range of 1000–2000 m and 50–110 °C. On the other hand, we noticed the fact that the permanent sequestration for CO2 capture should be encouraged at moderate temperature [12]. Therefore, based on the comprehensive consideration, the relevant mild (100–150 °C) geologic conditions will be attractive. A typical geologic CO2 sequestration reservoir should be located in saline aquifer or formations under cap rock, as shown in Fig 1. Because the solubility of scCO2 into the formation water or saline water is very small within such a deep saline aquifer [14], the great amount of scCO2 is immiscible in the liquid hot water in the aquifer or some formations; only a small amount of CO2 partially dissolving in the hot water should react with rock minerals. For the low solubility of scCO2 into the formation water or saline water, and for the density of scCO2 far lower than that of the formation water, the scCO2 fluid under cap rock tends to form a separated phase from the surrounding formation water or saline water, which is named as CO2-rich filed. Based on the above consideration, the interactions in scCO2/water/rock minerals systems in CO2-rich field should occur potentially in four reaction systems: water-free scCO2/rock reaction system, scCO2/vapor/rock reaction system where a little of liquid water (pore water) diffuse in the state of vapour, CO2/water/rock reaction system in the aquifer dissolving low concentration of CO2 adjacent to CO2-rich field, and water/rock reaction system far away from CO2 filed, as shown in Fig. 1.
Since the reactive behaviour of scCO2 under relevant moderate (100–150 °C) geologic conditions has not been understood very well, the investigation of interactions mechanism among the scCO2/water/rock minerals systems becomes essential to know the factors influencing this CO2 trapping technology. Here we present experimental evaluation of interactions in the scCO2/rock, scCO2/vapor/rock, CO2/water/rock and water/rock reaction systems under a simulated sequestration conditions at 100 °C suitable for underground storage of CO2. Comparison is made among the dissolution of quartz, biotite and granite involving water or gas-rock reactions. The importance of scCO2 pressure and the state of water is emphasized. The results of this study may be used to assess the hydrolysis behaviour of granite in the aquifer for CO2 sequestration, and the validity of CO2 trapping by means of the formation of the carbonate minerals as well as the further comprehend on the versatile problems of CO2 sequestration under geological environment.
Experimental
Starting rock materials
In this experiment, granite specimen taken from the surface of Iidate granite strata in Fukushima Prefecture of Japan was chosen as the geochemical and mineralogical representation of the igneous rock as well as one of the components in the aquifer. Quartz and biotite specimens collected from pegmatite deposit in Nellore, Andhra Pradesh, India, were select as the individual minerals for further understanding of that which minerals in the granite should play an important role in the dissolution of granite under for the simulated geological environment. The chemical composition of SiO2 in Quartz is larger than 99.5% with few impurities. The chemical oxides composition of biotite is presented in Table 1. The representative (igneous) granite specimen is composed of an interlocking mosaic of quartz, plagioclase and K-feldspar, with other minerals in a small quantity (including hornblende, biotite and opaque minerals). Its chemical oxides compositions and modal mineral abundance of Iidate granite are presented in Table 2.
The starting granite specimen was prepared by core boring of 16 mm in diameter from the mass of rock. Both of the coring granite samples and the quartz were broken into fragments approximately 3–5 mm in diameter to provide granular samples. The biotite sample was prepared by cutting the biotite specimen into slice with 3–5 mm in diameter. After washing by distilled water and impurity iron removal using magnetic separation, the rock samples prepared for subsequent experiments conducted in the scCO2 fluid/water/rock interaction systems were vacuum dried preliminarily at 105 °C for 24 h in 0.1 kPa.
Experimental apparatus and methods
Laboratory scCO2/water/rock interaction experiments were conducted in a hydrothermal autoclave apparatus consisting of a Hastelloy C reaction cell of 41 mL, as shown in Fig 2. A sample cup consisting of SUS316 mesh set to the middle section of reaction cell served as the sample holder of rock minerals. As a simulation of geologic CO2 sequestration environment, three reaction systems (scCO2/rock, scCO2/vapor/rock and CO2/water/rock reaction system) were achieved by addition of the rock minerals (quartz of 10 g, biotite of 5 g and granite of 10 g, respectively) to the sample holder, as well as the introduction of CO2 and water or not (Fig. 3a, c, d) after air in the reaction cell being purged by CO2 gas. The fourth reaction system was the water/granite system without introduction of CO2 gas (Fig. 3b), which was selected as a reference system compared to the CO2/water/rock reaction system so as to comprehend the contribution of CO2 to the physicochemical interactions in the scCO2/water/rock systems. The hydrothermal autoclave was treated at 100 °C for the desired time in an electric oven. After treatment, the hydrothermal autoclave was air cooled rapidly to room temperature. And then the residual CO2 gas was absorbed by bubbling into purified water as well as the reacted rock samples and the reactor cell were washed by purified water.
