Abstract
The synthesis and antibacterial properties of N-halamine copolymers are reported in this paper. 3-(4′-vinylbenzyl)-5,5-dimethylhydantoin (VBDMH) monomer and its copolymers with n-butyl methacrylate (BMA) were prepared under mild conditions. The effects of monomer feeds on the composition of the final copolymers and reaction conversion were investigated. It was found that VBDMH had higher reactivities than BMA in the copolymerization reactions and the reactivity ratios of VBDMH and BMA were calculated to be 8.91 and 0.42, respectively, according to the Fineman–Ross equation in the preparation of PBMA-co-VBDMH. Two chlorination methods were applied and compared in the process of polymers chlorination. After chlorination by tert-butyl hypochlorite, the polymers provided powerful antibacterial activities against Escherichia coli (E. coli). The surface morphologies of the chlorinated polymer films used in antibacterial assessment were observed by scanning electron microscope. The copolymers before and after chlorination were characterized with fourier transform infrared (FTIR) and 1H-nuclear magnetic resonance (1H-NMR). Their thermal properties were analyzed with thermogravimetric analysis (TGA) studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of the society, people are more care about the health nowadays and many diseases are caused by micro-organisms exist on material surface. The antibacterial modification of material surfaces is of great importance. Consequently, biocidal polymer materials have received much attention in recent years [1, 2]. Currently there are several sorts of antibacterial polymers such as polymers containing heavy metal cations, pyridinium and quaternary ammonium salt polymers, and N-halamine polymeric biocides, among which, N-halamine polymeric biocides have shown to be disinfectants which provide almost instant and total kill of a wide range of micro-organisms [3, 4]. In addition, their N-halamine structures possess several useful features including good stability for long-term use and storage over a wide temperature range and ability to be regenerated in a chlorine solution repeatedly [5–7]. Researchers have synthesized polymeric N-halamines through the chemical incorporation of cyclic amines into polystyrene or cellulose-containing fabrics and halogenation of the resultant polymers [8, 9]. Those novel polymers were proven biocidal, and their antibacterial activities were durable and regenerable. Sun et al. [10] synthesized monomer of VBDMH and copolymerized it with acrylonitrile, vinyl acetate, and methyl methacrylate, respectively. After chlorination, these copolymers exhibited powerful antibacterial activities. Till now, although there have been some important researches on N-halamine polymers [8, 10–12], types of antibacterial N-halamine polymers are still limited. Antibacterial mechanism and relationship between structure and antibacterial activity are still uncertain. Therefore, there is a need to continue researches for novel antibacterial N-halamine polymers as well as to put more efforts to get a better understanding of antibacterial mechanism.
In this study, VBDMH was synthesized and its copolymers with BMA were prepared and characterized. The copolymerization behaviors of VBDMH and BMA were studied. The copolymers were halogenated to obtain halamine structures. Their antibacterial properties against E. coli were investigated and the possible antibacterial mechanism was discussed.
Experimental
Materials
4-Vinyl benzyl chloride was purchased from Aldrich and used without further purification. 5,5-Dimethylhydantoin (DMH) was supplied by Shun Ye Corp. of China. Other reagents were all commercially obtained. BMA was washed with 10% sodium hydroxide solution and distilled before usage. Benzoyl peroxide (BPO) was recrystallized from methanol (MeOH)/chloroform twice before use.
Measurements
FTIR spectra were recorded on a Bruker Vector22 spectrometer. 1H-NMR spectra were recorded on a Bruker Avance 400/500 DMX NMR spectrometer. TGA measurements were performed on a Perkin–Elmer Pyris 1 thermogravimetric analyzer at a heating rate of 20 °C/min under a N2 atmosphere. The surface morphologies of the polymer films were observed with an FEI SIRION scanning electron microscope. Sample preparation included gold sputter coating for 8 min.
Synthesis of VBDMH
The monomer of VBDMH was synthesized according to reference as showed in Scheme 1 [10]. 6.4 g (0.05 mol) of DMH was dissolved in 25 cm3 of 10 wt% KOH solution, and then mixed with a solution of 7.5 cm3 (0.05 mol) of 4-vinyl benzyl chloride in 10 cm3 of MeOH. The mixed system was stirred for 3 h at 60 °C and the solvents were removed under reduced pressure at room temperature. The solid was recrystallized from MeOH/H2O (Volume ratio = 3/1) and dried in a vacuum oven to give a yield of 3 g (25.8%). 1H-NMR [(CD3)2CO, ppm]: 1.37(6H, CH3), 4.57 (2H, CH2), 5.21–5.81 (2H, CH2=), 6.70–6.76 (1H, =CH), 7.28 (1H, N–H), 7.30–7.44 (4H, phenyl).
