Abstract
Geologic carbon storage (GCS) has recently been drawing attention as an effective and sustainable method to reduce CO2 emissions to the atmosphere. The injection of CO2 is mainly conducted into brine-saturated reservoir formations, and assessment of the multiphase flow properties becomes essential to evaluate the injectivity and storage capacity for carbon storage. Characterization of the CO2/water flow system requires comprehensive knowledge of host rock’s relative permeability, capillary pressure, and wettability that are related to each other. Moreover, chemical reactions between the mineral grains and acidic mixture of CO2 and water may significantly affect the flow properties. In this study, a comprehensive experimental approach to characterize the chemical effect on the multiphase flow of CO2 in water-saturated reservoir rock is presented. One silica-rich formation—Berea sandstone, and two calcite-rich formations—Apulian and Indiana limestones, are selected to represent the reservoir materials. Robust experimental techniques for measurements of the relative permeability and degree of saturation based on the changes in poroelastic response are introduced. The relative permeability curves are determined, as it is shown that the curvatures and maximum degree of CO2 saturation change after CO2 treatment, especially for the limestones. In addition, the apparent contact angle increases and surface roughness decreases due to the chemical effect of CO2 injection on limestones. Finally, the advantages and limitations of the presented measurements are discussed and reported flow properties are compared to those predicted from the pore-scale analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Climate change is argued to be accelerated by CO2 among different greenhouse gases, highlighting the importance of mitigating its atmospheric emission (IPCC 2021). As part of the solution, geologic carbon storage (GCS) has been widely recognized as a sustainable and effective method to reduce the discharge of CO2, aiming to collecting it from stationary sources and injecting into subsurface formations for permanent sequestration (IPCC 2005). The injection is generally conducted into brine-filled porous reservoir rocks with potentially large storage capacities, mainly comprising of sandstones and limestones located at depths of at least 800 m (Orr 2004; US DOE 2010).
As CO2 is injected into the reservoir, the distribution of the aqueous fluid is rearranged laterally, followed by the shift of CO2 upward due to its density being smaller than the one of water (Johnson et al. 2005). It was observed that CO2 injected into Utsira sandstone in the North Sea propagated laterally over an area of ~ 10 km2, while the plume was also rising due to buoyancy (Chadwick et al. 2006). This transposition of the fluid can be described by understanding the hydraulic behavior of participating rock—one of the most important factors in assessing the formation’s suitability for most geo-energy projects. However, as CO2 injection is generally conducted into brine-saturated reservoir formations, the concept of permeability for single-phase flow cannot be directly applied for the multiphase fluid system (Fig. 1). Therefore, the relative permeability needs to be evaluated considering the interaction between the wetting and non-wetting fluids (Kopp et al. 2009; Bachu 2013). Characterization of the multiphase flow response for GCS projects involves assessment of viscous and capillary forces at the pore scale and the interfacial and wetting properties of the involved fluids (Fulcher et al. 1985). Moreover, as the injection of high-pressure CO2 introduces poromechanical deformation and chemical reaction, proper description of relative CO2 permeability in reservoir rock requires comprehensive understanding of the multiphysical processes (Rutqvist 2012; Rohmer et al. 2016; Vilarrasa et al. 2019).
The significance of accurately assessing the two-phase flow behavior has been highlighted by many laboratory-scale experiments, where the relative permeabilities were measured through various methods. Civan and Donaldson (1989) suggested a semianalytic approach to calculate the relative permeability for the immiscible displacement of two incompressible fluids based on the fractional-flow equation and an integro-differential equation (Donaldson et al. 1988; Bennion and Bachu 2005; Bachu and Bennion 2008), and Bachu and Bennion (2008) reported a series of core-scale experiments to evaluate the two-phase flow characteristics of brine and CO2 for various rock types, using the mass balance method to determine the degree of saturation and the relative permeability curve. Krevor et al. (2012; 2015) conducted tests on reservoir rock to measure the relative permeability under in situ conditions. The X-ray computed tomography (CT) method was utilized to determine the degree of saturation by scanning the fluid distribution in the pores, as other studies have also adopted this method (Perrin et al. 2009; Pini et al. 2012).
A different approach to evaluating the degree of CO2 saturation is based on accurate measurements of the ultrasonic wave velocities during the multiphase flow experiments (Shi et al. 2007; Falcon-Suarez 2020). In general, the changes in velocities and wave attenuation are shown to be correlated with the degree of non-wetting fluid saturation (Winkler and Plona 1982; Amalokwu et al. 2014). Akbarabadi and Piri (2013) conducted unsteady- and steady-state flow tests and determined the relative permeability curves, while the effect of hysteresis on capillary trapping was also examined for both supercritical and gaseous CO2. The integrated workflow to measure the relative permeability from unsteady-state flow was introduced by Berg et al. (2013, 2021). Permeability and capillary pressure curves could be iteratively extracted by history matching through the inverse modeling, but the solutions might not be unique due to some instability issues.
Despite the existing number of studies focused on measuring the CO2 relative permeability in porous rock, the experimental data remain to be dependent on the experimental setup and interpretation (Müller 2011; Chen et al. 2014; Pini and Krevor 2019). Many numerical simulations have also been conducted to predict the multiphase flow during CO2 injection (Chen et al. 1994; Juanes et al. 2006; Martinez et al. 2013). Pruess et al. 1999 introduced a multiphase fluid and heat flow code TOUGH2 and presented simulations of CO2 flow in brine aquifer considering the losses through fault discharge (Pruess and Garcia 2002). As TOUGH2 could not consider the effect of chemical reactions and mechanical stress, the code was expanded to include a nonisothermal reactive geochemical transport relationship and became TOUGHREACT (Xu and Press 2001; Xu et al. 2006). The analytical relationship between relative permeability and saturation were proposed by Brooks and Corey (1964), van Genuchten (1980), Burdine (1953), and Mualem (1976). However, comparing their prediction for CO2 relative permeability, saturation, and capillary pressure, significant discrepancies have been reported, thus experimental validation is needed (Oostrom et al. 2016).
CO2 injection causes the non-wetting fluid (CO2) to displace the wetting fluid (brine or water), so understanding the wettabilities of the participating fluids is extremely important since it affects the capillary pressure and degree of saturation (Anderson 1986). Wettability is the preference of a solid to be in contact with one fluid than the other, and depending on the fluid’s contact angle to the surface, the material can be classified as water/CO2/intermediate-wet (Abdallah et al. 1986). Different approaches to measuring the wettability of the multi-fluid system have been reported, as both the quantitative (contact angle measurement, Amott method, and USBM method) and qualitative methods (imbibition rates, relative permeability curves, microscope examination, and capillary pressure curves) have been introduced (Amott 1959; Craig 1971; Donaldson et al. 1969; Ionescu and Maini 1983). Among the suggested techniques, measurements of the contact angle can be selected as one of the most consistent for reporting the wettability since it is not affected by surfactants or other compounds and is determined by the balance of the interfacial forces of the multi-fluid system and the surface (Anderson 1986). Thus, many different approaches such as the tilting plate method, sessile drop and bubble method, vertical rod method, and cylinder method have been proposed to perform accurate contact angle measurements (Adamson and Gast 1967; Johnson 1969; Good 1979; Neumann and Good 1979; Drelich et al. 1996). These techniques have been already implemented to report the contact angles for the water/brine–CO2–mineral system at high pressure (Espinoza and Santamarina 2010) and even inside the rock pores using micro-CT images (Tudek et al. 2017).
Wettability explains the distribution of multiphase fluids in the pore space but does not directly describe the saturation state and needs to be considered along with the hydromechanical behavior of the system (Marsden 1965; Falode and Manuel 2014). Moreover, several factors such as the pore geometry, surface roughness, and heterogeneity can increase the complexity of assessing the wettability of the porous rock, as previous studies reported a wide range of contact angles for various materials (Drelich et al. 1996; Sarmadivaleh et al. 2015). Particularly, since reservoir formations are composed of micro-sized and rough mineral grains, the surface roughness should also be analyzed to accurately determine the wettability (Morrow 1990; Amirfazli et al. 1998; Baldacchini et al. 2006). Kaveh et al. (2014) performed laboratory experiments at representative reservoir conditions to establish a relationship between the surface roughness and contact angle, where it was found that the CO2 injection did not significantly affect the surface roughness, although its increase caused the contact angles to become slightly larger. Haeri et al. (2020) proposed that the contact angle increased with the surface roughness based on a series of tests with various sandstones. In contrast to the previous results, Al-Yaseri (2016) reported that for the CO2/brine/quartz system, the contact angles decreased with increase in the surface roughness in accordance with the Wenzel theory (1936) which can also be inferred from the measurements on the solid surfaces (Wang et al. 2013). To investigate these discrepancies, the effect of CO2 injection on the surface roughness and its relationship with the wettability requires further experimental assessment.
Another important factor to be considered is the chemical reaction caused by CO2 injection, as it alters the pore structure and directly affects mechanical and flow characteristics of the reservoir rock (Mazumder and Wolf 2008; Luquot and Gouze 2009; Grombacher et al. 2012; Su et al. 2019; Tarokh et al. 2020). Due to the challenges with direct experimental measurements, only a few recent studies have been focused on the chemical effect on the multiphase flow behavior, reporting the change in relative permeability curves due to dissolution of calcite in limestones (Kim et al. 2018; Niu and Krevor 2020).