Schematic of the four reaction systems in the reaction cell for simulation of the scCO2/water/rock reaction systems under the geologic CO2 sequestration conditions: (a) water-free scCO2/rock system; (b) water/rock reaction system without CO2; (c) scCO2/vapor/rock system, water of 1 mL introduced to form a saturated steam in the reaction cell; (d) CO2/water/rock system, rock mineral samples immersed into water of 30 mL, respectively
Dissolved element Na, K, Al, Si, Ca, Mg and Fe in the recovered solutions were determined using Optima 3300 SYS ICP-AES (PerkinElmer Ltd., Japan). The recovered solutions comprised the solution for the residual CO2 gas absorption plus the solution for washing rock samples and the residual solution in the reaction cell; 30 mL, 30 mL, 1, 30 mL recovered solution for (i) scCO2/rock, (ii) water/rock, (iii) scCO2/vapor/rock, (iv) CO2/water/rock reaction systems, respectively. The pH of the residual solution (at room temperature) was determined by a digital pH meter (Horiba pH meter D-52). The reacted rock sample was dried at 105 °C for 24 h in 0.1 kPa, reweighed and examined by SEM/EDX (HITACHI, S-4700T) to note any hydrothermal effects on the primary minerals, as well as the occurrence of any secondary minerals and their textures in the host rock.
Results and discussion
Effect of state of water on dissolution of rock minerals
A series of autoclave experiments to study the dissolution behavior of rock minerals (quartz, biotite and granite) were conducted in (i) scCO2/rock, (ii) water/rock, (iii) scCO2/vapor/rock, (iv) CO2/water/rock reaction systems at 100 °C for 48 h, as shown in Fig. 4.
Major elements in the recovered solution from quartz (a), biotite (b1) and (b2), and granite (c1) and (c2) systems treated at 100 °C for 48 h, respectively. scCO2; H2O(l), scCO2/H2O(g) and scCO2/H2O(l) in X axis title representing the following reaction system: (i) water-free scCO2/rock system, scCO2 of 10 MPa; (ii) water/rock reaction system, water of 30 mL introduced without CO2; (iii) scCO2/vapor/rock system, water of 1 mL introduced to form a saturated steam in the reaction cell filled with scCO2 of 10 MPa; (iv) CO2/water/rock system, rock minerals sample immersed into water of 30 mL without CO2, respectively
Quartz (SiO2) is one of main important components of granite in the aquifer, holding 37.1% (vol%) of mineral abundance in Iidate Granite. Figure 4a shows that the concentrations of Si both in the water/quartz and the CO2/water/quartz reaction system were approached to about 20 μmol/L (5 ppm) and about 3 μmol/L in the scCO2/vapor/quratz system, which suggests that the introduction of scCO2 into the water/quartz system has no significative influence on the dissolution of quartz in the hot water at 100°C. In the scCO2/quartz system, the dissolution of Si in the scCO2 fluid was hardly to determine. Therefore, quartz phase is present chemical stably in the scCO2/rock system because quartz could not dissolve into the scCO2 fluid without liquid water or vapor.
Biotite ((K, Na) (Mg, Ca, Mn, Fe2+)2.4 (Al,Fe3+)2.2 (Si,Ti)3.2O11.8 (OH)1.6) is one of the main components of the granite despite its lower mineral abundance (6.3 vol%) in the Iidate Granite, and is also the most important resource of Fe2+. The concentration analysis for the recovered solution was conducted in the four reaction systems of using biotite as the starting rock minerals (Fig. 4b1 and b2). Si, Mg and Ca are the major elements in the recovered solution in the related reaction system compared with Na, K, Al and Fe of low concentration. In the scCO2/bitiotite reaction system without water, elements in trace quantities were detected; no descried chemical interaction proceeded similar to that in the scCO2/quartz system. Compared to that in quartz reaction system, the elemental concentrations in scCO2/biotie system with water or vapor were far greater than that in the water/biotite system. In addition, the higher Si, Mg and Ca concentration (950, 300, 270 μmol/L, respectively) found in the scCO2/vapor/bitiote system instead of that in the scCO2/water/biotite system or the water/biotite system shows a surprising impact on the function of vapor. The result implies the difference of biotite dissolution mechanism attributing to the state of water.
