Abstract
In this study, TiO2 nanoparticles (NPs)-based catalysts were prepared for the photocatalytic removal of toluene as a model VOC from air under UV light. Expanded graphite (EG) and activated carbon (AC) as two sustainable supports were employed for immobilization of TiO2 NPs by sol–gel technique. In this approach, substrates were added to TiO2 sol–gel and heated up to 60 °C followed by calcination process at 400 °C to afford, EG/TiO2 and AC/TiO2. The studies show that much better results ensued for AC–TiO2 because of higher surface area, good nanoparticle distribution, and lower pore width. The products were characterized by X-ray diffraction, scanning electron microscope, energy dispersive X-ray, as well as N2 adsorption/desorption.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) such as toluene, benzene, xylene, etc. are a group of hydrocarbons emitted from building materials, furnishings, and many industrial processes that are greatly harmful to environment and human (Vandenbroucke et al. 2011; Ojala et al. 2011). Despite their deleterious effects, VOCs are still widely produced, used, and released to the atmosphere (Ojala et al. 2011). This issue has been a major concern for many researchers in recent decades, and they have tried to find efficient and cost-effective technologies for the removal of these compounds from the gas and water streams (Varshney et al. 2016; Pelaez et al. 2013; Virkutyte and Varma 2010; Virkutyte et al. 2010; Virkutyte and Varma 2012a, b; Virkutyte et al. 2012).
Recently, noble metal and metal oxides have been broadly applied for the elimination of VOCs due to their high thermal, chemical, and physical stabilities, high efficiency and activity, large surface area, and strong electron transfer abilities (Park et al. 2019; Sharma et al. 2019). Despite all the advantages, the catalytic activity was decreased due to the agglomeration of the nanoparticles. In fact, the nanoparticles used in the reaction, due to their very small size, cannot be recovered and remain in the reaction environment and caused various constraints. Therefore, to address this problem, heterogeneous catalysts have been developed that include stable support such as carbon nanotubes (CNTs), graphene, Al2O3, Fe3O4, etc. to stabilize the nanoparticles (Goudarzi and Salavati-Niasari 2018; Mahdiani et al. 2017, 2018; Salavati-Niasari and Bazarganipour 2007, 2008, 2009; Salavati-Niasari et al. 2010; Nasrollahzadeh et al. 2018, 2019a, b).
There has been a great deal of interest in oxidation processes using nanoparticle catalysts specially titanium dioxide (TiO2) in many scientific and industrial fields (Yang et al. 2009; Narayanaswamy et al. 2008). This unique semiconductor has attracted considerable attention (Choi et al. 2006; Verma et al. 2016) and has been shown to be one of the most suitable catalysts, having a strong oxidation activity. Moreover, its significant attributes such as chemical inactivity, low cost, non-toxic nature, compatibility with the environment, along with long-term stability and a lower energy band gap (3.02 eV) enabled many practical applications (Wang and Zhou 2011; Yap et al. 2011; Vega et al. 2011). Anatase, rutile, and brookite are prominent phases of TiO2 among which anatase and rutile have been primarily involved in environmental decontaminations (Williams and Kamat 2009). Anatase is usually found to be more active than rutile, but in recent years, the scientific literature suggests that the photocatalytic and photovoltaic properties of mixed phases of TiO2 nanoparticles are much preferred as compared to pure anatase TiO2, reducing electron–hole recombination (Wetchakun and Phanichphant 2008).
In order to improve specific surface area of TiO2 and its affinity toward VOCs molecules (due to the polar structure of the most semiconductors), and thereby attaining much higher activities, attention has been focused to coat it on adsorbent matrixes such as silica, zeolites, as well as carbon-structured materials (Colmenares et al. 2016, 2017) such as activated carbon (AC) and expanded graphite (EG); among these, AC and EG are considered as exceptional supports (Abedi et al. 2015a; Zarezade et al. 2011; Wang et al. 2009a; Arana et al. 2003). Thanks to the unique molecular structure, extremely high affinity for the most of VOCs, large surface area, microporous structure, availability in various forms and sizes, high adsorption capacity, and low cost, AC can be an ideal substrate (Ghaedi et al. 2011). EG has also a porous structure with typical apparent densities of 0.002–0.01 g cm−3 and has been used in wide-ranging practical applications in many fields such as hydrogen storage, sensors production, catalytic oxidation, biomedical treatment, as well as adsorption (Zhao and Liu 2009). In recent years, the ecological problems arising from the oil spills in the world have caused EG to be considered as a strong and powerful absorbent for water contaminants (Yue et al. 2010). Tsumura et al. studied the effect of TiO2 on decomposition of absorbed heavy oil and found that doping anatase-type TiO2 onto EG could markedly accelerate the decomposition of heavy oil under UV irradiation (Tsumura et al. 2002).