In this study, we present the experimental work to assess the multiphase flow characteristics of reservoir formations during CO2 injection. Berea sandstone, Apulian limestone, and Indiana limestone are selected as representative materials, and the properties of pristine- and CO2-treated specimens are compared. The relative permeability curves are determined by adopting a novel technique, where the poroelastic measurements are utilized to calculate the degree of saturation. Maximum and residual degree of saturation and the entry pressure for CO2 are evaluated, and the chemical effect of CO2 treatment is observed. The relative permeability curve measurements are compared with the predictions based on the application of the mercury intrusion porosimetry (MIP). In addition, microscopic measurements are conducted for the wettability and surface roughness, and further discussions on the potential implications for CO2 storage are provided.
2 Methods
In this section, the detailed information on the materials selected for this study is introduced. Then, the methods for the relative permeability measurement, CO2 treatment, and calculation of the degree of saturation from the poroelastic measurements are elaborated. In addition, the devices utilized to assess the microscopic properties—mercury intrusion porosimetry, microgoniometer, and profilometer, are explained.
2.1 Materials
Three isotropic and homogeneous porous materials—Berea sandstone, Apulian limestone, and Indiana limestone, are selected for this study. Berea sandstone is a sedimentary formation that overlies the Bedford and Ohio shale and underlies Sunbury shale, also known as Berea grit (Newberry 1874). It was formed in the late Devonian period and primarily consists of fine to medium-sized grains, being studied as the host rock for oil and natural gas (Collins 1979). The gray-colored sandstone dominantly consists of quartz (around 90% by mass), with minor inclusions of kaolinite, K-feldspar, and muscovite. The porosity φ is measured to be 0.22.
Apulian limestone is a glauconitic fossiliferous carbonate rock, formed in the mid-Cretaceous era, and originated from the Apulia region in Southern Italy. This formation mainly comprises calcite (more than 95%) with a small portion of plagioclase, glauconite, and iron oxide, representing the soft calcite-rich rock for this study. The matrix of Apulian limestone is constructed with 0.05–1 mm size fragmental calcitic foraminifera, which are calcitic mud (micrite) cementations with allochems. For this soft limestone, the porosity is measured to be the largest among the tested rock: φ = 0.37.
Indiana limestone is versatile for buildings and public structures, as it is widely recognized for its durability and stability (Shaffer 2020). It is formed with uniform carbonate grainstones during the Mississippian Subperiod of the Carboniferous and is composed mainly of calcite (more than 97%), with inclusions of quartz, aluminum oxide, and sulfur. This light-gray to bluish-gray rock has the porosity equal to 0.13.
For this study, deionized water is selected as the pore fluid instead of brine. Thus, we neglect the influence of salinity and solely focus on the chemical effect of the acidic mixture of CO2 and water on the rock minerals and pore structure. All the selected materials contain less than 1% of clay minerals (by mass) to minimize the chemical interaction of pore fluids with clays, and repeatability of the test data confirms that swelling and alteration processes can be neglected. CO2 is injected and maintained in the liquid state throughout all the tests to disregard any phase transitions and associated thermal effects on the rock properties. In addition, injection of liquid CO2 is energetically more efficient comparing to the supercritical fluid and should be investigated for near-wellbore multiphase flow (Rayward-Smith and Woods 2011; Vilarrasa et al. 2013). The capillary pressure–saturation curves and porosities for all materials are measured using the mercury intrusion porosimetry (MIP) method, with mercury being a non-wetting fluid in air-saturated samples (Fig. 2). The dominant pore sizes are determined as 20 μm, 2 μm, and 1 μm for Berea sandstone, Apulian limestone, and Indiana limestone, respectively. At least three samples are tested for each material to confirm the consistency of obtained results.
2.2 Multiphase Flow Test
The multiphase flow is defined as the simultaneous flow of more than one fluid through a porous media, as it becomes the case for CO2 injection in water-saturated rock in geologic carbon storage (Fig. 3). Although multiple fluids exist, characterization of the multiphase flow behavior triggers from defining the concept of intrinsic permeability. Permeability describes the capacity of the interconnected porous medium to allow fluid to penetrate and can be introduced based on the law of conservation of mass. If it is written in Darcy’s form for laminar and steady-state conditions, the flow rate q can be expressed as a function of viscosity of the fluid µf and the differential pressure dpf along the differential distance xi.
The parameter k in this equation can be measured when a constant differential pressure Δpf between the upstream and downstream sides of a specimen is applied: pfup–pfdown = Δpf. The flow rate is calculated as the volume of the fluid ΔV exiting the specimen over time Δt. If the viscosity of the fluid is known (µf = 0.001 Pa·s for pure deionized water at 22 °C), L is the length of the specimen, and A is the cross-sectional area perpendicular to the flow, then permeability can be calculated as
For the multiphase fluid flow, relative permeability kri = ki / k is introduced to describe the flow capability of the i-th fluid. By extending Eq. (2), the relative permeability can be calculated from the knowledge of the viscosity µιf, as well as the flow rate ΔVi/Δt and differential pressure Δpif in the i-th fluid (Bear 1988).
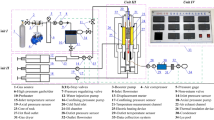
The two-phase flow test of water and CO2 is conducted in the core flooding device with the high-pressure CO2 injection setup, assuming that flow of the two fluids in the rock is steady-state and laminar. Right cylindrical specimens with diameter D = 50.8 mm and length L = 100–110 mm are inserted in the rubber viton membrane installed inside the device. The core holder allows imitation of high in situ stress conditions and is connected to four pressure controllers: the Stigma 500/700 controller (Sanchez Technologies, France, 70 MPa capacity) for the confining pressure, and three syringe pumps (Teledyne ISCO, USA, 25.9 MPa capacity) for the CO2 upstream, water upstream, and downstream pressures. Accurate measurements of the pore pressure are performed via two pressure transducers (Honeywell, USA, capacity 34.5 MPa) installed at the upstream and downstream sides of the specimen. As two syringe pumps are connected to the upstream for CO2 and water for the flow rate and pressure control, this setup allows two-phase flow experiments, while the CO2 treatment test can also be conducted by only having the CO2 syringe pump valve open (Kim and Makhnenko 2021).
The deformation of the specimen is assumed to be poroelastic, and the total mean stress P can be determined from calculating the induced axial stress σax applied via the passive restraint installed in the axial direction. By knowing the applied confining pressure σlat and the pore pressure pf, and measuring the Poisson’s ratio ν and Biot coefficient α from the mechanical tests, P can be calculated as (Kim and Makhnenko 2020a).
Full saturation with water is achieved before each flow test by adopting the back pressure saturation method (Lowe and Johnson 1960; Makhnenko and Labuz 2016). Then, the pore water is exposed to liquid CO2 for at least 4 h to guarantee that it becomes CO2-rich under the testing conditions. In the flow test, the two upstream pumps for water and CO2 are operated in the flow control regime with the prescribed rate of injection, while the downstream pump is maintained in constant pressure control. The ratio of the flow rates (water/CO2) for the upstream pumps is prescribed to be 100:0%, 80:20%, 50:50%, 20:80%, and 0:100%. As the two fluids are injected from the upstream, the pressure in each pump fluctuates at the beginning, while it eventually reaches a constant value that allows calculation of the differential pressure Δpfi for each fluid (i = w for water and i = c for CO2 in Eq. 3). The two-phase flow tests for reservoir rock are conducted for not more than an hour after reaching the steady-state flow condition to assure that the chemical reactions have minimal effect on the material properties. Moreover, as the relative permeability experiment starts from injection of water only and then increases the rate of CO2 injection, it is to be noted that this study focuses solely on the drainage process. After treating the specimens with CO2, it is completely removed from the pores by flushing the deionized water at low pressure before conducting any relative permeability measurements. Considering the pore size of the tested rock, and resaturating the treated specimens with water via the back pressure saturation technique, we affirm that the subsequent measurements of the mechanical and hydraulic properties are performed with presence of only the wetting fluid (Kim and Makhnenko 2021).
2.3 Saturation Calculation
In order to assess the storage efficiency for CO2 injection projects, a proper understanding of the degree of saturation for the two fluids (water and CO2) is required. In addition, as the pore compressibility can be affected due to CO2 injection, the poromechanical response needs to be coupled with the multiphase flow. The materials selected for this study all represent monomineralic and isotropic rock (the sandstone is silica-rich and limestones consist mainly of calcite) allowing to adopt the poroelastic theory introduced by Biot (1941) for the description of their mechanical behavior. In this study, a novel method to determine the degree of saturation is introduced based on the measurements of the fluid-saturated rock compressibility during the CO2 injection. After running a two-phase flow test and assuring that the total injected fluid volume of CO2 and water exceeds five times the pore volume of the specimen, both the upstream and downstream valves are closed simultaneously, imposing the undrained boundary condition. Then, the Skempton’s B coefficient is directly measured as an increase in pore pressure Δpf due to the application of the increment in the mean stress ΔP (Skempton 1954). At the same time, it can be expressed through other poroelastic parameters and the bulk modulus of the pore fluid that represents a mixture of water and CO2 - Kf, mix (Detournay and Cheng 1993).
Here, K is the drained bulk modulus, K′s is the unjacketed bulk modulus, K″s is the unjacketed pore modulus, and φ is the interconnected porosity. The drained bulk modulus K (Eq. 6) and unjacketed bulk modulus K′s (Eq. 7) are measured using the hydrostatic compression device, with strain gauges installed on a prismatic specimen with a linear dimension of at least 30 mm in three perpendicular directions. The bulk deformation is monitored during the loading/unloading steps, as the slope for the pressure versus volume strain (εv = ε1 + ε2 + ε3) is taken as the bulk modulus (Tarokh and Makhnenko 2019).
