Abstract
The supercritically treated TiO2-activated carbon (Sc-TiO2-AC) composites were studied for their adsorption and subsequent photocatalytic activities for acetaldehyde. Sc-TiO2-AC composites showed much higher decomposing ability for acetaldehyde compared to a noncomposite comprising a simple mixture of activated carbon and TiO2. This indicates that Sc-TiO2-AC composites have a synergetic effect of adsorption and photocatalytic decomposition of acetaldehyde. Meanwhile, acetaldehyde was found to be decomposed to CO2 as final product by Sc-TiO2-AC composites through the conversion to methanol, formaldehyde and acetic acid as intermediates. It was also found that many OH groups exist on the Sc-TiO2-AC composites. These OH groups can be assumed to take part in the photocatalytic degradation of acetaldehyde.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The widespread use of volatile organic compounds (VOCs) in both domestic and industrial areas has caused significant environmental problems, such as air pollution, water contamination, global warming and so on [1]. A useful approach to these problems is to develop efficient and novel materials for converting these toxic matters to harmless substances such as CO2 and H2O.
Photocatalytic treatment using UV-irradiated semiconductors has been promising and interesting in recent decades, which is capable of decomposing many kinds of VOCs by using UV light as an energy source under ambient temperature and pressure conditions. Among these semiconductors, TiO2 as a photocatalyst is one of the most popular and effective materials because of its chemical and physical stability, strong oxidation and reduction abilities, and nontoxicity. The photocatalytic activity of TiO2 varies depending on its crystal phase, crystallite size and crystallinity. It is known that anatase-form of TiO2 crystallite with small crystallite size and high crystallinity exhibits better photocatalysis [2].
On the other hand, it was clarified that wood charcoal or activated carbon is effective for removal of harmful substances in the environment by their excellent adsorption ability [3, 4]. Therefore, some researches on the preparation of TiO2-carbon composites that were composed of carbon as an adsorbent and the anatase-form of TiO2 crystallite as a photocatalyst have been reported [5–7]. Doi et al. [6] developed the carbonized TiO2-woody composites by thermal treatment where anatase-form of TiO2 crystallites was deposited in the wood lumen, cell wall and the surface of the carbonized wood particles, separately, which have the synergetic effect of adsorption and photocatalytic activity for formaldehyde. Huang and Saka [7] have applied the supercritical isopropanol treatment to prepare TiO2 crystallite-activated carbon composites for converting TiO2 gels in the composites to anatase-form of TiO2 instead of the thermal treatment. It was found that the supercritical treatment is effective for the conversion to anatase-form of TiO2 in the composites. The obtained TiO2-activated carbon composites have highly synergetic effects of adsorption and photocatalytic activities for formaldehyde.
In this study, the supercritically treated TiO2-activated carbon composites were prepared and studied for their adsorption and photocatalytic activities for acetaldehyde, one of the most common VOCs. Meanwhile, reaction intermediates and final products in the photocatalytic process of acetaldehyde were studied and the mechanism of the photocatalytic reactions was discussed.
Materials and methods
Preparation of samples
Supercritical treatment was carried out by a batch-type reaction system described elsewhere [8], where a 5-ml reaction vessel made of inconel-625 was fully charged with reactant and solvent.
Activated carbon (0.2 g) (AC: activated charcoal powder from wood sawdust washed with hydrochloric acid, Extra Pure Reagent, Nacalai Tesque) was added to a reactant of tetraisoproply titanate (TPT) (4 g) in 15 ml of the solvent, isopropanol. The mixture was, then, treated by ultrasonification for 30 min, and then decanted to remove the supernatant. Subsequently, the TPT-soaked AC was treated in air for the sol–gel reaction for 5 h to obtain TiO2-AC composites. The TiO2-AC composites were then treated with supercritical isopropanol at 295 °C, 12 MPa, which were above a critical temperature of isopropanol (T c = 235 °C) and a critical pressure of isopropanol (P c = 5.37 MPa), for various reaction times to obtain supercritically treated TiO2-AC (Sc-TiO2-AC) composites. We also examined the synergetic effect for a simple mixture of supercritically treated TiO2 (Sc-TiO2) granules with AC.