Adsorption has long been used as one of the most successful methods for air and water treatment. However, it does not mineralize the pollutants but simply prevents them from further recycling in the atmosphere. There also exists the problem of saturation and breakthrough with the adsorption process. Combining the both techniques, adsorption and the catalytic oxidation, has been proposed as a hybrid method to overcome some of the drawbacks and thriving performance for the removal of contaminants (Abedi et al. 2015b; Lee et al. 2004; Wang et al. 2009b; Sharma et al. 2019). Furthermore, magnetic, electrochemical, and photocatalytic properties of catalyst can be modified throughout coating some material (Goudarzi and Salavati-Niasari 2018; Mahdiani et al. 2017; Salavati-Niasari and Bazarganipour 2009).
Consequently, during this work, TiO2 nanoparticles were synthesized using sol–gel technique, and carbon-based adsorbents, i.e., activated carbon, and expanded graphite were deployed as supports for TiO2. The study focused on the synthesis and characterization of AC–TiO2 and EG–TiO2 nanocomposites using XRD, SEM, EDX, and BET; the photocatalytic efficiency of AC–TiO2 and EG–TiO2 was investigated under UV radiation for mineralization of toluene as a model compound.
Materials and methods
General
Expandable graphite powder with 300 μm diameter and 30 μm thickness was supplied by Beijing Invention Biology Engineering and New Material Co., China. Activated charcoal having granules with the mean diameter of 1.5 mm was purchased from Merck, Germany. Tetra n-butyl titanate [Ti(OBu)4] used as Ti precursor, PrOH, and acetyl acetone (with purity of 99.8%) as solvent, and toluene (with purity higher than 99%) used as air pollutant, were all obtained from Merck, Germany.
Expanded graphite was prepared using expandable graphite powder which was dried at 90 °C for 12 h, followed by heating up to 1050 °C for just 15 s.
The surface state and structure of the composites were observed by scanning electron microscopy (SEM) (Scam MV 2300, Cam type). Chemical characterization of the prepared nanostructures was also analyzed by dispersive X-ray spectroscopy (EDX). The crystalline nature of the samples was investigated by X-ray diffractometer (Philips powder diffractometer type PW 1373 goniometer) with Cu Kα (λ = 1.5405 Å) radiation source. The diffraction patterns were recorded in the 2θ range of 10°–60° with scanning speed of 2° min−1. The BET surface area was determined using the N2 adsorption data in the relative pressure (P/P0) range of 0.05–0.5 at 77 °K using a BELSORP-max nitrogen adsorption apparatus (Japan Inc.). Furthermore, the desorption isotherm was used to determine the pore size distribution using the density functional theory (DFT) method.
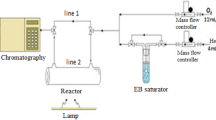
A quartz cylindrical glass tube with 3 cm diameter and 30 cm length was employed as a catalyst reactor. Some amount of glass wool was applied each time at both ends of the tube in order to prevent particles escaping from the inlet and outlet of the reactor. In the center of the reactor, an 8-W lamp with wavelength of 253.7 nm (Philips TUV 8 W/G8 T5 Bulb), 15 mm × 300 mm, was placed axially as a UV radiator. The distance between the lamp and the reactor wall was filled by EG–TiO2 or AC–TiO2 nanocomposites. The setup system consisted of several parts including air supply, air drying, air cleaning, air controlling and measuring, pollutant injection, mixing, decomposition, and analysis sections. A compressor was used to supply normal air in order to dilute the pollutant molecules. This air was subjected to flow through our experimental setup and controlled using several regulators and needle valves. It was then allowed to flow through a ceramic-activated carbon and a cellulosic ester filter to be cleaned from water vapor and any background air contaminants. Afterward, the toluene liquid was injected into the line as air pollutant (300 ppm) through a specified port using a motor-driven syringe pump (SP-510, JMS), from where it was allowed to be mixed inside a mixing chamber and could be accessed from the reactor. A temperature controller was used to keep the temperature of the system at 40 °C, making it possible for the liquid substance to vaporize conveniently. All the setup system was inspected to be thoroughly gastight.
