Abstract
LiFePO4 (space group: Pnma) was prepared by hydrothermal method at 170 °C. LiFePO4 was prepared from precursor solutions consisting of FeSO4 · 7H2O, (NH4)2HPO4, and three kinds of Li sources. LiCl, Li(CH3COO), and LiOH · H2O were used as Li sources. The pH of the precursor solution varied depending on Li source. The particle size, particle shape, and crystal texture of the obtained LiFePO4 changed depending on pH. The electrochemical properties of the prepared LiFePO4 were characterized as a cathode material for lithium batteries in an organic electrolyte at room temperature. The LiFePO4 particle prepared from the precursor solution with Li(CH3COO) was flake-like crystal (particle size: 1–2 μm) and had a preferred crystal orientation with a (020) texture. This LiFePO4 exhibited a discharge capacity of 147 mA h g−1, which was 85% of the theoretical capacity 170 mA h g−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
LiFePO4 has been prepared by various methods, in order to improve its electrochemical performance as a cathode material of rechargeable lithium batteries [1–6]. Usually, transition metal oxides such as LiCoO2 and LiMn2O4 have been utilized as cathode materials for rechargeable lithium batteries. However, present cathodes have been suffered from a safety problem, which is due to oxygen release from the transition metal oxides under abuse conditions [7]. The oxygen release causes a rapid temperature increase in the battery, which sometimes leads to an explosion of battery. In order to realize real safety of rechargeable lithium batteries, new type cathode materials have to be developed. Recently, phosphate compounds have been paid much attention in a sense of safety and cost of cathode materials. Especially, LiFePO4 has been intensively studied by many research groups [1–6]. The Li+ ion can be extracted/inserted from/into LiFePO4 at the electrode potential of 3.5 V versus Li/Li+, and the theoretical discharge capacity of LiFePO4 is 170 mA h g−1. In addition, iron compounds are usually very cheap compared with other transition metal compounds. A main problem of LiFePO4 is its low electronic conductivity. Several researchers reported that carbon-coating on LiFePO4 particle was effective in improving its electrochemical performance [8–14]. On the other hand, high crystalline small particles of LiFePO4 have been prepared by using various methods, such as hydrothermal process, sol–gel process, and so on [15–19]. In the case of hydrothermal process, the composition of precursor solution strongly influences on particle nature of LiFePO4. Moreover, the oxidation state of iron ion during hydrothermal treatment is very important to obtain LiFePO4 without any impurities [20]. In order to increase the utilization of LiFePO4 for charge and discharge, smaller particle should be prepared under suitable conditions because the shorter electronic conduction path is helpful in reducing ohmic resistance within the particle.
The crystal texture of LiFePO4 particle is also an important aspect, which is strongly related to electrode kinetics [21–24]. The Li+ ion diffusion takes place along b-axis in the crystal of orthorhombic LiFePO4 (space group: Pnma) during charge and discharge, and the charge transfer (Li+ ion transfer) takes place mainly on the (020) plane [24]. Therefore, if a crystal with a (020) texture is obtained, faster electrochemical reaction can be achieved.
In this study, LiFePO4 was prepared by hydrothermal method from FeSO4 · 7H2O, (NH4)2HPO4, and three kinds of Li sources. LiCl, Li(CH3COO), and LiOH · H2O were used as Li sources. The pH of the precursor solution varied depending on Li source. The particle size, particle shape, and crystal texture of the obtained LiFePO4 changed depending on the pH. The crystal characteristics and electrochemical properties of the prepared LiFePO4 were investigated. Especially, the effects of crystal texture of LiFePO4 on its electrochemical properties were discussed.