Synthesis of PBMA-co-VBDMH
A total of certain quantity of VBDMH, 1 cm3 of BMA, 0.0016 g of BPO were added to a three-necked, round-bottom flask with a condensator. The reagents were purged with argon for 15 min, and the flask was heated to 70 °C for 3 h with stirring. The solution was cooled to room temperature and was drop-added into 200 cm3 of dimethyl sulfoxide (DMSO). The polymers were precipitated out and filtered. After purification, they were dried in a vacuum oven to give a pure product. The product, PBMA-co-VBDMH, exhibited the following spectral data: 1H-NMR [(CD3)2CO, ppm]: 0.78, 0.94 (3H, CH3), 0.87 (3H, CH3), 1.17 (3H, CH2, CH), 1.28 (6H, CH3), 1.34 (2H, CH2), 1.54 (2H, CH2), 1.76–1.94 (2H, CH2), 3.85 (2H, CH2), 4.43 (2H, CH2), 7.06–7.19 (4H, phenyl), 7.26 (1H, N–H).
Synthesis of tert-butyl hypochlorite
Tert-butyl hypochlorite was synthesized according to reference as showed in Scheme 2 [13]. 500 cm3 of a 5 wt% sodium hypochlorite solution was placed in a round-bottomed flask equipped with a mechanical stirrer. The flask was placed in an ice bath and rapidly stirred until the temperature dropped below 10 °C. A solution of t-butyl alcohol (37 cm3, 0.39 mol) and glacial acetic acid (24.5 cm3, 0.43 mol) was added in a single portion to the rapidly stirred solution, and stirring was continued for about 10 min. The entire reaction mixture was poured into a separatory funnel. The low aqueous layer was discarded, and the oily yellow organic layer was obtained as product.
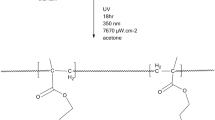
Halogenation of PBMA-co-VBDMH
The halogenation process was carried out according to Scheme 3 [8]. A total of 0.02 g of copolymer, dissolved in 2 cm3 of dichloromethane, and 1 cm3 of tert-butyl hypochlorite prepared as aforementioned, were added to a one-necked, round-bottom flask with a condensator. The reagents were stirred vigorously for 2 h and drop-added into 100 cm3 of petroleum ether. The polymer was precipitated out and filtered, dried in a vacuum oven to give a pure product.
The product, PBMA-co-VBDMH after chlorination, exhibited the following spectral data: 1H-NMR [(CD3)2CO, ppm]: 0.78, 0.94 (3H, CH3), 0.87 (3H, CH3), 1.17 (3H, CH2, CH), 1.28 (6H, CH3), 1.34 (2H, CH2), 1.54 (2H, CH2), 1.76–1.94 (2H, CH2), 3.85 (2H, CH2), 4.43 (2H, CH2), 7.06–7.19 (4H, phenyl).
Antibacterial assessment
The antibacterial activities of the chlorinated copolymers were evaluated with polymer films as following [10]: the substrate to be coated was washed, and dried before the polymer solution was added. Acetone solution of copolymer was added to coat the surface about 10 mm in diameter, and then the material coated with the polymer solution was heated in an oven until the solvent was removed. Onto the surface of the film, 10 μL of an aqueous suspension containing 107–108 colony forming units (CFU) per cm3 of E. coli was placed, and covered with preservative film. After 30 min contact with the bacterial suspension, the polymer film was immersed in 20 cm3 of a 0.02 N sodium thiosulfate aqueous solution, which completely neutralized spare active chlorine in the film without affecting bacterial growth. The resultant solution was vigorously shaken for 5 min. An aliquot of the solution was serially diluted, and plated onto a nutrient agar. The same procedure was also applied to an unchlorinated sample as a control. Viable bacterial colonies on the agar plates were counted after incubation at 37 °C for 24 h. Bacterial reduction is reported according to Eq. 1 [10]
where A is the number of bacteria counted from an unchlorinated polymer sample and B is the number of bacteria counted from chlorinated polymer sample.
Results and discussion
Synthesis and characterization of the VBDMH and PBMA-co-VBDMH
VBDMH was characterized by 1H-NMR and the characteristic peaks of VBDMH can be found at 1.37 (6H, CH3), 4.57 (2H, CH2), 5.21–5.81 (2H, CH2=), 6.70–6.76 (1H, =CH), 7.28 (1H, N–H), 7.30–7.44 (4H, phenyl), respectively.