Characterizations of samples
X-ray diffractograms were obtained by RINT2000 (Rigaku Denki) to examine the crystallographic structures of the TiO2 granules under Cu-Kα radiation (λ = 0.1542 nm) using a Kβ filter, operated at 40 keV and 30 mA, integrating five times. The average crystallite size of the anatase-form TiO2 in the composites was calculated by the following equation.
where λ is the radiation wavelength of the X-ray (Å), β is the integral width of the peak at 2θ = 25.3 (degrees), θ is the Bragg’s angle in the (101) plane, and K is Scherrer’s value (0.94) [9].
Degree of crystallinity of TiO2 crystallite in the composites was determined from the ratio of the integral intensity of crystalline portions to the total intensity of the samples over a range of 2θ = 18°–58°at the obtained X-ray diffractograms [10].
Infrared spectra in KBr matrices were measured on an FTIR-8000 spectrometer (Shimadzu) in the wave number range of 4000–500 cm−1.
Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) was made with a TGA-50 (Shimadzu) and a DTA-50 (Shimadzu), respectively. All the analyses were carried out in a air condition and a heating rate of 30 °C /min up to 800 °C, with a sample weight of around 40 mg. Al2O3 was used as the reference material in DTA.
Evaluation of adsorption and photocatalytic activities
To evaluate the adsorption and photocatalytic avtivities of the Sc-TiO2-AC composites on acetaldehyde, a previously developed evaluation apparatus was used [6].
An oven-dried sample (contains 10 mg of TiO2) was dispersed in ethanol and then spread over the glass surface of a petri dish 58 cm2 in area for 30 min at 105 °C and cooled to room temperature in a desiccator. The sample was then pretreated under UV light irradiation in the evaluation apparatus filled with nitrogen (purity: 99.95%) until carbon dioxide evolution stopped.
After the UV light was cut off, the gas inside the apparatus was substituted with 1.8 L gas composed of about 500 ppm of acetaldehyde in dried nitrogen. The gas was then circulated inside the apparatus at a flow rate of 3.6 L/min and a temperature of 26 °C. The treatment time was measured from when the circulation was started.
The UV light was turned off for the first 100 min in treatment time to examine adsorption behavior and then turned on to irradiate the sample. The light used for UV irradiation was from a Black lamp (Toshiba FL 6BL). For all samples, the light intensity in the reaction vessel was 0.49–0.50 mW/cm2 at a wavelength of 365 nm. Concentrations of acetaldehyde and its decomposed products were measured from the peak area of the chromatograms obtained with gas chromatography-mass spectrometry (GC-MS) (Shimadzu QP-5000A) by time-lag gas sampling.
To evaluate the photocatalytic effects of the Sc-TiO2-AC composites on acetaldehyde, the relative decomposition rate (RDR) was calculated from the following equation:
where A 400 is an incremental area of CO2 in the GC-MS chromatogram under UV light irradiation for 400 min, and A 400max is the maximum value among all the samples studied.
Results and discussion
Supercritically treated TiO2-activated carbon (Sc-TiO2-AC) composites
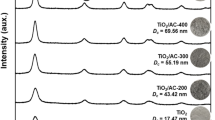
X-ray diffractograms of the Sc-TiO2-AC composites prepared for various reaction times were shown in Fig. 1. It is clear that anatase-form of TiO2 crystallite was formed in the prepared composites by supercritical treatment.
Table 1 shows the crystallite size and crystallinity of anatase-form TiO2 in the Sc-TiO2-AC composites with various reaction times. It was found that TiO2 crystallites with smaller crystallite size and lower crystallinity were obtained under the condition of the shorter reaction time. Various crystallite sizes and crystallities can be controlled by treatment time. These Sc-TiO2-AC composites with various crystallite sizes and crystallinities were evaluated in their photocatalytic effects for acetaldehyde.
Adsorption and photocatalytic activities of the Sc-TiO2-AC composites for acetaldehyde
Figure 2 shows the changes of acetaldehyde and carbon dioxide concentration for the Sc-TiO2-AC composites prepared for 5 min of reaction time, as evaluated before and after UV irradiation. Before UV irradiation, the constancy of CO2 and the decrease of acetaldehyde concentration were observed, which must be due to the adsorption for acetaldehyde by the composites. After UV irradiation, acetaldehyde decreased and disappeared completely in about 200 min of UV irradiation, and CO2 concentration increased gradually. This result indicates that acetaldehyde can be photocatalytically decomposed to CO2. After 500 min in treatment time, CO2 concentration reached around 1100 ppm. Therefore, 1000 ppm CO2 was found to be produced because the initial concentration of CO2 was 100 ppm approximately. Since 1 mol of acetaldehyde is decomposed to 2 moles of CO2, 500 ppm of acetaldehyde can provide about 1000 ppm of CO2 if all acetaldehyde molecules were oxidized into CO2. About 1000 ppm CO2 production, therefore, indicates that acetaldehyde was entirely decomposed into CO2.