Experimental
LiFePO4 was prepared by the hydrothermal method with three kinds of Li sources LiCl, LiCH3COO, and LiOH · H2O. The FeSO4 · 7H2O and (NH4)2HPO4 were used as Fe and P sources, respectively. These starting materials were dissolved into ultra pure water. This mixed solution (40 mL) was put into Teflon-lines Parr reactor (inner volume: 120 mL). Table 1 shows the molar ratio of each compound and concentration of FeSO4 for the hydrothermal process. Using these three different solutions, the hydrothermal process was performed at 170 °C for 12 h using a stainless steal autoclave. These processes were performed under N2 atmosphere in order to prevent the oxidation of Fe2+ to Fe3+ [25]. After filtration, the prepared sample was dried under vacuum at 100 °C for 1 h. The prepared samples were characterized with X-ray diffraction (XRD). The charge and discharge measurements of prepared samples were conducted using 2016 coin-type cells. The cathode was prepared from a mixture of prepared sample, Ketjen black, and PTFE with weight ratio of 80:15:5. The anode was lithium metal and a mixed solvent of ethylene carbonate and diethylcarbonate with volume ratio of 1:1 containing 1.0 mol dm−3 LiClO4 was used as the electrolyte. The charge and discharge tests were performed at 0.1 C rate (17 mA g−1-LiFePO4). The electrochemical test was conducted in an argon-filled glove box at room temperature.
Results and discussion
The pH of precursor solution depended on lithium salt used as a starting material. In the case of LiOH, the precursor solution was most basic condition, and the pH was 8.99. The precursor solution prepared using LiCl as Li source was most acidic and the pH was 3.44. When Li(CH3COO) was used as Li source, pH of the precursor solution was 5.11. The differences in Li source and pH of precursor solutions influenced on the particle shape of resulting LiFePO4. Figure 1 shows the scanning electron micrographs of hydrothermally synthesized samples. Using LiOH as a Li source, the smallest particles were prepared. The particles were agglomerated, and the particle size was less than 1 μm. Among the three samples, the largest particle was obtained using LiCl as Li source. The particle was very thin and flake-like crystal, which was different from that prepared in the solution containing LiOH. On the other hand, the precursor solution with Li(CH3COO) provided thick flake-like crystals. The particles were well dispersed, and the particle size was estimated to be 1–2 μm. In this way, the particle nature of LiFePO4 strongly depended on Li source and/or pH of the precursor solution.
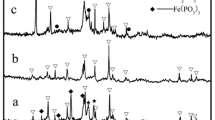
Figure 2 shows XRD patterns of LiFePO4 samples prepared from three precursor solutions. All of the peaks in three x-ray diffraction patterns can be assigned to those of orthorhombic LiFePO4 (space group: Pnma) reported in the literature [16–18]. Therefore, it can be said that three samples are the single phase of LiFePO4. However, the yield of LiFePO4 (conversion ratio of Fe2+ in the precursor to LiFePO4) depended on the acidic condition of the solution as shown in Table 1. When the pH of the precursor was higher than 5, the yield was more than 90%. However, the yield was as small as 55% in the case of precursor with LiCl because of the strong acid condition. Probably, the solubility of LiFePO4 would be higher under acidic condition pH <4 at high temperatures (∼170 °C).
Although the all XRD patterns in Fig. 2 were assigned to the LiFePO4 with olivine structure, the relative intensities of the peaks were completely different between three samples. The peak intensity ratio of the LiFePO4 prepared from the precursor solution with LiOH was very similar to that reported in the literature [11–14], and the particle had a random crystal orientation. Indeed, the obtained particles were agglomerated polycrystalline particles as shown in Fig. 1c. However, the peak intensity of (200) of the LiFePO4 prepared from the precursor solution with LiCl was strongest, which suggested that the LiFePO4 flake particle had a preferred crystal orientation with a (200) texture. On the other hand, the (020) peak of the LiFePO4 prepared from the precursor solution with Li(CH3COO) was strongest, which suggested that the LiFePO4 flake crystal had a preferred crystal orientation with a (020) texture. In this way, the crystal texture of LiFePO4 was strongly influenced by Li source and/or pH of the precursor solution. These results suggested that the preferred crystal growth orientation changed depending on the pH of solution. The crystal texture of particles gives significant effects on the electrochemical properties of LiFePO4. The following electrochemical measurements were conducted for these LiFePO4 samples.