The unjacketed pore modulus K″s is assumed to be equal to K′s for ideal porous media, so K′s = K″s is adopted in this study regarding the isotropy of the tested reservoir formations (Detournay and Cheng 1993). We acknowledge though that this assumption might be violated for Berea sandstone that has minor inclusions of other minerals (Makhnenko and Labuz 2016) and Apulian limestone that contains some non-connected pores (Tarokh and Makhnenko 2019).
With the knowledge of the poroelastic properties, the bulk modulus for the mixture of two fluids (water and CO2) - Kf, mix can be calculated from Eq. 5. Then, from the Wood’s (1930) formula, the degree of saturation for each fluid can be determined, while the bulk modulus of CO2 is calculated to be Kc = 0.07 GPa at the testing conditions (6.9 MPa and 22 °C) (Achenbach 1973) and bulk modulus of pure water Kw = 2.24 GPa.
The procedure to determine the saturation of the two fluids are repeated for each flow rate of H2O/CO2, where the relative permeabilities of the wetting and non-wetting fluids are measured. The relative permeability curve can be obtained by plotting the measured values of krw and krCO2 versus the degree of saturation for each of the fluids. This approach overcomes the limitations of other methods, such as the X-ray CT scanning, regarding the resolution, discretization effect, and testing conditions (Cnudde and Boone 2013) and can be used with the materials where pore sizes fall below the imaging resolution (e.g., shales).
For the discrete data points obtained from the multiphase flow measurements, the fitting is performed using the analytical relationships. The Brooks and Corey (1964) power law model is adopted, where the effective saturation parameter Se is introduced. Then, with the fitting exponent parameters for water and CO2, Nw and Nc, the relative permeabilities can be expressed as:
The parameter Se can be calculated from the knowledge of liquid saturation Sl, the residual liquid saturation Srl, and the maximum liquid saturation Smax, that is equal to 1 for the case of full water saturation.
The effective saturation parameter Se allows estimation of the capillary pressure Pc through Brooks–Corey fitting parameters: CO2 entry pressure Pe and pore size distribution index λ (Dullien 1992).
Although the Brooks–Corey model provides intuitive fitting of the relative permeability curves and information on the CO2 entry pressure, it cannot be directly connected to the saturation characteristics of a porous material. Therefore, van Genuchten model (1980) is used, since it adopts the same fitting parameters α, m, and n, for both capillary pressure and relative permeability curves.
Use of this model enables validation of the two different methods presented in this study—relative permeability and capillary pressure measurements. For van Genuchten model, certain restrictions can be imposed on the fitting parameters based on Mualem’s (1976) approach, where m can be expressed in terms of n as m = 1–1/n, while it should stay in the range of 0 < m < 1. However, for this study, we utilize three parameters independently to obtain a more accurate fitting.
2.4 CO2 Treatment
The effect of CO2 treatment on the multiphase flow behavior is explored by comparing the characteristics of the pristine and treated rock specimens. After reaching full saturation with deionized water, the pressure conditions are controlled to maintain 4.5 MPa effective mean stress and 6.9 MPa pore pressure. Then, liquid CO2 is injected from the upstream side at 7 MPa with the downstream valve closed. The upstream valve is being closed after injecting 40–50 mL of liquid CO2 to maintain an undrained condition, and the pore pressures at the upstream and downstream sides of the specimen are monitored. The readings on the pressure transducers indicated that the pore pressure slowly decreases to 6.2 MPa—the boundary value between liquid and gaseous CO2. Therefore, additional liquid CO2 is injected daily at the pressure of 7 MPa once the decrease in the pore pressure is observed.
Previous works reported that the duration time of CO2 treatment correlates with the degree of the change in the material properties (Kim and Makhnenko 2022; Shi et al. 2019). For this study, 21 days are selected as the treatment period, as we intend not to introduce any macroscopic damage to the materials (Tarokh et al. 2020). Furthermore, since Apulian limestone is recognized as a softer material that highly reacts with the acidic mixture of water and CO2, the treatment period is reduced to 3 days (Luquot and Gouze 2009; Kim et al. 2018).
2.5 Mercury Intrusion Porosimetry
The capillary pressure Pc for the CO2–water–rock system can be determined by utilizing mercury intrusion porosimetry (MIP) to measure the porosity and pore size distribution. Quantachrome Poremaster 60 from Anton Paar© is used to inject mercury in the pores of dry reservoir rock samples with a mass of approximately 1 gram. As liquid mercury has a high cohesive force, its volume can be accurately measured, while it is forced to intrude the pores by increasing the pressure up to 413.7 MPa. After reaching full saturation with mercury, the weight of the sample cell is accurately measured to calculate the bulk density of the rock sample. Then, with the knowledge of the density (ρ = 13.54 g/cm3), contact angle (θ = 140°), and surface tension (γ = 480.0 erg/cm2) of mercury, the pore size distribution can be determined as the function of the pore throat diameter d using Young–Laplace equation.
The degree of saturation for each corresponding pressure is calculated as the ratio of the injected mercury to the total interconnected pore volume. Finally, the capillary pressure curve can be obtained by plotting the capillary pressure vs. the degree of saturation. Application of the capillary pressure curves for CO2 injection requires converting the MIP data from the mercury/air/rock system to the CO2/water/rock system. The abbreviation n/w describes the non-wetting/wetting system for CO2/water, while m/a represents the mercury–air system. As a result, the capillary pressure for the CO2–water–rock system can be calculated as:
We employ the findings of Espinoza and Santamarina (2010), where the average interfacial tension and contact angle for CO2/water are measured as 32 mN/m and 20° for silica-rich materials and 32 mN/m and 40° for calcite-rich materials, respectively. Although some previous studies reported different contact angles for porous rock to that of its composing mineral (Lv et al. 2017; Scanziani et al. 2017; Tudek et al. 2017), we presume that the contact angle and interfacial tension of the tested rocks follow their dominant composing minerals. Nevertheless, based on the direct measurements of the apparent contact angle, further discussion regarding the effect of CO2 injection on wettability is presented.
2.6 Wettability
The wettability of the system can be assessed by measuring the contact angle θ, which is formed at an equilibrium state of the interfacial forces in the multiphase fluid system. Although in situ measurements of the wettability may differ from those at ambient conditions (Lv et al. 2017; Tudek et al. 2017), a robust method to evaluate the effect of CO2 treatment on the contact angle is adopted by taking high resolution images with a microgoniometer MCA-3 (Kyowa, Japan). The device is capable of accurately controlling the size of the microscopic droplets to be as small as 100 μm and can detect the droplet from both the top and the side for its movement and wettability. A high magnification lens is installed with the ability to monitor the droplet in a 12-fold zoom that allows a field of view from 75 × 56 to 910 × 680 μm, with a fast image capture system for accurate measurements. The drop deposition can be automatically recognized with the FAMAS software, and the apparent contact angle can be measured in the range of 0.1–80° with 0.01° resolution.
The specimens are prepared by cutting slices from rock cores with a 1–2 cm length and width and 0.5 cm depth, and then polishing their surface to maintain a uniform roughness. After washing it with deionized water, the rock is being completely dried before the contact angle measurements. The sessile drop method is used for this study as the microscopic droplets are applied to the flat and polished surface (McCaffery et al. 1970; Anderson 1986), after which the contact angles are measured using the microgoniometer. Dependence of the contact angle from the applied pressure, temperature, and salinity limits the understanding of the wettability. Thus, the effect of CO2 treatment on wettability is qualitatively investigated by measuring the apparent contact angles at room temperature, atmospheric pressure, and zero salinity. Moreover, the reported contact angles are related to the wettability of the air–water–rock system, as it is expected to be different to the results of the CO2–brine system. Additionally, at the microscopic scale, the advancing and receding contact angles are expected to be observed in the pores during multiphase flow and their values are, in general, pressure and temperature dependent (Tudek et al. 2017). However, due to the uncertainties associated with measuring the advancing/receding apparent contact angles in non-equilibrium state, such as wicking occurred during the movement and tilting of the rock surfaces, only the apparent contact angles at equilibrium are reported (Anderson 1986). Therefore, this assessment is mainly aimed at addressing the qualitative differences between the wettability of treated and untreated rock surfaces.
2.7 Surface Roughness
Characterization of the wettability is crucial for understanding the multiphase flow in porous rock, while the contact angle could also depend on the surface roughness (Wenzel 1949; Tamai and Aratani 1972; Morrow 1975). A qualitative approach to evaluate the effect of CO2 treatment on rock’s surface roughness is adopted, where the Sloan Dektak3ST Profilometer provides repeatable and accurate measurements of the height and 2D profile of the surface, ranging from a few nanometers to 100 microns. With a 2.5-micron radius diamond-tipped conical stylus, the contact technique is utilized to measure the surface topography without any damage, as it scans across the surface of the specimen. All specimens are precisely ground to take a shape of a disk with 2 cm in diameter and 1 cm thickness. Each measurement is conducted by scanning 1000 μm along the specimen surface, with N = 2000 data points collected per scan. Then, 12 scans are performed per specimen to obtain average representative data by selecting different starting positions for the stylus. The profilometer is set to the highest scanning resolution with 12 s scanning time duration for each measurement. The root-mean-square (RMS) method is utilized to calculate the surface roughness, where Zi is the measured height value and Zavg is the arithmetic mean for all height values.