Changes in acetaldehyde (CH3CHO) and carbon dioxide (CO2) concentrations depending on the treatment time before and after UV irradiation for the Sc-TiO2-AC composites, prepared by supercritical treatment for 5 min. Results of the simple mixture of Sc-TiO2 and AC are included for comparison (dash lines)
For comparison, results for the simple mixture (contain the same TiO2 content as in the composites: 10 mg) of Sc-TiO2 and AC were also shown in Fig. 2 as dash lines. Although acetaldehyde concentration decreased to zero after 500 min of treatment time, adsorbed acetaldehyde was not decomposed entirely into CO2. Therefore, the Sc-TiO2-AC composites must have highly synergetic effects of adsorption and subsequent photocatalytic activity on acetaldehyde.
Table 2 shows the relative decomposition rate (RDR) on acetaldehyde of the Sc-TiO2-AC composites. Just for comparison, RDR of the simple mixture of Sc-TiO2 and AC is also shown. It is clear that all the composites have larger RDRs than the simple mixture. More closer inspection shows that RDR increased with increasing the reaction time for the Sc-TiO2-AC composites. In general, crystallite size of TiO2 is known to affect its photocatalytic activity and smaller TiO2 crystallite exhibits better photocatalytic activity for many organic compounds [11, 12]. Meanwhile, it is also known that TiO2 with lower crystallinity shows lower photocatalytic activity [2]. This is why the Sc-TiO2-AC composite prepared for 1 min cannot attain the highest RDR even though it has smallest crystallite size, compared with those prepared for longer reaction time.
Photocatalytic decomposition pathways for acetaldehyde by the Sc-TiO2-AC composites
Gaseous compounds produced during UV irradiation can be analyzed by GC-MS during the evaluation of the photocatalytic activities of sample. However, liquid compounds are not measurable because those compounds exist on the surface of the sample. Therefore, the sample was washed with ethanol after UV irradiation. By GC-MS analysis on ethanol after washing the sample irradiated by UV light for 100 min, acetic acid, methanol and formaldehyde could be identified, which were thought to be adsorbed on the surface of the sample. In previous papers, acetic acid was found to be an intermediate in the photocatalytic decomposition of acetaldehyde with TiO2 [13, 14], and the mechanism of photocatalytic decomposition for acetaldehyde through acetic acid as an intermediate was proposed. Acetic acid found in this study is, therefore, thought to be one of the intermediates in the process of photocatalytic decomposition for acetaldehyde.
Gaseous methanol and formaldehyde could be analyzed quantitatively by GC-MS during the evaluation of the photocatalytic activities for acetaldehyde as shown in Fig. 3, while gaseous acetic acid could not be detected. Both methanol and formaldehyde increased with UV irradiation and reached their maximum concentrations after 100 min of UV irradiation, and then disappeared completely at about 400 min of the UV irradiation that may be subsequently decomposed into CO2. The concentrations of methanol and formaldehyde detected were relatively low. It is probably due that they exist partly in gas phase, but they were mainly adsorbed on the surface of the sample and decomposed quickly to CO2 with UV irradiation. These results indicate that methanol and formaldehyde are also intermediates of acetaldehyde decomposition.
Mechanism of photocatalytic decomposition for acetaldehyde by the Sc-TiO2-AC composites
When UV light with shorter wavelength than 380 nm irradiates the TiO2 photocatalyst, the highly mobile hole-electron pairs can be created. In the absence of the hole and electron scavengers, most of them recombine with each other within a few nanoseconds. If the scavengers or surface defects are present to trap the holes or electrons, the recombination can be prevented and the subsequent photocatalytic reactions caused by the hole-electron may be dramatically enhanced [15]. Commonly, holes were trapped by water molecules adsorbed on the surface to produce hydroxyl radicals (OH·) which have strong oxidative ability for many organic substances and play an important role in the photocatalysis. Meanwhile, oxygen as scavengers for electrons also can play a significant role in the reaction with electrons, and O −2 , O· and O− being the products of this reaction can take part in the next oxidation and reduction reactions [16, 17].
