Abstract
In this paper the results of secondary phases determination in a SAF 2205 duplex steel, heat treated at 780, 850 and 900 °C for 10–40 min, are presented. χ-phase is the first phase to precipitate at all the temperatures, followed by σ-phase. The main drop of toughness is related to very low contents of χ-phase and σ-phase. Hardness is not a sensitive parameter to low amounts of secondary phases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Duplex stainless steels are ferritic-austenitic steels that combine good mechanical and corrosion properties. However, owing to their susceptibility to formation of dangerous intermetallic phases, the use of duplex stainless steels have to be restricted, especially in the temperature range over 500 °C. Generally this embrittlement is attributed mainly to the formation of σ-phase, but also to other intermetallic phases such as χ and R-phase as well as carbides and nitrides [1].
Therefore, the formation of the dangerous phases and their influence on the toughness of duplex stainless steels (DSS) have been extensively examined [2 – 4].
However, at now, there is not a complete and definitive description of the secondary phases formation in DDS and several different precipitation sequences of various intermetallic phases (σ-phase, χ-phase and carbides) have been suggested. The sequence more frequently indicated [2 , 4–7], also in the most recent researches, consist in the precipitation of σ-phase occurring before the precipitation of χ-phase, sometimes followed by carbides.
Other authors [8 , 9] suggest that the precipitation of σ-phase is associated with the precipitation of χ-phase, meaning that χ particles precipitate before σ particles and only few authors detect the carbides as the first phase [10 – 12].
In all cases the precipitation occurs at the α/γ interfaces. Some discrepancies of results could derive from differences in thermal aging procedures, or from the different metallographic techniques used for secondary phase identification. Perhaps this is due to the difficulty in separately quantifying the volume fractions of σ and χ, by means of image analysis on optical micrographs.
It has been demonstrated that, over a certain amount (between 5 and 10%) of σ-phase, the toughness of duplex steels is reduced to values too low for practical applications, but some features of the early stages of precipitation are not yet well understood. Recently it has been suggested [2] that also contents not detectable by metallographic techniques can determine an abrupt decrease of the toughness.
Therefore many standards, relating to manufacturing and welding of DSS, require “no intermetallic phases” in the microstructure. Consequently the question arises on the choice of the technique right for defining a DSS “without intermetallic phases”, because OM, SEM or TEM allow to recognize the dangerous phases precipitation at different sensitivity levels.
Usually the embrittlement effect is measured by reporting the impact energy decrease versus ageing temperature and time. However, it is well known that the kinetics of intermetallic precipitation is markedly affected both by the plastic deformation and by the temperature of the solution treatment. Therefore, the critical time for σ, and other phase formation, seems to be dependent both on the solution treatment temperature and on the cooling rate of the subsequent quenching.
Besides the presence and the content of the intermetallic phases, it was suggested [2] that also their morphology could play a role in the embrittlement. Clearly the influence of a number of small precipitates at the grain boundaries should be different from that of fewer, coarse particles. The morphology depends on nucleation and growth conditions, i.e. on temperature and time of aging.
The relationship between all these various phases and the initial abrupt impact toughness reduction still have to be investigated in depth. Therefore, the attempt to obtain further information on the correlation between the type and the content of the various intermetallic phases with the toughness of the steel after aging would seem to be useful.
In a previous paper [13], the authors verified that backscattered electron microscopy (SEM-BSE), on unetched samples, is more accurate than OM in detecting and measuring secondary phases with image analysis. The measurements of secondary phases, with OM after etching with different reagents (Murakami, Groesbeck) and SEM-BSE on the same area of a sample, evidenced that the effects of the etching reagents depend on the amount of secondary phases, with overestimation of low quantities and underestimation of high amounts.
In samples with low content of secondary phases the corrosion products of the etchant enhance the contours and the image analysis overestimates the areas, while in samples with high amounts of σ and χ phases, they are not completely etched and the image analysis underestimates the corresponding areas.
The SEM-BSE method is based on the atomic number contrast: the secondary phases are brighter than ferrite and austenite, according to their content of element with high atomic number (Z), as Mo and Cr.
The different Mo concentrations of χ (11–14%) and σ (7–9%) allow each phase to be accurately identified, especially at the early stages of precipitation.
The present investigation is aimed to determine separately the amount of χ and σ-phase, in order to describe the sequence and kinetic of the early stages of precipitation and to correlate the obtained data with the Charpy impact test results for a 2205 DSS after isothermal aging, in the critical temperature range 780–900 °C. A discussion on the effect of small amounts of secondary phases on the impact toughness is presented.
Experimental method
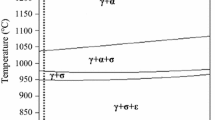
The as received material was a wrought 2205 DSS rod (30 mm) in the solution-annealed condition (1050 °C for 30 min., W.Q.), with composition reported in Table 1.