3 Results
3.1 Poroelastic Properties
The knowledge on the poroelastic properties of reservoir rock is needed to calculate the degree of saturation with CO2 and report the relative permeability curve. Pristine- and CO2-treated specimens are tested in the hydrostatic compression cell under the jacketed and unjacketed conditions to calculate the corresponding bulk moduli measured during the unloading stage (Fig. 4). For the jacketed test on Apulian limestone, the pressure is increased up to 30 MPa, since preceding studies reported pore collapse of this material around 39 MPa (Tarokh and Makhnenko 2019). For the other two materials, the jacketed compression test is conducted up to 50 MPa. The results for all materials show that the drained bulk moduli increase gradually to reach a constant value, indicating that all the microcracks and oblique pores at the early compression stage are being closed. For Berea sandstone, the drained bulk modulus decreases from 12.1 to 10.3 GPa. For Apulian limestone, the jacketed bulk modulus K decreases (e.g., from 6.3 to 5.0 GPa at P = 30 MPa), meaning the material is getting more compressible due to the treatment process. The measurements for Indiana limestone show a similar result to Apulian limestone in terms of CO2 treatment—the decrease in drained bulk modulus by 20%, from 29.5 to 23.1 GPa (at P = 30 MPa pressure). It is to be noted that for Berea sandstone and Indiana limestone, the bulk moduli of the pristine- and CO2-treated specimens are almost identical at low mean stress values (0–4 MPa), while for Apulian limestone, the effect of CO2 treatment can already be observed from the low mean stress indicating high compressibility of the material (Kim and Makhnenko 2022).
Accurate measurements of the unjacketed bulk modulus K′s are also required to calculate the degree of saturation, and the results of unjacketed experiments are reported in Kim and Makhnenko (2021). All the unjacketed moduli are measured to be constant during the loading and unloading stages. K′s for Berea sandstone remained the same after CO2 treatment, being equal to 30 GPa. For Apulian limestone, the unjacketed bulk modulus decreased from 42.7 to 34.2 GPa, and for Indiana limestone, K′s also decreased by 20%, from 65.9 to 54.9 GPa (Table 1). The decrease in the unjacketed moduli of limestones is explained by the observed dissolution and precipitation of calcite caused by the chemical reaction with the acidic mixture of water and CO2. However, it is to be noted that apparent precipitation may be related to the particle migration and rearrangement of calcite particles, which can be suggested as another reason for the creation of the new non-connected pores (Mangane et al. 2013). It appears that CO2 treatment causes increases in the total porosity, as well as in the non-connected porosity in calcite-rich rock, making the overall solid matrix response more compliant (Kim and Makhnenko 2021).
For this study, the measurements of the Skempton’s B coefficient are utilized to calculate the degree of saturation for the relative permeability curves. The B values are measured for the pristine- and CO2-treated reservoir rock specimens at P′ = 4.5 MPa and are reported as functions of the degree of saturation, that is calculated from Eq. (5). The Skempton’s B coefficient for Berea sandstone decreases after the treatment from 0.77 to 0.67, while it increases from 0.47 to 0.53 for Apulian limestone and 0.32 to 0.40 for Indiana limestone. For limestones, calcite dissolution has been reported as the main reason for the increase in the B values, since the porosity increases and rock becomes more compliant (Kim and Makhnenko 2021). On the other hand, for Berea sandstone, the reduction in the undrained bulk modulus Ku along with the drained bulk modulus K is observed, and explained by the stress corrosion cracking, hence B= (Ku−K)/(1−Κ/Κ′s)/Ku can decrease (Tarokh et al. 2020).
3.2 Relative Permeability
Understanding the flow properties of reservoir rock in the water/CO2 system is crucial for CO2 injection, as it is closely related to the injectivity and storage efficiency. For the multiphase flow behavior, the capillary pressure and relative permeability are essential factors that describe the two-phase fluid displacement in the pore space. This section examines the effect of CO2 treatment on the multiphase characteristic curves of the reservoir rock. The relative permeability is measured using the core flooding apparatus via the novel method that allows assessing the CO2 saturation from changes in the undrained compressibility of rock upon CO2 injection.
The relative permeability curves are determined before and after CO2 treatment. Prior to conducting the multiphase flow experiments, the intrinsic permeabilities for pristine- and CO2-treated specimens are accurately measured at P′ = 4.5 MPa. For Berea sandstone, the intrinsic permeability is 1.9 × 10− 13 m2 for the pristine state, and it increases slightly to 2.1 × 10− 13 m2 after the CO2 treatment. The permeability of pristine Apulian limestone is measured to be 7.5 × 10− 15 m2, changing to 8.2 × 10− 15 m2 for the treated specimen. Similarly, for Indiana limestone, the intrinsic permeability increases from 8.0 × 10− 14 to 8.4 × 10− 14 m2 after the treatment. For all tested materials, the intrinsic permeability slightly increases due to the CO2 treatment, while an interpretation is different for the sandstone and limestones. For the limestones, calcite dissolution appears to increase the porosity, and hence the permeability—similar results are reported in a number of studies (Bennion and Bachu 2008; Luquot and Gouze 2009; Kim and Makhnenko 2021). For Berea sandstone, the stress corrosion cracking is assumed to be the main mechanism affecting the permeability without any significant increase in porosity (Tarokh et al. 2020).
The values of Skempton’s B coefficient are measured during the two-phase flow experiments at each injection rate (10:0, 8:2, 5:5, 2:8, 0:10 for H2O/CO2), and the degree of CO2 saturation is calculated from Eq. 5. The pristine- and CO2-treated poroelastic properties measured at the same effective mean stress (P′ = 4.5 GPa) are adopted in the calculations for each corresponding material. At the same time, we assume that the poroelastic parameters (K, K′s, and φ) remain constant at different flow rates. Calculated Skempton’s B coefficients decrease significantly right after starting CO2 injection, also being different for the pristine- and CO2-treated rock (Table 2). Then, the intrinsic permeability value is taken for each pristine- and CO2-treated material to calculate the relative permeability from Eq. 3. Finally, with the degree of saturation calculated from the measurements of the poroelastic parameters and changes in Skempton’s B values (Eqs. 5 and 8), the relative permeability curves are reported (Figs. 5, 6 and 7). The results are fitted to the power law functions in terms of the degree of saturation, similar to the Brooks–Corey model. The fitting parameter Nw is determined using the least-squares method for all materials. The additional fitting is provided for van Genuchten model (Eqs. 12 and 13), where the set of fitting parameters (α, n, and m) is reported in Table 1.
For Berea sandstone, CO2 treatment does not seem to significantly affect the relative permeability curves, with the exponent Nw remaining constant at 2.9 and slight increase in the maximum degree of CO2 saturation from 0.70 to 0.73 (Fig. 5). For Apulian limestone, the maximum degree of CO2 saturation increases from 0.68 to 0.80, while the exponent Nw decreases from 3.8 to 1.3, indicating that the shape of the relative permeability curve turned from a power law to a quasi-linear function (Fig. 6). The relative permeability curves for Indiana limestone are presented in Fig. 7, showing Nw decreasing from 6.3 to 5.2 and the maximum degree of CO2 saturation increasing from 0.69 to 0.75 after the treatment. The values of Sw are reported with 5% accuracy where the main error comes from the determination of the Skempton’s B coefficient within the core flooding apparatus at high degree of CO2 saturation (Kim and Makhnenko 2021). Each experiment is duplicated and the variation between the measured parameters appears to be less than 3%, while the average results are provided in the figures and tables. This study suggests that the multiphase flow response for sandstones is not affected by CO2 injection, while for limestones, the exponent parameter Nw decreases as the maximum degree of saturation for CO2 increases.
In addition to the Brooks–Corey model, van Genuchten model is applied for fitting the relative permeability curves for all materials. The α values are reported to increase due to CO2 injection, as they are mostly related to the CO2 entry pressure. Although, when the relative permeability vs. saturation relationship becomes approximately linear after the CO2 treatment of Apulian limestone (Fig. 6b), van Genuchten model fails to provide a good fit, unlike the Brooks–Corey model.
CO2 treatment appears to cause an increase in the maximum degree of CO2 saturation for all tested materials, although it requires more detailed analyses on the elements that may affect the capillary pressure, such as the wettability and the surface roughness. Further investigations and validation of the measured relative permeability curves are presented in the discussion section, where the capillary pressure curves are coupled with the relative permeability measurements.
4 Microscopic Measurements
Characterization of the multiphase flow in rock oftentimes relies on the assumption that the microscale properties generally remain constant after CO2 treatment. However, a number of studies have reported the chemical effect of CO2 at a microscale (Farokhpoor et al. 2013; Al-Anssari et al. 2017). Thus, the parameters that directly affect the relative permeability–wettability and surface roughness have to be measured before and after CO2 treatment (Kaveh et al. 2014; Haeri et al. 2020).
4.1 Wettability
Experiments are conducted to assess the effect of CO2 treatment on the wettability of reservoir rock, as it is directly related to the changes in capillary pressure and relative permeability. For reservoir formations, pores on the surface may affect the contact angle measurements as they can be considered as defects or surface rugosity. By using the microgoniometer, the apparent contact angles for the air/water system are measured for the pristine- and CO2-treated reservoir rock (Fig. 8). More than twenty measurements are taken for each sample at different locations, and the average values are reported in this study with the overall variations being within ± 5° for each material. For Berea sandstone, the CO2 treatment causes a slight increase in the contact angle from 31 to 34°, so the effect is minor staying within the accuracy range. However, the contact angle increases from 52 to 75° for Apulian limestone and from 55 to 83° for Indiana limestone, revealing a significant effect of CO2 treatment. It is to be noted that the measured contact angles are apparent angles that are based on the average plane of the surface for air/water system, as it does not quantitatively characterize the true contact angle in the microscale pores for the CO2–water–rock system. However, it can be argued that these measurements provide a qualitative understanding of the effect of CO2 treatment on the wettability of the reservoir rock.