After the steady state conditions were established, the effluent gas was sampled and subjected to analysis using a gas chromatograph (GC/FID, Shimadzu-2010), equipped with a SGE capillary column with the inner diameter of 0.22 mm, film diameter of 0.25 µm, and length of 25 m. A Varian 3800 GC equipped with a Saturn 2200 mass spectroscopy system (GC/MS), with the same column mentioned above, was also used during our experiments. Temperature programming with both GCs was as follows: 80 °C for 1 min and then increased by 12 °C min−1 up to 180 °C and finally kept constant there for 5 min. 100 µl of effluent gas was removed each time using a sampling syringe.
During all experiments, the UV lamp was turned on for 20 min after steady state conditions and then the removal efficiency was analyzed.
Preparation of TiO2
TiO2 particles were prepared through modified sol–gel process using tetra n-butyl titanate as the precursor. Ti(OBu)4 was diluted with PrOH as solvent, and the resultant mixture was stirred at room temperature for 1 h. Acetylacetone (AcAc) as stabilizer was added dropwise to the mixed solution under stirring, and the resultant mixture stirred for another 2 h. Afterward, HNO3 aqueous solution (2 or 3 droplets of HNO3 in 1.8 ml of H2O) was added under stirring to ensure that the pH of sol–gel solution maintained around 2. Finally, the solution was heated under reflux at 75 °C for 8 h to obtain a colorless crystalline sol. At this stage, the temperature was kept constant and strictly controlled to avoid instant gelation. The volume ratios of PrOH, H2O, and AcAc to Ti(OBu)4 were 40, 100, and 1, respectively (Hu et al. 2009).
Preparation of AC–TiO2 and EG–TiO2 nanocomposites
For the preparation of AC–TiO2 and EG–TiO2 nanocomposites, AC and EG were firstly added to TiO2 sol followed by subjecting the mixtures to sonication in an ultrasound bath for 30 min and the contents were maintained at room temperature for 2 days. Afterward, the mixtures were filtered and then dried at 60 °C for 5 h. The samples were finally calcined at 400 °C for 2 h. The flowcharts of TiO2 sol, AC–TiO2, and EG–TiO2 nanocomposites, in terms of preparation procedure sequences, are presented, in Fig. 1a–c, respectively. Image of synthesized materials was taken by digital camera and included in Fig. 2.
Results and discussion
The EG–TiO2 and AC–TiO2 nanocomposites were synthesized and then characterized using several techniques in order to determine how the structure of composites affects their catalytic activity under UV irradiation. It has been recognized in the literature that the catalytic activity of a catalyst can be influenced by many factors such as adsorption properties and surface area of the substrate, Ti amount, pore size, TiO2 crystalline phase etc. (Arana et al. 2003; Jafari et al. 2011; Jaleh and Shahbazi 2014; Yu et al. 2002). In keeping with these reports, the synthesis and characterization of EG–TiO2 and AC–TiO2 complexes were carried out.
Surface analysis of composites
The morphologies of EG and AC, before and after TiO2 coating, are provided in Fig. 3. Lamellar structure of EG is shown in Fig. 3a, and the pore structure of EG is found to be distributed uniformly, having a large number of thin and flat layers. The loaded TiO2 particles could also be clearly observed over the EG substrate in Fig. 3b. The particles are in nano-scale range but have different shapes and sizes as depicted in Fig. 3b, the dispersion and distribution of TiO2 particles being not uniform. Zhang et al. (2005) reported that more reactive sites can be expected in catalysts with higher particle distribution degrees. It can also be seen from the figure that the morphology of EG after TiO2 coating was dramatically changed; some of the thin layers disappeared, joining and sticking together. The surface macroscopic alteration can be attributed to two factors; the wet process of sol–gel technique and the calcinations temperature. Due to the soft and fragile nature of EG, the sol–gel wet process can affect its structure adversely, reducing the surface area significantly. Although the calcination process can positively influence catalyst properties, it can also be considered as a detrimental factor for catalyst substrate and even catalyst phase transition (Ao and Lee 2005). In Fig. 3c, AC has a relatively smooth surface, having many holes and cavities along it. However, after deposition and calcination, TiO2 particles are well attached and dispersed over the AC surface, making it much uniform and smooth (Fig. 3d). The distribution of TiO2 particles over AC is also more uniform as compared to EG surface. According to Afanasov et al. (2009), the distribution pattern can have a considerable impact on the pollutant removal efficiency, since the contaminant molecules firstly adsorbed on substrate surface and then transferred to the nanoparticles for decomposition.