Compared to other granite systems, the elemental concentrations were greatest in the scCO2/vapor/granite system (Fig. 4c1 and c2); the Ca concentration took an unimaginable maximum of 13650 μmol/L (550 ppm) in a short reaction time of 48 h; In addition, Si, Na and Mg were major elements from the dissolution of granite, with concentrations of about 3400, 3300 and 1700 μmol/l (95, 76 and 40 ppm) respectively. In the scCO2/granite without water, only a trace elemental concentrations less than 0.05 ppm were detected in the recovered solution, suggests no effective dissolution of granite in the scCO2 fluid without water. In addition, introduction of scCO2 fluid into the water/granite system shows a more severe dissolution of granite for Ca Mg and Si, nevertheless there is no distinct alternation for Na, K and Al.
In all the scCO2/rock (quartz, biotite and granite) systems without water, no evident chemical alternation occurred contributing to the dissolution of rock minerals in the scCO2 fluids. Consequently, rock minerals exist chemical stably in the scCO2 fluids without water under the current experimental conditions for CO2 sequestration.
In the case of introduction of water or vapor, elements dissolved from both quartz and biotite were too low to be responsible for the occurrence of high concentration elements dissolved in the residual solution in the granite system. Under the acid scCO2 hydrothermal conditions of 100 °C, the dissolution of quartz is not active because the dissolution is a releasing process of acid (H4SiO4). The high concentrations of Ca and Na with low concentrations of K and Fe in the granite system suggest most of Ca, Si and Na leached from granite were originated from the hydrolysis of plagioclase phase (Na, Ca) (Al, Si)4O8 in Iidate granite. The presence of Al with very low concentration less than 8 μmol/L in the residual solution implies the possibility of generation of secondary minerals consisting of some aluminosilicates in the scCO2/granite/system.
The presence of highest concentrations elements in the residual solution from the scCO2/vapor/granite system reveals that the vapor provides stronger reactivity for the granite hydrolysis process than do the liquid water under the same experimental hydrothermal conditions of temperature and CO2 pressure. If taken into account the volume of water introduced into the scCO2/vapor/granite system was 1 mL (about 1 g), the dissolved amount of elements in the vapor system were a several times less than that in the scCO2/water/granite with addition of 30 mL water. However, in the scCO2/vapor/granite system, introduction of about 0.025 g water could generate a saturated steam in the reaction cell of 41 mL. Therefore, large amount of 1 mL water only exist in the liquid state in the bottom of the reaction cell, and the minute quantity of water (0.025 g) in the form of the vapor could diffuse into the scCO2 fluid above. We infer that in the scCO2/vapor/granite system, the vapor in the scCO2 fluid was absorbed and formed a very thin water film on the porous surface of granite for surface absorption. At the same temperature the free energy of reaction of the vapor was relatively higher than that of the liquor, and the diffusion of CO2 in the thin water film should be easier than that in the scCO2/water/granite system which had low solubility of CO2. Moreover, the dissolution of granite should be enhanced by the diffusion of CO2 which was discussed in the next section. Therefore, the vapor shows greater reactivity with granite than the liquid water does.
Effect of CO2 pressure on the dissolution of rock minerals
Figure 5a shows a slight reduction for the Si concentration in the residual solution from the CO2/water/quartz system with the increase of CO2 pressure ( \( {\text{P}}_{{{\text{CO}}_{{\text{2}}} }} \)). Release of Si comes from the hydrolysis of quartz:
This reaction gives acid H4SiO4 and releases [SiO4]4− anion, that is inactive under the current experimental conditions of 100 °C. Obviously, the solubility of CO2 in the water/quartz system was enhanced with the increase of CO2 pressure in the reaction cell [10, 12], and made the solution become more acidic and holdback the formation of H4SiO4. Thus, in the scCO2/water/granite system, quartz phase was not responsible for the increasing dissolved Si in the residual solution from Iidate granite.
As shown in Fig. 5b1, b2, c1 and c2, the concentrations for most of elements except for Al were encouraged by the introduction of CO2 into the water/granite system, as well as depended on the pressure of CO2. Of interest is the dissolved behavior of Al, which shows a greater concentration (about 23 μmol/L) in the residual solution from the water/granite system than that (less that 9 μmol/L) in the CO2/water/granite system. It strongly suggests that Al was fixed on the granite samples in the presence of CO2, which was attributed to the deposition of some aluminosilicates (i.e., kaolinite) based on the following reactions:
Therefore, the introduction of CO2 accelerated the hydrolysis of plagioclase phase in the Iidate granite.