The copolymers of VBDMH and BMA were obtained and their structures were confirmed by FTIR. The FTIR spectrum of PBMA-co-VBDMH shows characteristic absorption bands of CH3 at 2,960 cm−1, CH2 at 2,873 cm−1, C=O at 1,727 cm−1, and C–O at 1,151 cm−1. The absorption bands of amide groups of the VBDMH structure can be observed at 1,776 cm−1. The structures of the copolymers were further confirmed from their 1H-NMR spectrum: 7.26 ppm (N–H), 7.06–7.19 ppm (aromatic protons), and 4.43 ppm (methylene group). All these data suggest that the copolymer of VBDMH and BMA have successfully synthesized.
Polymerization behaviors
The effects of the monomer feed on reaction conversion and copolymer composite were investigated and the results are listed in Fig. 1. The VBDMH molar contents in the copolymers were determined by their 1H-NMR spectra. For example, in the 1H-NMR spectrum of PBMA-co-VBDMH (Fig. 3), the 3.85 ppm peak is attributed to CH2 of the BMA component in the side groups, whereas the 4.43 ppm peak is attributed to the methylene protons of the VBDMH component in the side groups. Consequently, the VBDMH molar content in PBMA-co-VBDMH copolymer was calculated from Eq. 2
where M VBDMH represents the molar content of VBDMH in the PBMA-co-VBDMH copolymer (%) and A a and A bare the relative peak areas of the 3.85 and 4.43 ppm peaks, respectively. From Fig. 1, it can be seen that the conversion was increased with the increment of the initial molar ratio of VBDMH and the molar content of VBDMH in the PBMA-co-VBDMH increased with the increase of the initial molar ratio of VBDMH. The copolymerization behaviors of BMA and VBDMH were further studied by calculating the reactivity ratios of VBDMH and BMA. Since the conversion is less than 10%, the Fineman–Ross equation (Eq. 3) was used in this study to calculate the reactivity ratios [14]. The data collected for calculation were shown in Table 1. After linear simulation, r 1 (reactivity ratio of VBDMH) showed to be 8.91 as the slope and r 2 (reactivity ratio of BMA) showed to be 0.42 as the intercept. The higher reactivity of VBDMH would cause more VBDMH component in copolymer, and this is helpful to enhance the antibacterial property of copolymer since VBDMH is a potential antibacterial component
where R = [M 1]/[M 2] ≈ [M 1]o/[M 2]o, corresponding to the concentration ratio of the monomers. ρ = d[M 1]/d[M 2], corresponding to the concentration ratio of the copolymer components. M 1 represents VBDMH and M 2 represents BMA.
Characterization of the halogenated PBMA-co-VBDMH
After reacted with tert-butyl hypochlorite, the hydantoin groups in the copolymers were fully transformed into N-halamine structures according to Scheme 3. Figure 2 shows the FTIR spectra of the copolymers in the region of 1,500–2,300 cm−1 before and after chlorination. Before the chlorination reaction, the copolymers display bands around 1,776 cm−1, attributable to the amide carbonyl structures of the hydantoin ring. After the reaction, the 1,776 cm−1 bands disappear, and instead, new bands at 1,791 cm−1 appear. These 15 cm−1 differences of vibrations between the samples strongly indicate that the hydantoins have been transformed into N-halamine structures [10]. The transformation of hydantoin to halamine structures was further confirmed with 1H-NMR studies. Figure 3 shows the 1H-NMR spectrum of the chlorinated polymers. As expected, after chlorination the peak of hydrogen in amide around 7.26 ppm disappeared, strongly indicating that the N–H group of the hydantoin amide had been transferred into N–Cl structures, in agreement with Scheme 3.
It was reported that polymers with hydantoin structures could be chlorinated to halamine structures using sodium hypochlorite solution. In our study, this chlorination method was tried by casting polymer into films and treating it with sodium hypochlorite solution. However, it was found that the chlorination was not successful enough, as there was no obvious difference in the FTIR and 1H-NMR spectra of the copolymers before and after chlorination. This result may be caused by the poor contact of polymers with sodium hypochlorite solution in the solid state: only the hydantoin structure on the surface of the polymer could contact with the solution, while, the interior hydantoin structure of polymers had no chance to contact with the solution therefore had not been transformed into halamines. Facing such a result, we used another method to chlorinate the polymers. Tert-butyl hypochlorite is an organic reagent which can be homodispersed in dichloromethane. By dissolving polymers in dichloromethane and mixing the polymer solution with tert-butyl hypochlorite, the hydantoin structure of polymers can sufficiently contact with tert-butyl hypochlorite, therefore can be chlorinated completely. The experimental result demonstrates that this is a feasible way. By using tert-butyl hypochlorite, the hydantoin structures had been completely chlorinated into halamines, as can be seen in Figs. 2, 3. So the Cl loading can be calculated from the compose of the copolymer, and the results are listed in Table 2.