Additionally, as the tested materials in this study are mainly composed of quartz or calcite, the contact angles of the pristine- and CO2-treated mineral crystals are measured (Fig. 9). The contact angle for pristine quartz is 40°, and it increases to 42° after the treatment. For calcite, the contact angle changes from 78 to 83°, indicating that the effect of CO2 treatment on the contact angle of minerals is significantly smaller than that for the porous rock that is composed of these minerals. This implies that the change in the contact angle for reservoir rock cannot result only from that of the minerals but can also be attributed to the changes in the surface roughness. The measured apparent contact angles of deionized water on mineral crystals also showed to be within the range of the reported values from other studies, where the contact angles for quartz were reported to be 26–50° (Lamb and Furlong 1982; Janczuk and Zdziennicka 1994; Drelich et al. 1996; Kowalczyk et al. 1996; Deng et al. 2018). For calcite, the contact angles are between 50 and 85°—larger than those of quartz (Okayama et al. 1997; Kowalczuk et al. 2017; Alnoush et al. 2021). Detailed procedures for the CO2 treatment of crystal minerals are elaborated in Kim and Makhnenko (2021).
Apparent contact angle measurements for a pristine- and b CO2-treated Berea sandstone, c pristine- and d CO2-treated Apulian limestone, and e pristine- and f CO2-treated Indiana limestone. All measurements are conducted for the air/water/rock interface at ambient room temperature (22 °C) and pressure
4.2 Surface Roughness
One of the main reasons the apparent contact angle differs from the true contact angle is the surface roughness. Particularly, for porous rock formations, pores contribute to surface rugosity, which requires thorough investigation (Cassie and Baxter 1944). As the surface roughness is highly dependent on the specimen preparation, in this study, it is evaluated through the parameter Rq—the root-mean-square (RMS) average of the profile heights over the evaluation length of 1000 μm. Moreover, to investigate the effect of CO2 treatment on the surface roughness, the ratio of the Rq values of the pristine- and CO2-treated rock (Rq, pris/Rq, CO2) is reported. Ten to twelve scans are made for each specimen, and the average value of Rq, pris/Rq, CO2 is calculated, while only the surface variation for a single measurement is presented in Fig. 10. Rq, pris/Rq, CO2 values appear to be larger than 1 for all materials, indicating that CO2 treatment smoothens the surface at the microscale. It appears that the chemical effect is more pronounced for the limestones, where the Rq, pris/Rq, CO2 values are reported as 2.4 and 2.2 for Apulian and Indiana limestones, while the value for Berea sandstone is 1.1.
Wenzel’s relation predicts that a decrease in surface roughness would lead to a reduction in wettability, hence the increase in contact angle, as it is observed in this study for the CO2-treated specimens (Wenzel 1936). Considering Young’s equation (Eq. 14), this effect can be intuitively verified, as the capillary pressure curve for water shifts down—toward smaller capillary pressure values, while the contact angle increases. Moreover, as the preceding study showed that the dominant pore sizes for the tested limestones increased after the CO2 treatment (Kim and Makhnenko 2021), the surface roughness measurements are in agreement with the theory of Cassie and Baxter (1944), stating that the apparent contact angles for porous surfaces should increase with the pore size (Fig. 11).
In summary, the reported results align with the previous analyses on the wettability and surface roughness, indicating that the CO2 treatment smoothens the rock surface, resulting in larger apparent contact angles for the wetting fluid. Therefore, this implies that the surface becomes more wet with respect to the non-wetting phase, which causes the increase in the maximum CO2 saturation.
5 Discussion
In the previous sections, the effect of CO2 treatment on the poroelastic and multiphase flow response is experimentally determined, indicating that the compressibilities of limestones increase after the treatment, while for the sandstone the effect is less pronounced. The relative permeability measurements suggest that the maximum degree of CO2 saturation increases for all materials. The evaluation of the fitting parameters for the relative permeability curves shows that the Brooks–Corey exponents decrease for limestones, implying that the CO2 treatment increases the relative permeability of CO2 at the same degree of saturation. In this section, further analyses on the capillary pressure–saturation curves are presented. The capillary pressure curves for pristine- and CO2-treated specimens are reported by adopting the information on the residual water saturation that can be obtained from the relative permeability measurements.
5.1 Residual Water Saturation–Capillary Pressure Curve
Although accurate measurements from the MIP method allow characterizing the capillary pressure for the mercury/air system, it cannot be directly applied to the water/CO2 system (Eq. 15), because the residual CO2 saturation also needs to be considered (Bennion and Bachu 2005). The residual liquid saturation Srl is the irreducible degree of saturation for the wetting phase, indicating the amount of the latter one that remains in the pores whichever are the pressure and flow rate of the non-wetting fluid. While the existing studies on CO2 relative permeability and saturation mostly rely on X-ray computed tomography (Krevor et al. 2012, 2015; Akbarabadi and Piri 2013), this technique has a number of limitations in terms of the possibility to simulate the in situ stress conditions, as well as in terms of the available image resolution that neglects the presence of submicron-scale pores (Elkhoury et al. 2019). In this study, a robust method to estimate the residual saturation from the relative permeability curve is adopted. As the selected reservoir formations have relatively large pores and almost no inclusions of clay minerals, it is assumed that the effect of hysteresis is minor, which is confirmed by the experimental observations. Thus, the fitting curves for the discrete measurements of relative permeabilities are utilized to determine the residual saturation.
From the relative permeability measurements, the equation for the fitting curves can be obtained by the least-squares method, which allows the calculation of the effective saturation parameter Se at zero relative permeability. Subsequently, the residual water saturation Srl can be obtained using Eq. (10) for Se. For Berea sandstone, Srl is equal to 0.1 for pristine- and CO2-treated specimens. The residual water saturation is calculated to be 0.08 and 0.07 for pristine Apulian limestone and Indiana limestone, respectively, and these values decrease to almost zero (≈ 0.01) after CO2 treatment. These observations are in accordance with the previous studies, where the residual saturation is reported to be in the range of 0.05–0.11 for reservoir rock (Bennion and Bachu 2008; Krevor et al. 2012; Pini and Krevor 2019; Niu and Krevor 2020). The decrease in the Srl values for limestones implies that higher CO2 saturation can be achieved after the CO2 treatment. As the residual liquid saturation values are obtained, the capillary pressure–saturation curves for the CO2/water system can now be determined. By changing the minimum value of water saturation with respect to the measured residual saturation of CO2, the capillary pressure–saturation curve can be proportionally reformed.
For Berea sandstone, the water saturation decreases at 0.004 MPa capillary pressure, and no significant effect of the CO2 treatment is observed. The CO2 entry pressure of Apulian limestone decreases due to the treatment from 0.04 to 0.01 MPa. This indicates that the non-wetting fluid (liquid CO2 in this case) can more easily intrude into the pores. However, the effect is less pronounced for Indiana limestone, as its CO2 entry pressure decreases only slightly from 0.009 to 0.008 MPa. Considering the microscopic measurements, the alteration of the residual water saturation–capillary pressure curve due to CO2 treatment is in agreement with the wettability and surface roughness changes in limestones (Sect. 4.1 and 4.2). As the contact angles increase with the decreasing surface roughness, the limestones become more CO2-wet, meaning that the CO2 saturation becomes larger at the same capillary pressure after the treatment, with the CO2 entry pressure decreasing (Table 3).
The van Genuchten parameters (α, m, and n) assessed from the relative permeability measurements (see Table 1) are utilized to validate the capillary pressure–saturation curves obtained from the MIP tests (Fig. 12). The two methods show an excellent agreement, so it can be argued that the introduced approach for reporting the relative permeability curve can provide reliable results. The only discrepancy is observed at low water saturation for Berea sandstone, which may be caused by a few factors. Firstly, it is the difference between specimen sizes for the core flooding (10 cm long) and MIP (< 1 cm linear dimension) tests. Berea sandstone contains grains with sizes 70–400 μm and pores and cracks with sizes up to 0.1 mm, while for the limestones they are an order of magnitude smaller. The small size of MIP specimens with relatively large pores and cracks on its surface may affect the saturation curve measurements (Churcher et al. 1991). Secondly, errors in determining the fitting curves can provide another explanation for the disagreement between the two methods. The relative permeability curve is highly sensitive to the value of the entry pressure of the non-wetting fluid, where a major decrease in the degree of saturation occurs. However, as few studies have reported, van Genuchten model has some limitations in accurately assessing the capillary pressure and saturation at low wetting fluid saturation conditions (Krevor et al. 2012; Pini and Krevor 2019). The pore distribution results obtained from MIP experiments cannot always be directly used to evaluate the relative permeability curves, and direct capillary pressure measurements at low saturation levels are suggested (Oostrom et al. 2016).
While the adopted testing approaches are different, it is important to note that the results obtained in this work are shown to be consistent with the existing studies for the pristine materials. For the relative permeability curves of sandstone and limestones, the Brooks–Corey exponents were reported to be within the range of 2.5–6 and the maximum degree of CO2 saturation was 0.3 ± 0.1 (Perrin et al. 2009; Krevor et al. 2012; Akbarabadi and Piri 2013; Bachu 2013; Berg 2013; Oostrom et al. 2016). The results for the saturation–capillary pressure curves also match well with the previously reported studies.
5.2 Limitations of the Approach
In this study, a novel method is introduced to characterize the relative permeability curve by estimating the degree of saturation of the two fluids as the poromechanical responses are accurately measured. Although this method offers significant advantages, there are some limitations that need to be discussed.
The degree of saturation for the multiphase flow experiment is calculated by measuring the Skempton’s B coefficient, where it is assumed that the pore fluid is evenly distributed in the pores (Eq. 5). However, CO2 saturation could be larger in the upstream and gradually decrease toward the downstream (Krevor et al. 2012; Berg et al. 2013; Chen et al. 2014). Although the undrained condition is maintained for a sufficient time (10 min) to redistribute the fluids before the measurements, this may affect the reported B values and cause errors in evaluating the degree of saturation of CO2.