According to Eq. (4), the calcite generation was dependent upon the hydrogen ion concentration, CO2 pressure and Ca ion concentration. In fact, the formation of calcite and the dissolution reactions of H2CO3 (HCO −3 , CO 2−3 ) in Eq. (3), and the release of Ca from the dissolution of the plagioclase were concurring or/and competing reactions. In the water/granite system, the release of Ca was encouraged by the introduction of CO2 as suggested in Eq. (2). In the initial dissolution of the granite, the release rate of Ca from the dissolution of plagioclase was dominant which made the equilibrium of Eq. (3) tend to right side and promoted the formation of calcite. Therefore, the concentration of Ca was increased with the increase of CO2 pressure even if the solution became acidic, as shown in Fig. 5c2. On the other hand, Fig. 6 shows that with the increase of CO2 pressure the pH for the residual solution tended to a sequential decrease which should contribute to the dissolution of calcite in the acidic solution. Finally, the competing reactions between formation and dissolution of calcite reached an equilibrium state, and then the concentration of Ca and the pH of solution showed slightly modification at CO2 pressure of 15 MPa (Figs. 5c2, 6). Therefore, an endless formation of calcite could not be expected in such acid conditions in the case of the CO2-rich /water/granite system [15].
Deposition of secondary minerals and CO2 mineral trapping
In the case of the scCO2/water or vapor/quartz or biotite and the water/granite system, any evident alternation of the rock surface texture had not been seen by our exclusive SEM investigation, which could attribute to their sluggish hydrolysis process under the current mild hydrothermal conditions.
For the scCO2/water/granite system, our SEM/EDX observation reveals the presence of an aluminosilicate (kaolinite?) that was not present in the starting granite when granite was hydrothermal treated at 100 °C and the scCO2 fluid of 10 Mpa for 96 h (Fig. 7a). The aluminosilicate phase was characterized by randomly oriented, fine flocculent platelets. Nevertheless, no distinct carbonate minerals were found on the granite sample in the system during such a short reaction period, which attributed to the relatively lower concentration of Ca in the scCO2/water/granite system. As suggested by Eq. (4), lower Ca and acidic solution was not beneficial to the stable formation of calcite. Many researchers focusing on the mineral trapping process under the reservoir conditions suggested that times for precipitation of the various carbonates should be on the order of hundreds of years, and mineral trapping conversion of CO2 to carbonate minerals may contribute significantly to CO2 sequestration within saline aquifers but only in the very long term [8, 15, 16]. Our results from the scCO2/water/granite system also verify carbonate minerals not present in a short term under the mild experimental conditions.
A calcium-rich carbonate mineral (calcite?) formed in the scCO2/vapor/granite system experiment undertaken at 100 °C and CO2 of 10 MPa for 48 h, as shown in Fig. 7b. The secondary mineral phase occurred as grained on plagioclase and biotite phase in the granite samples. The result is far away from the previous option that conversion of CO2 to stable carbonate minerals was expected to be slow and was a long-term reaction process. Obviously the remarkable phenomenon of carbonate minerals occurring during such a short reaction period has not been understood yet. In the residual solution of 1 mL, the Ca took the maximum concentration with 13650 μmol/L (550 ppm); concentrations of Mg and Fe were about 1700 and 45 μmol/L (40 and 2.5 ppm), respectively. The entire M2+ (Ca, Mg and Fe) originated from the hydrolysis of granite. We considered the fact that in the scCO2/vapor/granite system only a very small proportion of water in the form of vapor could diffuse into the scCO2 fluid, and then was absorbed to form the very thin water film on the porous granite surface. At the same temperature the free energy of reaction of the vapor was relatively higher than that of the liquor, and the diffusion of CO2 into the thin water film should be easier than that in the scCO2/water/granite system which had low solubility of CO2. Consequently, the vapor shows greater reactivity with granite than the liquid water does. Therefore, it is conceivable that the concentrations of M2+ on the granite surface should be one or two even more orders of magnitude greater than that in the residual solution. Based on the Eq. (4), higher concentrations of M2+ were beneficial to the formation of carbonate minerals. Additionally, the presence of accumulational elements dissolved from the granite sample made the ionic strength of the aqueous water film layer on the granite surface larger, which contributed to the aqueous layer becoming more basic and promoted the formation of carbonate minerals. Since the concentrations of Mg and Fe in the residual solution were too low and far less than that of Ca, calcite (CaCO3) should precipitate instead of MgCO3 and FeCO3 based on their solubility product constants, Ksp (MgCO3 > CaCO3 > FeCO3) [17]. Thus, a meaningful CO2 minerals trapping process should occur in the scCO2/vapor/granite system under the geologic CO2 sequestration conditions during a short reaction period. The injection of CO2-rich fluid into such a mild geothermal system (100 °C) should be certainly encouraged by electric energy developers and investors. Nevertheless, the system was lack of long-term experimental valuation. The risk is never neglectable that the deposition of carbonate and secondary minerals near the injection site may lead to lower the permeability of the granite in the aquifer and hold back further CO2 injection. Additionally, the long-term stability and quantity of carbonate minerals need to be carefully accessed.