Isothermal ageing treatments of annealed specimens were carried out in the conditions reported in Table 2.
The relatively short ageing times have been chosen as we are interested in measuring low amounts of secondary phases in order to investigate their precipitation kinetics and effects on mechanical properties.
Different phases have been identified by SEM examination of unetched samples, using the backscattered electron (BSE) signal, on the basis of atomic number contrast effect: the ferrite appears slightly darker than austenite, while the secondary phases are lighter. The amounts of secondary phases have been determined using image analysis software on SEM-BSE micrographs (10 fields, 1000×). The contribution of each phase to total volume fractions was determined. The SEM operated at 25 kV; the BSE detector was set to maximize the atomic number contrast, allowing ferrite, austenite and secondary phases to be identified. The chemical composition of the phases was determined by SEM-EDS on unetched samples.
The volume fractions of ferrite and austenite in a solution treated sample have been measured on three longitudinal and three transversal sections (20 fields for each section) by image analysis on light micrographs at 200×, after etching with the Beraha reagent (reaction time, 10 s).
In order to examine the morphology of the precipitates with SEM-SE the samples were etched with a solution of 30 ml HCl, 30 ml HNO3, 45 ml Glycerol and 2 drops of HF.
Instrumented Charpy–V impact specimens were prepared in the standard form of 10 × 10 × 55 mm3. Impact test was carried out at room temperature.
The hardness test has been performed with a Vickers micro indenter with a load of 0.3 kg.
Results and discussion
Solution treated material
The banded structure of elongated γ islands is observed in the longitudinal section, while the isotropic structure of ferrite and austenite grains is displayed on the transverse section. No secondary phases were detected. The values of volume fractions of ferrite and austenite, measured on longitudinal and transverse sections (200×), are reported in Table 3.
The differences between transversal and longitudinal data are less than individual standard deviations. All the quantifications were performed on the transverse sections. The ferrite and austenite volume fractions are typical for a 2205 alloy in the solution treated condition.
Heat-treated samples
Microstructure
In the SEM-BSE images ferrite and austenite appear in the background: with the ferrite darker than austenite. The secondary phases appear as small bright regions, with the χ-phase brighter than σ.
780 °C ageing: The first precipitates appear after 30′ ageing and become more evident after 40′, as in Fig. 1. The small bright particles were identified as χ by the EDS (Table 4), just within the beam resolution limit.
850 °C ageing: χ-phase appears after about 10′, while the σ-phase after about 20′: in any case at 850 °C the first secondary phase present is the χ-phase. After 25′ the χ and σ phases are both present: χ-phase is always at the boundaries ferrite/austenite and ferrite/ferrite. The σ-phase penetrates the ferrite or grows along the austenite/ferrite boundaries.
900 °C ageing: Also at this temperature the first phase present is the χ-phase, generally decorating the grain boundaries.
By increasing the treating time, the amount of χ increases and also the σ-phase appears, in the form of coarser precipitates at the austenite/ferrite boundary, but growing into the ferrite (Fig. 2).
Although σ particles are, at the beginning, less numerous than χ-phase particles, they are coarser, and grow more rapidly, quickly arriving almost to the same volume fraction. By increasing the holding time, σ-phase grows to large particles, moving from the boundaries into the ferrite, embedding some small χ particles. This seems to show the progressive transformation of χ to σ, occurring at 900 °C (Fig. 3).
In the samples at low treating times (sample treated at 950 °C for 20′), we can observe that the preferential sites, where χ-phase small particles precipitate, are the triple points connections of γ/α boundaries.
The morphology of the two phases is more evident in the secondary electron microscopy SEM-SE micrographs, on etched samples (Figs. 4 and 5), with the typical butterfly sized σ-phase. The localization of the new phases at the ferrite/austenite boundaries is well known [1].
Generally the secondary phases formation at grain boundaries and the growth into ferrite is justified by diffusion behaviour of the elements involved in the transformation: Mo diffusion coefficient is higher than Cr and Ni and is also higher in ferrite than in austenite [4].
Moreover, recent studies assert that at 900 °C χ-phase, formed at the lowest soaking times, is a meta-stable phase [3 , 9] and is completely transformed into σ-phase after a long isothermal ageing. Our results confirm that the transformation of χ to σ is faster at 900 °C, as the diffusion coefficients of Mo and Cr are higher than at 850 °C and the lattice arrangement from cubic χ to tetragonal σ is favoured. These considerations and some SEM observations suggest that χ particles could act as preferential sites for the σ-phase nucleation [1].