The novel method introduced in this study calculates the degree of water/CO2 saturation by measuring the poroelastic properties, as the accuracy and validation of this technique need to be discussed. From the measurement of Skempton’s B coefficient, the bulk modulus of the mixed fluid consisting of CO2 and water (Kf, mix) is calculated (Eq. 5). Since the B values significantly decrease as CO2 is injected, the uncertainty in calculated Kf, mix values increases, resulting in larger errors for the reported degree of saturation. Also, for the non-wetting fluid saturation above 20%, the wetting fluid may not exist as a continuous phase in the pores, limiting the application of the Wood’s formula and potentially causing uneven pore pressure distribution (Dullien 1992). The applicability of this method for anisotropic materials that contain multiple minerals may also be an issue, as it provides uncertainty in calculating the applied mean stress (Eq. 4) and may violate the ideal porous media assumption of K′s=K″s increasing the errors in calculated Skempton’s B coefficients (Eq. 5).
By duplicating the experiments and considering the accuracy of the experimental devices, the relative errors in determining the material properties are calculated as follows: 3% for the porosity, 5% for permeability, 3% for Skempton’s B coefficient, 3% for the bulk moduli, and 10% for the degree of saturation. It is to be noted that the fitting parameters for the relative permeability curve (e.g., Brooks–Corey exponent) may differ by up to 50% due to the error range in the degree of saturation, so the average value is taken for comparison.
The hysteresis of the CO2/water relative permeability curve for drainage and imbibition is another potential issue that has been reported in a few studies (Juanes et al. 2006; Bennion and Bachu 2007; 2008). This effect is argued to be minor for the tested reservoir rock, where we only concentrate on the drainage procedure (injection of the non-wetting fluid), assuming no hysteresis for the capillary pressure and relative permeability.
Lastly, the difference between the laboratory and the field scales needs to be considered. Despite accurate laboratory measurements of relative permeability and characterization of the microscopic properties, these findings cannot be directly applied to field-scale problems, as the preferential flow paths such as wormholes, cracks, and faults significantly affect the hydraulic behavior (Szymczak and Ladd 2009; Elkhoury et al. 2013). It is apparent that the uncertainty range increases with scale, and thus, the non-uniqueness and variation in relative permeability measurements must be taken into account, which can be determined through inverse modeling based on “history matching” for unsteady-state conditions (Berg et al. 2013, 2021). Moreover, as no direct measurements of relative permeability have been reported at the field scale, efforts to upscale the laboratory measurements have been introduced for the reservoir simulations (Crotti and Cobeñas 2001; Benson and Doughty 2006; Kamali and Hussain 2017), but further research needs to be conducted regarding validation of the proposed methods.
6 Conclusion
This study characterizes the effect of CO2 treatment on the multiphase flow characteristics of reservoir rock by comprehensively considering various factors such as poroelastic deformation, capillary pressure, wettability, and surface roughness. By selecting one silica-rich and two calcite-rich porous rocks, the chemical effect on the type of the dominant mineral is examined. Different experimental methods—core flooding, mercury intrusion porosimetry, microgoniometry, and profilometry, are introduced to characterize the changes in relative permeability, capillary pressure, wettability, and surface roughness of reservoir formations during CO2 injection. A robust technique is implemented to evaluate the relative permeability curve from the measurements of the changes in poroelastic response upon CO2 flow in the rock, and the results are compared with independent evaluations based on the direct capillary pressure measurements. The capillary pressure curves are affected due to the chemical effect caused by CO2 injection in water-saturated limestones, where the exponent values for the Brooks–Corey model decrease, implying that the relative CO2 permeability increases with maximum CO2 saturation. On the other hand, the exponent value is reported to remain constant for the sandstone, while its maximum CO2 saturation slightly increases. A qualitative analysis of the contact angle and the surface roughness is conducted, showing that the CO2 treatment smoothens the surface for limestones and increases their CO2 wettability, while the effect is almost unnoticeable for the sandstone. This implies that CO2 treatment may affect the relative permeability and wettability, highlighting the importance of adopting accurate multiphase flow parameters for reservoir models. In conclusion, this study thoroughly investigates the effect of CO2 treatment on the multiphase flow characteristics of reservoir rock and suggests that the chemical effect can increase the CO2 injectivity in calcite-rich formations.
References
Abdallah, W., Buckley, J.S., Carnegie, A., Edwards, J., Herold, B., Fordham, E., Graue, A., Habashy, T., Seleznev, N., Signer, C., Hussain, H.: Fundamentals of wettability. Technology. 38, 1125–1144 (1986)
Achenbach, J.: Wave Propagation in Elastic Solids. Elsevier, Amsterdam (1973)
Adamson, A.W., Gast, A.P.: Physical Chemistry of Surfaces, vol. 15. Interscience publishers, New York (1967)
Akbarabadi, M., Piri, M.: Relative permeability hysteresis and capillary trapping characteristics of supercritical CO2/brine systems: an experimental study at reservoir conditions. Adv. Water Resour. 52, 190–206 (2013)
Al-Anssari, S., Arif, M., Wang, S., Barifcani, A., Lebedev, M., Iglauer, S.: Wettability of nano-treated calcite/CO2/brine systems: implication for enhanced CO2 storage potential. Int. J. Greenh. Gas Control. 66, 97–105 (2017)
Al-Yaseri, A.Z., Lebedev, M., Barifcani, A., Iglauer, S.: Receding and advancing (CO2 + brine + quartz) contact angles as a function of pressure, temperature, surface roughness, salt type and salinity. J. Chem. Thermodyn. 93, 416–423 (2016)
Alnoush, W., Sayed, A., Solling, T.I., Alyafei, N.: Impact of calcite surface roughness in wettability assessment: interferometry and atomic force microscopy analysis. J. Pet. Sci. Eng. 203, 108679 (2021)
Amalokwu, K., Best, A.I., Sothcott, J., Chapman, M., Minshull, T., Li, X.Y.: Water saturation effects on elastic wave attenuation in porous rocks with aligned fractures. Geophys. J. Int. 197(2), 943–947 (2014)
Amirfazli, A., Kwok, D.Y., Gaydos, J., Neumann, A.W.: Line tension measurements through drop size dependence of contact angle. J. Colloid Interface Sci. 205(1), 1–1 (1998)
Amott, E.: Observations relating to the wettability of porous rock. Trans. AIME. 216(01), 156–162 (1959)
Anderson, W.: Wettability literature survey-part 2: Wettability measurement. J. Pet. Technol. 38(11), 1246–1262 (1986)
Bachu, S.: Drainage and imbibition CO2/brine relative permeability curves at in situ conditions for sandstone formations in western Canada. Energy Proced. 37, 4428–4436 (2013)
Bachu, S., Bennion, B.: Effects of in-situ conditions on relative permeability characteristics of CO2-brine systems. Environ. Geol. 54(8), 1707–1722 (2008)
Baldacchini, T., Carey, J.E., Zhou, M., Mazur, E.: Superhydrophobic surfaces prepared by microstructuring of silicon using a femtosecond laser. Langmuir 22(11), 4917–4919 (2006)
Bear, J.: Dynamics of Fluids in Porous Media. Courier Corporation (1988)
Bennion, B., Bachu, S.: Drainage and imbibition relative permeability relationships for supercritical CO2/brine and H2S/brine systems in intergranular sandstone, carbonate, shale, and anhydrite rocks. SPE Reserv. Eval. Eng. 11, 487–496 (2008)
Bennion, B., Bachu, S.: Relative permeability characteristics for supercritical CO2 displacing water in a variety of potential sequestration zones. In SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers (2005)
Bennion, D.B., Bachu, S.: Permeability and relative permeability measurements at reservoir conditions for CO2-water systems in ultra low permeability confining caprocks. In: EUROPEC/EAGE Conference and Exhibition, p. 106995. SPE (2007)
Benson, S.M., Doughty, C.: Estimation of field-scale relative permeability from pressure transient tests. In Proceedings, EPA CO2SC Workshop, Lawrence Berkeley National Laboratory, Berkeley (2006)
Berg, S., Oedai, S., Ott, H.: Displacement and mass transfer between saturated and unsaturated CO2–brine systems in sandstone. Int. J. Greenh. Gas Control. 12, 478–492 (2013)