Figure 3 shows the charge and discharge curves of LiFePO4 prepared from three different precursor solutions. The largest discharge capacity was achieved using Li(CH3COO) as a Li source of LiFePO4, which was 147 mA h g−1. On the other hand, LiFePO4 samples prepared from precursor solutions with LiCl and LiOH exhibited lower discharge capacities. As mentioned above, all LiFePO4 samples prepared by hydrothermal method in this study did not contain any impurities, so that all samples were expected to show discharge capacities near to the theoretical one (170 mA h g−1). However, the discharge capacity depended on Li source utilized as a starting material for hydrothermal synthesis of LiFePO4. The differences among three samples were particle size, particle shape, and crystal texture. Particle size of active material usually influences on charge-discharge performance, because the Li+ ion diffusion and electronic conduction take place within the particle. In fact, as reducing the particle size, the diffusion length of Li+ ion and electronic conduction path are decreased, which reduce the diffusion resistance for Li+ ion and electrical resistance within the particle [11]. In the case of LiFePO4, crystallographic phase transition takes place during charge and discharge, so that phase boundary diffusion may occur within the particle [16]. The rate of boundary diffusion depends on particle size and shape. By the way, LiFePO4 is an olivine-type compound, and anisotropic crystal structure is involved [23]. Consequently, the crystal orientation of LiFePO4 particle gives significant effects on its charge–discharge process. The Li+ ion diffusion takes place along b-axis in the crystal of LiFePO4 during charge and discharge [21–24]. Furthermore, the charge transfer (Li+ ion transfer) takes place mainly on the (020) plane [24]. Therefore, the electrode reaction rate of LiFePO4 can be improved by a preferential crystal orientation. If a tiny crystal with a (020) texture is obtained, faster electrochemical reaction can be achieved. In this way, the charge and discharge characteristics of LiFePO4 should be discussed considering at least two factors, i.e., crystal texture and particle size. The LiFePO4 prepared from the precursor solution with Li(CH3COO) had a larger particle size than that prepared from the precursor solution with LiOH. However, the LiFePO4 crystal prepared from the precursor solution with Li(CH3COO) had a preferred orientation with a (020) texture while the LiFePO4 prepared from the precursor solution with LiOH had random crystal orientations. The discharge capacity of LiFePO4 prepared from the precursor solution with LiCl was the smallest among the three LiFePO4 samples, due to the largest particle size and the (200) crystal texture. This crystal texture is not suitable for Li+ ion insertion and extraction, because the Li+ ion diffusion scarcely occurs along a-axis and the charge transfer does not take place on the (200) plane but (020) [24]. LiFePO4 prepared from the precursor solution with LiOH had the smallest particle size, however, the crystal texture of the sample was random. The smaller particle of the LiFePO4 is suitable for faster electrode reaction, but the poor (020) texture of LiFePO4 is not good. From these results, it can be said that the crystal texture of LiFePO4 particle is more dominant factor for the electrochemical performance. The crystal texture of LiFePO4 particle was strongly influenced by the Li source and/or pH of the precursor solution. It was revealed that the weak acidic condition (pH ≈5) was preferable to obtain flake-like crystals of LiFePO4 with a (020) texture, which showed high electrochemical reactivity.
Figure 4 shows charge and discharge curves of LiFePO4 prepared from the precursor solution with Li(CH3COO) at various charge and discharge rates. The discharge capacity decreased with increasing charge and discharge rate. This is due to polarization in cathode. At 2.0 C rate, the discharge capacity was 32 mA h g−1. This is smaller value compared with those of other cathode materials at 2.0 C (LiCoO2, LiMn2O4, and so on) [26, 27]. The electronic conductivity of LiFePO4 is smaller than those of other cathode materials. This property is not good for the high rate charge-discharge performance of lithium battery. In order to overcome this problem, smaller particles of LiFePO4 with a (020) texture should be prepared. The particle size of LiFePO4 does not only depend on Li source but also concentration of Fe2+ and PO 3−4 ions in the precursor solution. Further optimization of hydrothermal condition is now in progress in our group and will be reported in due course.