Elemental and pore size analysis
A quantitative EDX spectrum was obtained using SEM–EDX line scan technique to determine the elemental composition of samples. The analysis provides the percent by weight (wt%) and the percent number of atoms (at.%) of each identified element which are listed in Table 1 for EG–TiO2 and AC–TiO2. The results show that the nanocomposites comprise C, O, and Ti. Carbon atoms are the dominant element in both EG–TiO2 and AC–TiO2 samples accompanied by the O atoms in the second place followed by the minor quantities of Ti and with no detection of any other impurity. Surprisingly, nearly the same amount of Ti was detected in both EG–TiO2 and AC–TiO2, which was not supported by the primary examination of SEM images (Fig. 3b, d); aggregation may well be the reason for this discrepancy. A significant shrinkage has been observed in EG structure after exposing to the wet medium of sol–gel, leading the TiO2 particles to be accumulated on some parts of EG surface. Furthermore, as Table 1 shows, the % of O was also different in the two nanocomposites, much higher in AC–TiO2. According to Szymański et al. (2002), substrate surface carboxylic groups can strongly participate in catalytic destruction of hydrocarbons (Abedi et al. 2014). Therefore, an optimum concentration of O would be highly valued.
The samples were also subjected to nitrogen adsorption–desorption measurements in order to characterize the pore structures of two nanocomposites, and results are shown in Fig. 4; all samples, e.g., EG, EG–TiO2, AC, and AC–TiO2 display an IV-type isotherm accompanied by the presence of hysteresis loops, indicating mesoporous structure patterns. The BET specific surface area and the total pore volume of bare substrates and nanocomposites are shown in Table 2.
As expected, the volume of AC–TiO2 is slightly lower than that of AC, indicating the deposition of TiO2 onto the template pores. For EG–TiO2, this volume reduction was more noticeable which can be explained by the EG shrinkage as a result of introduction into the aqueous medium of sol.
The pore size distribution of samples was calculated by density functional theory (DFT) method (Fig. 5), which totally supported the BET results. From Fig. 5, it can be concluded that TiO2 coating can lead to the pore width reduction especially for EG–TiO2. It appears that all points of the two curves (AC and AC–TiO2) coincide in the Fig. 5, implying no deposition of TiO2 into AC, but with a magnification view, a difference of 135–190 nm appears between the two curves which can be attributed to a thin layer of TiO2 nanoparticles. The pore width for EG–TiO2 obtained in the range between 4 and 25 nm, while the distribution was much less for AC–TiO2 (1–8 nm). The last result can strongly affect the catalytic activity rate.
Structural analysis
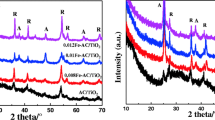
Figure 6 presents the X-ray diffraction patterns of some selected samples. The diffractogram shows the characteristic peaks of EG (2θ = 26.4°), and AC (2θ = 26) which were accompanied by a mixture of anatase and rutile TiO2 peaks in different amounts, locating at 2θ = 25.1°, 37.6°, 47.7°, 54.8°, 59.8°, and 2θ = 27.1°, 41.0°, 62.6°, respectively. An overlap is seen from Fig. 6 between the main peak of AC and that of anatase TiO2 at 2θ = 25.1 corresponding to the (101) plane, which is also considered to be the main peak of TiO2. Due to the presence of substrate species and also badly crystallized TiO2, a non-smooth diffractogram shape was obtained for all samples. According to Addamo et al. (2004), the phase type and properties of nanoparticles depend strongly on preparation conditions such as sol preparation sequence and calcination temperature; calcination temperatures higher than 400 °C may specially lead to the formation of much better crystallized anatase TiO2. But, at the same time, higher temperatures result in higher aggregation and thereby reducing surface area of nanoparticles and also loss of TiO2 surface hydroxyl groups which is fully destructive for catalytic activities.
Toluene mineralization
The effect of catalyst was investigated on toluene conversion in the presence of UV light, and our investigations were performed with both bare substrates and substrates coated by TiO2.
Assuming the production of CO2 and H2O, as well as oxygen and hydroxyl radicals as dominant agents in toluene mineralization, the possible pathway is depicted in Fig. 7.