Berg, S., Unsal, E., Dijk, H.: Non-uniqueness and uncertainty quantification of relative permeability measurements by inverse modelling. Computers and Geotechnics. 132, 103964 (2021)
Biot, M.A.: General theory of three-dimensional consolidation. J. Appl. Phys. 12, 155–164 (1941)
Brooks, R.H., Corey, A.T.: Hydraulic Properties of Porous Media. Hydrology Papers 3. Colorado State University, Fort Collins, CO (1964)
Burdine, N.T.: Relative permeability calculations from pore size distribution data. Trans. Am. Inst. Mining Metall. Eng. 198, 71–78 (1953)
Cassie, A.B.D., Baxter, S.: Wettability of porous surfaces. Trans. Faraday Soc. 40, 546–551 (1944)
Chadwick, A., Arts, R., Eiken, O., Williamson, P., Williams, G.: Geophyiscal monitoring of the CO2 plume at Sleipner, North Sea. In: Advances in the Geological Storage of Carbon Dioxide, pp. 303–314. Springer, Dordrecht (2006)
Chen, X., Kianinejad, A., DiCarlo, D.A.: An experimental study of CO2-brine relative permeability in sandstone. In: SPE-169137-MS. Society of Petroleum Engineers, Richardson, TX, US (2014)
Chen, Z., Ewing, R.E., Espedal, M.: Multiphase flow simulation with various boundary conditions. Comput. Methods Water Resour. 925–932 (1994)
Churcher, P.L., French, P.R., Shaw, J.C., Schramm, L.L.: Rock properties of Berea sandstone, Baker dolomite, and Indiana limestone. Society of Petroleum Engineers, Anaheim (1991)
Civan, F., Donaldson, E.C.: Relative permeability from unsteady-state displacements with capillary pressure included. SPE Form. Eval. 4(02), 189–193 (1989)
Cnudde, V., Boone, M.N.: High-resolution X-ray computed tomography in geosciences: a review of the current technology and applications. Earth Sci. Rev. 123, 1–17 (2013)
Collins, H.C.: The Mississippian and Pennsylvanian (Carboniferous) systems in the United States. United States Geological Survey, Ohio (1979)
Craig, F.F.: The Reservoir Engineering Aspects of Waterflooding. Monograph Series. Society of Petroleum Engineers of AIME, Pennsylvania(1971)
Crotti, M.A., Cobeñas, R.H.: Scaling up of laboratory relative permeability curves. An advantageous approach based on realistic average water saturations. In SPE Latin American and Caribbean Petroleum Engineering Conference Mar 25. 2001 (2001)
Deng, Y., Xu, L., Lu, H., Wang, H., Shi, Y.: Direct measurement of the contact angle of water droplet on quartz in a reservoir rock with atomic force microscopy. Chem. Eng. Sci. 177, 445–454 (2018)
Detournay, E., Cheng, A.H.-D.: Fundamentals of poroelasticity. In: Fairhurst, C. (ed.) Comprehensive Rock Engineering, vol. II, pp. 113–171. Pergamon, Oxford (1993)
Donaldson, E.C., Civan, F., Alam, U.: Relative permeabilities at simulated reservoir conditions. SPE. Reserv. Eng. 3(04), 1323–1327 (1988)
Donaldson, E.C., Thomas, R.D., Lorenz, P.B.: Wettability determination and its effect on recovery efficiency. Soc. Pet. Eng. J. 9(01), 13–20 (1969)
Drelich, J., Miller, J.D., Good, R.J.: The effect of drop (bubble) size on advancing and receding contact angles for heterogeneous and rough solid surfaces as observed with sessile-drop and captive-bubble techniques. J. Colloid Interface Sci. 179(1), 37–50 (1996)
Dullien, F.A.L.: Porous Media Fluid Transport and Pore Structure. Acad. Press Inc, San Diego, California (1992)
Elkhoury, J.E., Ameli, P., Detwiler, R.L.: Dissolution and deformation in fractured carbonates caused by flow of CO2-rich brine under reservoir conditions. Int. J. Greenh. Gas Control. 16, 203–215 (2013)
Elkhoury, J.E., Shankar, R., Ramakrishnan, T.S.: Resolution and limitations of X-ray micro-CT with applications to sandstones and limestones. Transp. Porous Med. 129(1), 413–425 (2019)
Espinoza, D.N., Santamarina, J.C.: Water-CO2‐mineral systems: Interfacial tension, contact angle, and diffusion—Implications to CO2 geological storage. Water Resour. Res. 46(7) (2010)
Falcon-Suarez, I.H., Papageorgiou, G., Jin, Z., Muñoz‐Ibáñez, A., Chapman, M., Best, A.I. (2020) CO2‐brine substitution effects on ultrasonic wave propagation through sandstone with oblique fractures. Geophys. Res. Lett. 47(16), e2020GL088439
Falode, O., Manuel, E.: Wettability effects on capillary pressure, relative permeability, and irredcucible saturation using porous plate. J. Pet. Eng. (2014)
Farokhpoor, R., Bjørkvik, B.J., Lindeberg, E., Torsæter, O.: CO2 wettability behavior during CO2 sequestration in saline aquifer-an experimental study on minerals representing sandstone and carbonate. Energy Proced. 37, 5339–5351 (2013)
Fulcher, R.A., Ertekin, T., Stahl, C.D.: Effect of capillary number and its constituents on two phase relative permeability curves. J. Pet. Technol. 37, 249–260 (1985)
van Genuchten, M.T.: A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 44(5), 892–898 (1980)
Good, R.J.: Contact angles and the surface free energy of solids. In: Surface and Colloid Science. Springer, Boston, MA (1979)
Grombacher, D., Vanorio, T., Ebert, Y.: Time-lapse acoustic, transport, and NMR measurements to characterize microstructural changes of carbonate rocks during injection of CO2-rich water. Geophysics 77(3), WA169–WA179 (2012)
Haeri, F., Tapriyal, D., Sanguinito, S., Shi, F., Fuchs, S.J., Dalton, L.E., Baltrus, J., Howard, B., Crandall, D., Matranga, C., Goodman, A.: CO2–Brine contact angle measurements on Navajo, Nugget, Bentheimer, Bandera Brown, Berea, and Mt. Simon sandstones. Energy Fuels 34(5), 6085–6100 (2020)
IPCC.: Climate Change 2021: The physical science basis. In: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press (2021)
IPCC: IPCC Special Report on Carbon Dioxide Capture and Storage. Cambridge University Press (2005)
Ionescu, E., Maini, B.B.: A Review of Laboratory Techniques for Measuring Wettability of Petroleum Reservoir Rocks. US Department of Energy, Technical Report. (1983)
Janczuk, B., Zdziennicka, A.: A study on the components of surface free energy of quartz from contact angle measurements. J. Mater. Sci. 29(13), 3559–3564 (1994)
Johnson, R.E.: Wettability contact angles. Surf. Colloid Sci. 2, 85–153 (1969)
Johnson, J.W., Nitao, J.J., Morris, J.P.: Reactive transport modeling of caprock integrity during natural and engineered CO2 storage. In: Carbon Dioxide Capture for Storage in Deep Geologic Formations, pp. 787–813. Elsevier, Oxford, UK (2005)
Juanes, R., Spiteri, E.J., Orr Jr, F.M., Blunt, M.J.: Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 42, W12418 (2006)
Kamali, F., Hussain, F.: Field-scale simulation of CO2 enhanced oil recovery and storage through SWAG injection using laboratory estimated relative permeabilities. J. Pet. Sci. Eng. 156, 396–407 (2017)
Kaveh, N.S., Rudolph, E.S.J., Van Hemert, P., Rossen, W.R., Wolf, K.H.: Wettability evaluation of a CO2/water/bentheimer sandstone system: contact angle, dissolution, and bubble size. Energy Fuels 28(6), 4002–4020 (2014)
Kim, K., Makhnenko, R.Y.: Coupling between poromechanical behavior and fluid flow in tight rock. Transp. Porous Med. 135(2), 487–512 (2020)
Kim, K., Makhnenko, R.Y.: Changes in rock matrix compressibility during deep CO2 storage. Greenh. Gases Sci. Technol. 11(5), 954–973 (2021)
Kim, K., Makhnenko, R.Y.: Short- and long-term response of reservoir rock induced by CO2 injection. Rock. Mech. Rock. Eng. 55 (11), 6605–6625 (2022). https://doi.org/10.1007/s00603-022-03032-1
Kim, K., Vilarrasa, V., Makhnenko, R.Y.: CO2 injection effect on geomechanical and flow properties of calcite-rich reservoirs. Fluids. 3(3), 66 (2018)
Kopp, A., Class, H., Helmig, R.: Investigations of CO2 storage capacity in deep saline aquifers. Part 1: dimensional analysis of flow processes and reservoir characteristics. Int. J. Greenh. Gas Control 3(3), 263–276 (2009)
Kowalczuk, P.B., Akkaya, C., Ergun, M., Janicki, M., Sahbaz, O., Drzymala, J.: Water contact angle on corresponding surfaces of freshly fractured fluorite, calcite and mica. Physicochem. Prob. Min. Process. 53, 192–201 (2017)
Kowalczyk, D., Slomkowski, S., Chehimi, M.M., Delamar, M.: Adsorption of aminopropyltriethoxy silane on quartz: an XPS and contact angle measurements study. Int. J. Adhes. Adhes. 16(4), 227–232 (1996)
Krevor, S., Blunt, M.J., Benson, S.M., Pentland, C.H., Reynolds, C., Al-Menhali, A., Niu, B.: Capillary trapping for geologic carbon dioxide storage–from pore scale physics to field scale implications. Int. J. Greenh. Gas Control. 40, 221–237 (2015)
Krevor, S.C., Pini, R., Zuo, L., Benson, S.M.: Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resour. Res. 48(2) (2012)
Lamb, R.N., Furlong, D.N.: Controlled wettability of quartz surfaces. J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases 78(1), 61–73 (1982)
Lowe, J., Johnson, T.C.: Use of back pressure to increase degree of saturation of triaxial test specimens. In: Proceedings of the Research Conference on Shear Strength of Cohesive Soils, pp. 819–836. ASCE, Boulder (1960)