Conclusion
LiFePO4 was prepared by hydrothermal method using three different Li sources, LiOH, Li(CH3COO), and LiCl. The pH of the precursor solution varied depending on the Li source. The particle morphology and preferred crystal growth orientation of LiFePO4 were strongly influenced by Li source and/or pH of the precursor solution. The electrochemical performance of LiFePO4 depended on the crystal texture. The LiFePO4 crystal with a (020) texture exhibited the highest charge and discharge capacities among the three samples. The discharge capacity of LiFePO4 was 147 mA h g−1 measured at 0.1 C rate (17 mA g−1), however, the discharge capacity decreased with increasing discharge rate and became less than 40 mA h g−1 at 2.0 C rate. This may be due to low electronic conductivity of LiFePO4. In order to improve the electrochemical reactivity of LiFePO4, smaller crystals with a (020) texture should be prepared by optimizing the hydrothermal condition.
References
Sheu SP, Yao CY, Chen JM, Chiou YC (1997) J Power Sources 68:533
Thackeray MM, de Kock A, Rossouw MH, Liles D, Bittihu R, Hoge D (1992) J Electrochem Soc 139:363
Inaba M, Doi T, Iriyama Y, Abe T, Ogumi Z (1999) J Power Sources 81:554
Yamada O, Ishikawa M, Morita M (2000) Electrochim Acta 45:2197
Lu C, Lin S (2001) J Power Sources 97:458
Deiss E, Haringer D, Novak P, Haas O (2001) Electrochim Acta 46:4185
Dahn JR, Fuller EW, Obrovac M, von Sacken U (1994) Solid State Ionics 69:265
Ravet N, Goodenough JB, Besner S, Simoneau M, Hovington P, Armand M (1999) Abstract 127, The Electrochemical Society and the Electrochemical Society of Japan Meeting Abstracts, 99–2, Honolulu
Prosini PP, Zane D, Pasquali M (2001) Electrochim Acta 46:3517
Ravet N, Chouinard Y, Magnan JF, Besner S, Gauthier M, Armand M (2001) J Power Sources 97:503
Huang H, Yin S-C, Nazer LF (2001) Electrochem Solid-State Lett 4:A170
Croce F, Epifanio AD, Hassoun J, Deptula A, Olczac T, Scrosati B (2002) Electrochem Solid-State Lett 5:A47
Chen Z, Dahn JR (2002) J Electrochem Soc 149:A1184
Myung S, Komaba S, Hirosaki N, Yashiro H, Kumagai N (2004) Electrochim Acta 49:4213
Eftekhari A (2001) Electrochim Acta, 47:495
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188
Yamada A, Chung SC, Hinokuma K (2001) J Electrochem Soc 148:A224
Yang S, Zavalij PY, Wittingham MS (2001) Electrochem Commun 3:505
Yang S, Song Y, Zavalij PY, Whittingham MS (2002) Electrochem Commun 4:239
Dokko K, Shiraishi K, Kanamura K (2005) J Electrochem Soc 152:A2199
Islam MS, Driscoll DJ, Fisher CAJ, Slater PR (2005) Chem Mater 17:5085
Morgan D, Van der Ven A, Ceder G (2004) Electrocehm Solid-State Lett 7:A30
Amin P, Balaya P, Maier J (2007) Electrochem Solid-State Lett 10:A13
Chen G, Song X, Richardson TJ (2006) Electrochem Solid-State Lett 9:A295
Dokko K, Koizumi S, Shiraishi K, Kanamura K (2007) J Power Sources 165:656
Abraham KM, Pasquariello DM, Willstaedt EM (1998) J Electrochem Soc 145:482
Shin Y, Manthiram A (2004) J Electrochem Soc 151:A204
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanamura, K., Koizumi, S. & Dokko, K. Hydrothermal synthesis of LiFePO4 as a cathode material for lithium batteries. J Mater Sci 43, 2138–2142 (2008). https://doi.org/10.1007/s10853-007-2011-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2011-1