Conclusions
Our experiments were conducted to understand the physicochemical interactions in the scCO2/water/rock reaction systems using a hydrothermal reaction cell at 100 °C. The results suggest that the hydrolysis of plagioclase phase should be mainly responsible for the elements dissolved from the Iidate granite samples. The hydrolysis of the plagioclase was encouraged in the presence of CO2 in the water/granite system, and generated an unknown aluminosilicate. No distinct chemical alternations occurred in the scCO2/granite system without water, which indicates that rock minerals exist chemical stably in the water-free scCO2 fluids system under the current mild experimental conditions. The highest concentration of Ca occurred in the scCO2/vapor/granite system combining with the SEM investigation results of calcite deposit, suggest that a meaningful CO2 minerals trapping process should be potential in this system under the geologic CO2 sequestration conditions during a short reaction period.
Our work is ongoing; it aims to identify the optimum geologic conditions including reasonable organization of aquifer and cap rock that will allow for safe and reliable subsurface disposal of CO2.
References
Johansson T, Williams R, Ishitani H, Edmonds J (1996) Energy Policy 24:985
Manabe S, Stouffer R (1993) Nature 364:215
White S, Allis R, Moore J, Chidsey T, Morgan C, Gwynn W, Adams M (2005) Chem Geo 217:387
Hitchen B (1996) In: Aquifer disposal of carbon dioxide, hydrologic and mineral trapping. Geoscience Publishing Ltd., Sherwood, Alberta, Canada, p 12
Editorial (2005) Chem Geo 217:183
Bachu S, Gunter W, Perkins E (1994) Energy Convers Manag 35:269
Koide H, Takahashi M, Tsukamoto H, Shindo Y (1995) Energy Convers Manag 36:505
Tianfu X, John A, Karsten P (2005) Chem Geo 217:295
Gunter W, Wiwchar B, Perkins E (1996) In: Hitchon B (ed) Autoclave experiments and geochemical modeling of Chapter 8. Geoscience Publishing Ltd., Sherwood, Alberta, Canada, p 115
Liu L, Suto Y, Greg B, Yamasaki N, Hashida T (2003) Energy Convers Manage 44:1399
Kaszuba J, David R, Marjorie G (2003) Appl Geochem 18:1065
Suto Y, Liu L, Yamasaki N, Hashida T (2007) Appl Geochem 22:202
Span R, Wagner W (1996) J Phys Chem Ref Data 25:1509
Suto Y, Liu L, Hashida T, Yamasaki N (2001) In: Proceedings of the sixth international symposium on hydrothermal reactions & fourth international conference on solve-thermal reactions. Kochi, Japan, p 309
Hitchon B, Gunter W, Gentzis T, Bailey R (1999) Energy Convers Manage 40:825
Soong Y, Goodman A, McCarthy-Jones J, Baltrus J (2004) Energy Convers Manage 45:1845
David R (2003–2004) In: CRC handbook of chemistry and physics, 84th edn. CRC Press, LLC, pp 8–120
Acknowledgements
This work was supported by the 21st Century COE Program Grant of the International COE of Flowing Dynamics from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Prof. N. Yamasaki of Graduate School of Environmental Studies, Tohoku University, for operation of hydrothermal apparatus. We are also grateful to Prof. T. Adschiri of Institute of Multidisciplinary Research for Advanced Materials, Tohoku University for providing valuable advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, H., Fujii, T., Takisawa, R. et al. Experimental evaluation of interactions in supercritical CO2/water/rock minerals system under geologic CO2 sequestration conditions. J Mater Sci 43, 2307–2315 (2008). https://doi.org/10.1007/s10853-007-2029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2029-4