However, even though water or oxygen was absent in the photocatalytic reaction system in this study, the Sc-TiO2-AC composites showed the good effects of photocatalytic decomposition on acetaldehyde. To elucidate the photocatalytic mechanism of the Sc-TiO2-AC composites, fourier-transform infrared (FTIR) and thermal analyses were made on the Sc-TiO2 powders.
Figure 4 shows the FTIR spectrum of the Sc-TiO2 powders. The peaks around 3490 and 1660 cm−1 observed are due to the stretching and bending mode of the OH groups, respectively. This shows that Sc-TiO2 has OH groups in itself. However, no any peaks that belong to isopropoxy groups from tetraisopropyl titanate were found. Figure 5 shows thermogravimetric (TG) and differential thermal analysis (DTA) curves of the Sc-TiO2 powders. The large endothermic peak at 100 °C in DTA curve with much weight loss in TG curve belongs to the dehydration of adsorbed water. The exothermic peak at 270 °C accompanied by a little weight loss is not attributable to the decomposition of the isopropoxy groups but that of OH groups in Sc-TiO2. The exothermic peak at 460 °C with no weight loss is probably due to the transformation of anatase-form to rutile-form of TiO2 crystallite. From these results in Figs. 4 and 5, many OH groups are proved to exist on the Sc-TiO2 powders and thus exist on the TiO2 in the Sc-TiO2-AC composites.
These surface OH groups are assumed to be able to trap the photogenerated holes to create hydroxyl radicals, which play an active role in the photocatalytic reaction. With these hydroxyl radicals, the decomposition of acetaldehyde can be initiated. Subsequently, methanol, formaldehyde and acetic acid as intermediates were formed, and then decomposed into carbon dioxide and water as final products. The produced water will be another source for the hydroxyl radicals and further take part in the decomposition for acetaldehyde. Thus the Sc-TiO2-AC composites can have the highly photocatalytic effects for acetaldehyde as shown in Fig. 2 even though both water and oxygen were not added to the reaction system in this study.
Conclusions
The Sc-TiO2-AC composites have highly synergetic effects of adsorption and photocatalytic activities on acetaldehyde. Acetaldehyde can be decomposed into carbon dioxide through the conversion to methanol, formaldehyde and acetic acid as intermediates. Many OH groups exist on the Sc-TiO2-AC composites. They are thought to be able to initiate the photocatalytic reaction for acetaldehyde even in the absent of water and oxygen condition.
References
Xu W, Raftery D (2001) J Phys Chem B 105:4343
Hayashi H, Torii K (2002) J Mater Chem 12:3671
Saka S, Doi M (1998) Mater Sci Res Int 4:249
Takeda N, Torimoto T, Sampath S et al (1995) J Phys Chem 99:9986
Torimoto T, Okawa Y, Takeda N et al (1997) J Photochem Photobiol A: Chem 103:153
Doi M, Saka S, Miyafuji H et al (2000) Mater Sci Res Int 6:15
Huang B, Saka S (2003) J.Wood Sci 49:79
Saka S, Dadan K (2000) Res Process 47:95
“Handbook of X-ray Diffractometer” (Rigaku denki Co., 1999) p 78
Bhuiyan MTR, Hirai N, Sobue N (2001) J Wood Sci 47:336
Zhang Z, Wang C, Zakaria R et al (1998) J Phys Chem B 102:10871
Almquist C B, Biswas P (2002) J Catal 212:145
Sopyan I, Watanabe M, Murasawa S et al (1996) J Photochem Photobio A: Chem 98:79
Muggli DS, Mccue JT, Falconer JL (1998) J Catal 173:470
Park DR, Zhang J, Ikeue K, et al (1999) J Catal 185:114
Pera J, Ollis DF (1992) J Catal 136:554
Fujishima A, Hashimoto K, Watanabe T (1997) In “TiO2 photo-catalysis fundamentals and applications. CMC, Co. Ltd., p 151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, H., Miyafuji, H. & Saka, S. Photocatalytic activities and mechanism of the supercritically treated TiO2-activated carbon composites on decomposition of acetaldehyde. J Mater Sci 41, 8295–8300 (2006). https://doi.org/10.1007/s10853-006-1009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1009-4