Luquot, L., Gouze, P.: Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 265, 148–159 (2009)
Lv, P., Liu, Y., Wang, Z., Liu, S., Jiang, L., Chen, J., Song, Y.: In situ local contact angle measurement in a CO2–brine–sand system using microfocused X-ray CT. Langmuir 33(14), 3358–3366 (2017)
Makhnenko, R.Y., Labuz, J.F.: Elastic and inelastic deformation of fluid-saturated rock. Phiosl. Trans. R Soc. A 374, 20150422 (2016)
Mangane, P.O., Gouze, P., Luquot, L.: Permeability impairment of a limestone reservoir triggered by heterogeneous dissolution and particles migration during CO2‐rich injection. Geophys. Res. Lett. 40(17), 4614–4619 (2013)
Marsden, S.S.: Wettability-its measurement and application to waterflooding. J. Jpn. Assoc. Pet. Tech. 30(1), 1–10 (1965)
Martinez, M.J., Newell, P., Bishop, J.E., Turner, D.Z.: Coupled multiphase flow and geomechanics model for analysis of joint reactivation during CO2 sequestration operations. Int. J. Greenh. Gas Control 17, 148–160 (2013)
Mazumder, S., Wolf, K.H.: Differential swelling and permeability change of coal in response to CO2 injection for ECBM. In SPE Asia Pacific Oil and Gas Conference and Exhibition. OnePetro (2008)
McCaffery, F.G., Mungan, N.: Contact angle and interfacial tension studies of some hydrocarbon-water-solid systems. J. Can. Pet. Technol. 9(03) (1970)
Morrow, N.R.: Wettability and its effect on oil recovery. J. Pet. Technol. 42(12), 1476–1484 (1990)
Morrow, N.R.: The effects of surface roughness on contact: angle with special reference to petroleum recovery. J. Can. Pet. Technol. 14(04) (1975)
Mualem, Y.: New model for predicting hydraulic conductivity of unsaturated porous media. Water Resour. Res. 12(3), 513–522 (1976)
Müller, N.: Supercritical CO2-brine relative permeability experiments in reservoir rocks—literature review and recommendations. Transp. Porous Med. 87(2), 367–383 (2011)
Neumann, A.W., Good, R.J.: Techniques of measuring contact angles. In: Surface and Colloid Science, pp. 31–91. Springer, Boston, MA (1979)
Newberry, J.S.: Report of the Geological Survey of Ohio. Nevins Myers 2, 1 (1874)
Niu, B., Krevor, S.: The impact of Mineral dissolution on drainage relative permeability and residual trapping in two Carbonate Rocks. Transp. Porous Med. 131(2), 363–380 (2020)
Okayama, T., Keller, D.S., Luner, P.: The wetting of calcite surfaces. J. Adhes. 63(1–3), 231–52 (1997)
Oostrom, M., White, M.D., Porse, S.L., Krevor, S.C., Mathias, S.A.: Comparison of relative permeability–saturation–capillary pressure models for simulation of reservoir CO2 injection. Int. J. Greenh. Gas Control 45, 70–85 (2016)
Orr, F.M. Jr.: Storage of carbon dioxide in geologic formations. J. Pet. Technol. 56(9), 90–97 (2004)
Patton, J.B., Carr, D.D.: The Salem Limestone in the Indiana Building-Stone District. Indiana Geological Survey, Kolkata (1982)
Perrin, J.C., Krause, M., Kuo, C.W., Miljkovic, L., Charoba, E., Benson, S.M.: Core-scale experimental study of relative permeability properties of CO2 and brine in reservoir rocks. Energy Proced. 1(1), 3515–3522 (2009)
Pini, R., Krevor, S.C., Benson, S.M.: Capillary pressure and heterogeneity for the CO2/water system in sandstone rocks at reservoir conditions. Adv. Water Resour. 38, 48–59 (2012)
Pini, R., Krevor, S.: Laboratory studies to understand the controls on flow and transport for CO2 storage. In: Science of Carbon Storage in Deep Saline Formations, pp. 145–180. Elsevier (2019)
Pruess, K., Garcia, J.: Multiphase flow dynamics during CO2 disposal into saline aquifers. Environ. Geol. 42, 282–295 (2002)
Pruess, K., Oldenburg, C.M., Moridis, G.: TOUGH2 User’s Guide, Version 2.0. Report LBNL-43134. Lawrence Berkeley National Laboratory, Berkeley, CA, USA (1999)
Rayward-Smith, W.J., Woods, A.W.: Some implications of cold CO2 injection into deep saline aquifers. Geophys. Res. Lett. 38(6) (2011)
Rohmer, J., Pluymakers, A., Renard, F.: Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth Sci. Rev. 157, 86–110 (2016)
Rutqvist, J.: The geomechanics of CO2 storage in deep sedimentary formations. Geotech. Geol. Eng. 30(3), 525–551 (2012)
Saad, N., Cullick, A.S., Honarpour, M.M.: Effective relative permeability in scale-up and simulation. In: Low Permeability Reservoirs Symposium. OnePetro (1995)
Sarmadivaleh, M., Al-Yaseri, A.Z., Iglauer, S.: Influence of temperature and pressure on quartz–water–CO2 contact angle and CO2–water interfacial tension. J. Colloid Interface Sci. 441, 59–64 (2015)
Scanziani, A., Singh, K., Blunt, M.J., Guadagnini, A.: Automatic method for estimation of in situ effective contact angle from X-ray micro tomography images of two-phase flow in porous media. J. Colloid Interface Sci. 496, 51–59 (2017)
Sedaghatinasab, R., Kord, S., Moghadasi, J., Soleymanzadeh, A.: Relative permeability hysteresis and capillary trapping during CO2 EOR and sequestration. Int. J. Greenh. Gas Control. 106, 103262 (2021)
Shaffer, N.R.: Indiana limestone: America’s building stone. Geol. Soc. Lond. Special Publ. 486(1), 77–101 (2020)
Shi, Z., Sun, L., Haljasmaa, I., Harbert, W., Sanguinito, S., Tkach, M., Goodman, A., Tsotsis, T.T., Jessen, K.: Impact of Brine/CO2 exposure on the transport and mechanical properties of the Mt Simon sandstone. J. Pet. Sci. Eng. 177, 295–305 (2019)
Shi, J.Q., Xue, Z., Durucan, S.: Seismic monitoring and modelling of supercritical CO2 injection into a water-saturated sandstone: interpretation of P-wave velocity data. Int. J. Greenh. Gas Control. 1(4), 473–480 (2007)
Skempton, A.W.: The pore-pressure coefficients A and B. Geotechnique 4(4), 143–147 (1954)
Su, E., Liang, Y., Zou, Q., Niu, F., Li, L.: Analysis of effects of CO2 injection on coalbed permeability: implications for coal seam CO2 sequestration. Energy Fuels 33(7), 6606–6615 (2019)
Szymczak, P., Ladd, A.J.: Wormhole formation in dissolving fractures. J. Geophys. Res. Solid Earth. 114(B6) (2009)
Tamai, Y., Aratani, K.: Experimental study of the relation between contact angle and surface roughness. J. Phys. Chem. 76(22), 3267–3271 (1972)
Tarokh, A., Makhnenko, R.Y.: Remarks on the solid and bulk responses of fluid-filled porous rock. Geophysics. 84(4), WA83–WA95 (2019)
Tarokh, A., Makhnenko, R.Y., Kim, K., Zhu, X., Popovics, J.S., Segvic, B., Sweet, D.E.: Influence of CO2 injection on the poromechanical response of Berea sandstone. Int. J. Greenh. Gas Control. 95, 102959 (2020)
Tudek, J., Crandall, D., Fuchs, S., Werth, C.J., Valocchi, A.J., Chen, Y., Goodman, A.: In situ contact angle measurements of liquid CO2, brine, and Mount Simon sandstone core using micro X-ray CT imaging, sessile drop, and Lattice Boltzmann modeling. J. Pet. Sci. Eng. 155, 3–10 (2017)
U. S. Department of Energy: Carbon Sequestration Atlas of the United States and Canada, 3rd edn. US DOE, Washington, D.C. (2010)
Vilarrasa, V., Silva, O., Carrera, J., Olivella, S.: Liquid CO2 injection for geological storage in deep saline aquifers. Int. J. Greenh. Gas Cont. 14, 84–96 (2013)
Vilarrasa, V., Makhnenko, R.Y., Rutqvist, J.: Field and laboratory studies of geomechanical response to the injection of CO2. In: Science of Carbon Storage in Deep Saline Formations. pp. 209–236. Elsevier, Oxford (2019)
Wang, S., Edwards, I.M., Clarens, A.F.: Wettability phenomena at the CO2–brine–mineral interface: implications for geologic carbon sequestration. Environ. Sci. Technol. 47(1), 234–241 (2013)
Wenzel, R.N.: Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 28(8), 988–994 (1936)
Wenzel, R.N.: Surface roughness and contact angle. J. Phys. Chem. 53(9), 1466–1467 (1949)
Winkler, K.W., Plona, T.J.: Technique for measuring ultrasonic velocity and attenuation spectra in rocks under pressure. J. Geophys. Res. Solid Earth 87(B13), 10776–10780 (1982)
Wood, A.B.: A Textbook of Sound. G. Bell and Sons, London (1930)
Xu, T., Pruess, K.: Modeling multiphase non-isothermal fluid flow and reactive geochemical transport in variably saturated fractured rocks: 1 methodology. Am. J. Sci. 301, 16–33 (2001)
Xu, T., Sonnenthal, E.L., Spycher, N., Pruess, K.: TOURGHREACT: a simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media. Comput. Geosci. 32, 145–165 (2006)
Acknowledgements
The experimental data are available upon request. K. Kim’s research was supported by Technip FMC Educational Fund Fellowship. P. Kundzicz is thankful for the REU scholarship provided by the CEE department at UIUC. R.Y. Makhnenko acknowledges the support from US DOE through Carbon SAFE Illinois Corridor Project DE-FE0031892. Xiao Yan has assisted with the contact angle measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, K., Kundzicz, P.M. & Makhnenko, R.Y. Effect of CO2 Injection on the Multiphase Flow Properties of Reservoir Rock. Transp Porous Med 147, 429–461 (2023). https://doi.org/10.1007/s11242-023-01916-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-023-01916-6