Figure 8 shows the photocatalytic activity of EG/TiO2 and AC/TiO2 for the degradation of toluene as a function of time; a relatively low activity was observed in removal efficiency (RE) after 2 min of UV radiation, and it then picked up after 5 min, rocketing by almost 60% for AC/TiO2. Furthermore, in the presence of AC/TiO2 total oxidation of toluene was obtained at 20 min of UV exposure. As far as the EG/TiO2 is concerned, the first signs of activation appeared after 5 min under UV radiation, and no catalytic behavior is seen from the figure at lesser times. From this point onwards, a gradual enhancement was observed in the catalytic activity, and nearly the total decomposition of toluene was achieved after 30 min.
As can be seen from Fig. 8, AC/TiO2 catalyst was more active relative to EG/TiO2 which may be attributed to the stronger adsorption ability of AC. As Fig. 8 represents, prior to TiO2 impregnation, the toluene breakthrough was much higher with the bare EG, indicating the greater capacity of AC to adsorb toluene. This may be more significant with prepared catalysts because the structure of EG/TiO2 appeared to be much denser compared to the structure of bare EG, meaning a lower surface area for EG/TiO2, while no significant change occurred with the AC after impregnation.
It has been widely recognized that catalyst support can influence the catalytic performance significantly (Ao and Lee 2003; Ao et al. 2008). Ao and Lee (2003) prepared TiO2 anatase loaded on AC and used it as catalyst for decomposition of phenols wherein phenol compounds were adsorbed on AC initially and then moved to the surface of TiO2 continuously which improved the catalytic oxidation remarkably. In agreement with this, our findings also showed that the effect of catalyst template can be significant, and a high level of catalytic activity was observed during the present work. The damaging effects of calcination could be counteracted by higher rates of adsorption and providing more organic compounds close to nanoparticles as well as the dispersion of TiO2 particles on the surface of AC (Li et al. 2007).
A strong relationship was observed between the time and removal efficiency (RE), and the trend for RE is obviously upwards in all cases regardless of the catalyst type (Fig. 8). Total oxidation of toluene was obtained after 20 min and 30 min with AC/TiO2 and EG/TiO2, respectively. Compared to the earlier studies (Schmidt-Szałowski et al. 2011; Yang et al. 2010; Zeng et al. 2010), much lesser time is needed to reach a stable and high catalyst activity, especially using AC/TiO2. Notably, the level of activation with AC/TiO2 was considerably high at 5 min, while no significant activity was observed with EG/TiO2. This can be probably explained by the thorough dispersion of nanoparticles over AC surface as confirmed by SEM. As we surmised previously, a good distribution of TiO2 particles was obtained on AC, while the pattern for EG was sporadic and was not much favorable. With higher quantities of nanoparticles dispersion over the substrate, much more hydroxyl groups could be available for redox reactions (Mul et al. 2001). Furthermore, a greater degree of aggregation estimated for EG/TiO2 can be another reason for its lower RE.
Conclusion
The EG–TiO2 and AC–TiO2 were successfully prepared by sol–gel method with the morphology for EG and AC, as observed by SEM analysis, being lamellar and smooth, respectively; TiO2 was well deposited on the surface of substrates. The XRD analysis confirmed that TiO2 supported on carbon bases contains two different phases: anatase and rutile. Moreover, the EDX analysis showed that the elements are C, O, and Ti and no other elemental impurities were discerned. AC–TiO2 showed a high catalytic activity, and nearly 100% of toluene was converted on its surface after 20 min of UV radiation. The catalytic activity of EG–TiO2 was very strong after 30 min, while not appreciable at lesser exposure times. We anticipate this study will help researchers design the appropriate preparative procedure and its processing, including calcination to attain the desirable traits for supported TiO2.