Thermal properties of the polymers
The thermal stabilities of the copolymers were determined by TGA and the results are shown in Figure 4. From Fig. 4, it can be seen that the copolymers have good thermal stabilities. The degradations of the copolymers commence at higher temperature than the PBMA homopolymer. The decomposition mechanism of PBMA is depolymerization [10, 15], and the presence of the VBDMH component in the copolymer may inhibit such a depolymerization.
Antibacterial properties of the chlorinated PBMA-co-VBDMH
The antibacterial properties of the chlorinated copolymer films were examined against E. coli, and the results are shown in Table 2. All of the samples showed inactivation of E. coli after a contact time of 30 min, but the reduction rate was different.
The process of antibacterial action of the chlorinated polymer is illustrated in Fig. 5. Because the hydrolysis equilibrium constant of compound with hydantoin structures is very low (less than 10−6 order), which resulted in limited “free chlorine” (HClO) liberated into aqueous solution as reported [16], the chlorinated copolymers mostly serve as contact biocides in aqueous solution. When E. coli contact with the immobile chlorine on the surface of the chlorinated copolymer, chlorine makes the membrane abrupt by changing the permeability of cell membrane, the content in the cell seeps, and leads to the death of cells [4, 16, 17].
It was found that with 30 min contact time, chlorinated PBMA-co-VBDMH could provide reduction of E. coli beyond 90% as can be seen in Table 2. Because the chlorinated copolymers mostly serve as contact biocides in aqueous solution as mentioned above, the Cl loading and the hydrophilicity are main factor that effect its antibacterial activity. The surface morphology of the chlorinated PBMA-co-VBDMH in this paper is shown in Fig. 6. It can be seen that the chlorinated copolymer film of PBMA-co-VBDMH was full of micropores which is help to improve the contact area and promote the antibacterial activity. So the relative low antibacterial activity was mainly caused by the less hydrophilicity of PBMA-co-VBDMH.
Conclusions
VBDMH and its copolymers with BMA were prepared under mild conditions. The molar ratio of VBDMH in final copolymer was increased with the increase of the initial molar ratio of VBDMH. According to the Fineman–Ross equation, the reactivity ratios of VBDMH and BMA were calculated to be 8.91 and 0.42, respectively. After chlorinated by tert-butyl hypochlorite, the hydantoin structures in the copolymers were completely transformed into N-halamines, providing the samples with antibacterial activities against E. coli. The copolymers have good thermal stabilities and the presence of the VBDMH component enhances the degradation temperature of the copolymers. The resultant polymers showed powerful antibacterial activities against E. coli.
References
Lin J, Winkelmann C, Worley SD, Kim J, Wei C-I, Cho U, Broughton RM, Santiago JI, Williams JF (2002) J Appl Polym Sci 85(1):177
Chen Y, Wang L, Jiang S, Yu HJ (2003) J Polym Mater 20(3):279
Jiang S, Wang L, Yu HJ, Chen Y (2005) React Funct Polym 62:209
Worley SD, Sun G (1996) Trends Polym Sci 4(11):364
Sun G, Chen TY, Habercom MS, Wheatley WB, Worley SD (1996) Water Res Bull 32:793
Sun G, Chen TY, Worley SD (1996) Polymer 37:3753
Worley SD, Sun G (1996) Polym Mater Encyclopedia 1:550
Eknoian MW, Putman JH, Worley SD (1998) Ind Eng Chem Res 37:2873
Sun G, Xu X (1998) Textile Chem Colorist 30(6):26
Sun YY, Sun G (2001) J Polym Sci 39:3348
Sun YY, Sun G (2001) J Appl Polym Sci 80:2460
Sun YY, Sun G (2002) Macromolecules 35:8909
Baumgarten HE (1973) Organic syntheses, vol. 5. John Wiley and Sons, New York, p 183
Fineman M, Ross SD (1950) J Polym Sci 5:259
Hawkins WJ (1984) Polymer degradation and stabilization. Springer-Verlag, Berlin
Xue GB (1986) Practical sterilization study, 2 edn. The People’s Military Medical Publishing Company, Shanghai, p 45
Chen YJ, Worley SD, Kim J, Wei CI, Chen TY, Suess J, Kawai H, Williams JF (2003) Ind Eng Chem Res 42:5715
Acknowledgements
The authors thank Yuanming Ying for performing the microbiological evaluation of the polymers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, L., Yu, H. et al. Synthesis, characterization, and antibacterial activities of novel N-halamine copolymers. J Mater Sci 42, 4018–4024 (2007). https://doi.org/10.1007/s10853-006-1412-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1412-x