References
Abedi K, Ghorbani-Shahna F, Jaleh B, Bahrami A, Yarahmadi R (2014) Enhanced performance of non-thermal plasma coupled with TiO2/GAC for decomposition of chlorinated organic compounds: influence of a hydrogen-rich substance. J Environ Health Sci Eng 12:119
Abedi K, Ghorbani-Shahna F, Bahrami A, Jaleh B, Yarammadi R (2015a) Effect of TiO2–ZnO/GAC on by-product distribution of CVOCs decomposition in a NTP-assisted catalysis system. Pol J Chem Technol 17:32–40
Abedi K, Ghorbani-Shahna F, Jaleh B, Bahrami A, Yarahmadi R, Haddadi R, Gandomi M (2015b) Decomposition of chlorinated volatile organic compounds (CVOCs) using NTP coupled with TiO2/GAC, ZnO/GAC, and TiO2–ZnO/GAC in a plasma-assisted catalysis system. J Electrostat 73:80–88
Addamo M, Augugliaro V, Dipaola A, García-López E, Loddo V, Marcí G, Molinari R, Palmisano L, Schiavello M (2004) Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 catalysts. J Phys Chem B 108:3303–3310
Afanasov I, Shornikova O, Avdeev V, Lebedev O, Van Tendeloo G, Matveev A (2009) Expanded graphite as a support for Ni/carbon composites. Carbon 47:513–518
Ao C, Lee S (2003) Enhancement effect of TiO2 immobilized on activated carbon filter for the photodegradation of pollutants at typical indoor air level. Appl Catal B Environ 44:191–205
Ao C, Lee S (2005) Indoor air purification by photocatalyst TiO2 immobilized on an activated carbon filter installed in an air cleaner. Chem Eng Sci 60:103–109
Ao Y, Xu J, Fu D, Shen X, Yuan C (2008) Low temperature preparation of anatase TiO2-coated activated carbon. Colloids Surf A Physicochem Eng Asp 312:125–130
Arana J, Doña-Rodrı́ J, Rendón ET, Icabo CG, González-Dı́ O, Herrera-Melián J, Perez-Pena J, Colón G, Naví J (2003) TiO2 activation by using activated carbon as a support: part I. Surface characterisation and decantability study. Appl Catal B Environ 44:161–172
Choi H, Kim YJ, Varma RS, Dionysiou DD (2006) Thermally stable nanocrystalline TiO2 photocatalysts synthesized via sol–gel methods modified with ionic liquid and surfactant molecules. Chem Mater 18:5377–5384
Colmenares JC, Varma RS, Lisowski P (2016) Sustainable hybrid photocatalysts: titania immobilized on carbon materials derived from renewable and biodegradable resources. Green Chem 18:5736–5750
Colmenares JC, Varma RS, Nair V (2017) Selective photocatalysis of lignin-inspired chemicals by integrating hybrid nanocatalysis in microfluidic reactors. Chem Soc Rev 46:6675–6686
Ghaedi M, Tavallali H, Montazerozohori M, Mousavi SD, Khodadoust S, Soylak M (2011) A novel nanometer pore size acorn based activated carbon modified with 2-(4-methoxybenzylideneamino) thiophenol for the preconcentration of copper and zinc ions and their determination in some food samples. Fresen Environ Bull 20:2785–2793
Goudarzi M, Salavati-Niasari M (2018) Using pomegranate peel powders as a new capping agent for synthesis of CuO/ZnO/Al2O3 nanostructures: enhancement of visible light photocatalytic activity. Int J Hydrog Energy 43(31):14406–14416
Hu CY, Xu YJ, Duo SW, Zhang RF, Li MS (2009) Non-covalent functionalization of carbon nanotubes with surfactants and polymers. J Chin Chem Soc 56:234–239
Jafari S, Azizian S, Jaleh B (2011) Adsorption kinetics of methyl violet onto TiO2 nanoparticles with different phases. Colloids Surf A Physicochem Eng Asp 384:618–623
Jaleh B, Shahbazi N (2014) Surface properties of UV irradiated PC–TiO2 nanocomposite film. Appl Surf Sci 313:251–258
Lee DK, Kim SC, Cho IC, Kim SJ, Kim SW (2004) Photocatalytic oxidation of microcystin-LR in a fluidized bed reactor having TiO2-coated activated carbon. Sep Purif Technol 34:59–66
Li Y, Zhang S, Yu Q, Yin W (2007) The effects of activated carbon supports on the structure and properties of TiO2 nanoparticles prepared by a sol–gel method. Appl Surf Sci 253:9254–9258
Mahdiani M, Sobhani A, Salavati-Niasari M (2017) Enhancement of magnetic, electrochemical and photocatalytic properties of lead hexaferrites with coating graphene and CNT: sol–gel auto-combustion synthesis by valine. Sep Purif Technol 185:140–148
Mahdiani M, Soofivand F, Ansari F, Salavati-Niasari M (2018) Grafting of CuFe12O19 nanoparticles on CNT and graphene: eco-friendly synthesis, characterization and photocatalytic activity. J Clean Prod 176:1185–1197
Mul G, Zwijnenburg A, Van Der Linden B, Makkee M, Moulijn JA (2001) Stability and selectivity of Au/TiO2 and Au/TiO2/SiO2 catalysts in propene epoxidation: an in situ FT-IR study. J Catal 201:128–137
Narayanaswamy A, Mcbride J, Swafford LA, Dhar S, Budai JD, Feldman LC, Rosenthal SJ (2008) Synthesis and characterization of porous TiO2 with wormhole-like framework structure. J Porous Mater 15:21–27
Nasrollahzadeh M, Issaabadi Z, Sajadi SM (2018) Fe3O4@SiO2 nanoparticle supported ionic liquid for green synthesis of antibacterially active 1-carbamoyl-1-phenylureas in water. RSC Adv 8(49):27631–27644
Nasrollahzadeh M, Issaabadi Z, Sajadi SM (2019a) Green synthesis of Cu/Al2O3 nanoparticles as efficient and recyclable catalyst for reduction of 2,4-dinitrophenylhydrazine, Methylene blue and Congo red. Compos B Eng 166:112–119
Nasrollahzadeh M, Issaabadi Z, Sajadi SM (2019b) Green synthesis of the Ag/Al2O3 nanoparticles using Bryonia alba leaf extract and their catalytic application for the degradation of organic pollutants. J Mater Sci Mater Electron 30(4):3847–3859
Ojala S, Pitkäaho S, Laitinen T, Koivikko NN, Brahmi R, Gaálová J, Matejova L, Kucherov A, Päivärinta S, Hirschmann C (2011) Catalysis in VOC abatement. Top Catal 54:1224–1256
Park SJ, Das GS, Schütt F, Adelung R, Mishra YK, Tripathi KM, Kim T (2019) Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater 11(1):8
Pelaez M, Baruwati B, Varma RS, Luque R, Dionysiou DD (2013) Microcystin-LR removal from aqueous solutions using a magnetically separable composite N-doped TiO2 catalyst under visible light irradiation. Chem Commun 49:10118–10120
Salavati-Niasari M, Bazarganipour M (2007) Effect of single-wall carbon nanotubes on direct epoxidation of cyclohexene catalyzed by new derivatives of cis-dioxomolybdenum (VI) complexes with bis-bidentate Schiff-base containing aromatic nitrogen-nitrogen linkers. J Mol Catal A Chem 278(1–2):173–180
Salavati-Niasari M, Bazarganipour M (2008) Covalent functionalization of multi-wall carbon nanotubes (MWNTs) by nickel (II) Schiff-base complex: synthesis, characterization and liquid phase oxidation of phenol with hydrogen peroxide. Appl Surf Sci 255(5):2963–2970
Salavati-Niasari M, Bazarganipour M (2009) Synthesis, characterization and catalytic oxidation properties of multi-wall carbon nanotubes with a covalently attached copper (II) salen complex. Appl Surf Sci 255(17):7610–7617
Salavati-Niasari M, Davar F, Bazarganipour M (2010) Synthesis, characterization and catalytic oxidation of para-xylene by a manganese (III) Schiff base complex on functionalized multi-wall carbon nanotubes (MWNTs). Dalton Trans 39(31):7330–7337
Schmidt-Szałowski K, Krawczyk K, Sentek J, Ulejczyk B, Górska A, Młotek M (2011) Hybrid plasma-catalytic systems for converting substances of high stability, greenhouse gases and VOC. Chem Eng Res Des 89:2643–2651
Sharma M, Joshi M, Nigam S, Shree S, Avasthi DK, Adelung R, Srivastava SK, Mishra YK (2019) ZnO tetrapods and activated carbon based hybrid composite: adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution. Chem Eng J 358:540–551
Szymański GS, Karpiński Z, Biniak S, Światkowski A (2002) The effect of the gradual thermal decomposition of surface oxygen species on the chemical and catalytic properties of oxidized activated carbon. Carbon 40:2627–2639
Tsumura T, Kojitani N, Umemura H, Toyoda M, Inagaki M (2002) Composites between photoactive anatase-type TiO2 an adsorptive carbon. Appl Surf Sci 196:429–436
Vandenbroucke AM, Morent R, De Geyter N, Leys C (2011) Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J Hazard Mater 195:30–54
Varshney G, Kanel SR, Kempisty D, Varshney V, Agrawal A, Sahle-Demessie E, Varma RS, Nadagouda MN (2016) Nanoscale TiO2 films and their application in remediation of organic pollutants. Coord Chem Rev 306:43–64
Vega AA, Imoberdorf GE, Mohseni M (2011) Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid in a fluidized bed photoreactor with composite template-free TiO2 photocatalyst. Appl Catal A Gen 405:120–128
Verma S, Nasir Baig RB, Nadagouda MN, Varma RS (2016) Titanium-based zeolitic imidazolate framework: chemical fixation of carbon dioxide. Green Chem 18:4855–4858
Virkutyte J, Varma RS (2010) Fabrication and visible-light photocatalytic activity of novel Ag/TiO2−xNx photocatalyst. New J Chem 34:1094–1096
Virkutyte J, Varma RS (2012a) Synthesis and visible light photoactivity of anatase Ag and garlic loaded TiO2 nanocrystalline catalyst. RSC Adv 2:2399–2407
Virkutyte J, Varma RS (2012b) Visible light activity of Ag-loaded and guanidine nitrate-doped nano-TiO2: degradation of dichlorophenol and antibacterial properties. RSC Adv 2:1533–1539
Virkutyte J, Baruwati B, Varma RS (2010) Visible light induced photobleaching of methylene blue over melamine doped TiO2 nanocatalyst. Nanoscale 2:1109–1111
Virkutyte J, Jegatheesan V, Varma RS (2012) Visible light activated TiO2/microcrystalline cellulose nanocatalyst to destroy organic contaminants in water. Bioresour Technol 113:288–293
Wang S, Zhou S (2011) Photodegradation of methyl orange by photocatalyst of CNTs/P-TiO2 under UV and visible-light irradiation. J Hazard Mater 185:77–85
Wang Q, Liang X, Qiao W, Liu C, Liu X, Zhan L, Ling L (2009a) Preparation of polystyrene-based activated carbon spheres with high surface area and their adsorption to dibenzothiophene. Fuel Process Technol 90:381–387
Wang X, Liu Y, Hu Z, Chen Y, Liu W, Zhao G (2009b) Degradation of methyl orange by composite photocatalysts nano-TiO2 immobilized on activated carbons of different porosities. J Hazard Mater 169:1061–1067
Wetchakun N, Phanichphant S (2008) Effect of temperature on the degree of anatase-rutile transformation in titanium dioxide nanoparticles synthesized by the modified sol–gel method. Curr Appl Phys 8:343–346
Williams G, Kamat PV (2009) Graphene-semiconductor nanocomposites: excited-state interactions between ZnO nanoparticles and graphene oxide. Langmuir 25:13869–13873
Yang X, Zhang X, Ma Y, Huang Y, Wang Y, Chen Y (2009) Superparamagnetic graphene oxide-Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem 19:2710–2714
Yang L, Luo S, Li Y, Xiao Y, Kang Q, Cai Q (2010) High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 pn heterojunction network catalyst. Environ Sci Technol 44:7641–7646
Yap PS, Lim TT, Srinivasan M (2011) Nitrogen-doped TiO2/AC bi-functional composite prepared by two-stage calcination for enhanced synergistic removal of hydrophobic pollutant using solar irradiation. Catal Today 161:46–52
Yu JC, Yu J, Ho W, Jiang Z, Zhang L (2002) Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem Mater 14:3808–3816
Yue X, Zhang R, Zhang F, Wang L (2010) Decomposition of crude oil absorbed into expanded graphite/TiO2/NiO composites. Desalination 252:163–166
Zarezade M, Ghasemi S, Gholami MR (2011) The effect of multiwalled carbon nanotubes and activated carbon on the morphology and photocatalytic activity of TiO2/C hybrid materials. Catal Sci Technol 1:279–284
Zeng J, Liu S, Cai J, Zhang L (2010) TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. J Phys Chem C 114:7806–7811
Zhang X, Zhou M, Lei L (2005) Preparation of photocatalytic TiO2 coatings of nanosized particles on activated carbon by AP-MOCVD. Carbon 43:1700–1708
Zhao M, Liu P (2009) Adsorption of methylene blue from aqueous solutions by modified expanded graphite powder. Desalination 249:331–336
Acknowledgements
The authors would like to thank the physics department of Bu-Ali Sina University for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirm that no conflict of interest arise with regard to the research leading to this paper, nor with publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaleh, B., Gandomi Rouzbahani, M., Abedi, K. et al. Photocatalytic decomposition of VOCs by AC–TiO2 and EG–TiO2 nanocomposites. Clean Techn Environ Policy 21, 1259–1268 (2019). https://doi.org/10.1007/s10098-019-01702-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-019-01702-3