In any examined samples no diffuse carbides formation was detected, only small and rare carbide particles were found, generally associated with σ particles, near the ferrite/austenite boundary. This seems to be related to some particular or local situations, not the general feature controlling the formation mechanism of secondary phases. The very low carbon content (C = 0.03wt.%) of the 2205 steel justifies this conclusion.
Composition
The mean compositions of σ-phase and χ-phase, determined by energy dispersive spectrometry (EDS), are reported in Table 4 and are in good agreement with literature data [9], despite the χ particle size is close to the beam resolution. The observed compositions do not seem dependent on both temperature and holding time: variations are inside the statistical errors.
Secondary phases formation kinetic
The possibility to quantify the χ-phase and σ-phase content variation during the holding time allows the description of the kinetic of the precipitation at different temperatures.
However the reliability of the quantitative measurements is largely dependent on the actual phase content. At the beginning of the precipitation it is reasonable to identify the first small particles as σ or χ-phase, but it is more difficult to obtain a reliable quantification of the total content. In 780 °C treated samples the small size of precipitates allows only a qualitative estimation of very low χ-phase contents, approximately less than 0.3%, for holding time higher than 30′ (see Fig. 1). Therefore, for the lowest ageing temperature, 780 °C, it was not possible to define a precipitation kinetic, besides the identification of the χ as the first precipitating phase.
The quantitative measurement of secondary phases amount was actually possible only on samples treated at 850 and 900 °C.
Figures 6 and 7 show the volume fractions of each phase and their sum. As the holding time increases, and whatever the temperatures, the total amount of secondary phases increases, at higher rate at 900 °C, but the trend of the two phases is different at the two considered temperature.
At 850 °C the first precipitates (tiny particles of χ-phase at the grain boundary) appear after 10′ but they are quantitatively measurable only after 20′ of heat treatment. For holding times longer than 25′ the percentages of both phases show a steep increase and for 30′ and 40′ an increment of both formation speed and volumetric fraction is evident. At this temperature the formation kinetic of χ-phase is favoured. After 30′, χ and σ fractions are approximately the same, while after 40′ σ-phase is approximately 1/5 of the entire volumetric fraction.
At 900 °C the percentage of σ-phase increases with time, while the percentage of χ-phase decreases after 30′. After 25′, χ and σ fractions are approximately the same, after 30′ the percentage of σ-phase is approximately three times the percentage of χ-phase, while at 40′ σ-phase becomes 9/10 of the total.
An attempt to analyse the quantitative data on the intermetallic phase precipitation at 850 and 900 °C by an Avrami-type equation was performed:
According to this equation the volume fraction of precipitating phases depends on two parameters: the exponent n, which can vary from ∼1 to 4, and the constant k, which depends on the nucleation and growth rates and therefore on the temperature [ 14 ].
Our results concern only the first stages of the intermetallic phases precipitation, as shown in Fig. 8; in this limited range both the σ and the total intermetallic phases volume fractions can be rather well-fitted according to this exponential law, with n = 4.11 and lnk = −32.31. In the computation the maximum σ-phase content (25 vol.%) is a literature value for a DSS [4].
This result doesn’t appear immediately justified, because the Avrami equation is strictly valid only for transformations where no diffusion of alloying elements is needed, that is without change of composition between the parent and the new phases. This is not a case of intermetallic precipitation in DSS: a transformation that seems to have a general diffusion-controlled growth.
However the agreement of the first stages of precipitation data with an Avrami-type equation could be justified considering that this equation is often valid for the early stages of diffusion controlled transformations, as in the case considered, when the transformation is less or not completely controlled by the diffusion. But another justification can be suggested, considering the essential role which is played in this transformation by the grain boundaries, which could be not only the preferred sites for the new phases nucleation but also the preferred paths of alloying elements diffusion. As it is well known, the diffusion at grain boundaries is faster than general diffusion, and allows a more rapid growth of grain boundaries precipitates. This could justify that the early stages of the intermetallic phases precipitation occur following an Avrami-type equation, strictly valid only for growths not controlled by the general diffusion.
Impact toughness
The influence of secondary phases on toughness was studied through Charpy impact tests carried out at room temperature. An attempt has been made to correlate the toughness to volume fraction of intermetallic phases since impact toughness depends on the amount and types of intermetallic phases.
At 780 °C for an ageing time of 40′, phase quantification (only χ-phase was visible at 2500×) could not be carried out, because the precipitates were too small, but it was verified that impact value was lower than in the as received material.
Figure 9 shows the impact energy values versus secondary phase volume fractions. The solubilized material has mean impact energy of 250 J, but only 0.5% of secondary phases seems sufficient to reduce the value to about 100 J. A further drop occur at 1%, when the impact energy is about 50 J, and the final severe deterioration of toughness is induced by higher values (volume fractions > 1.5–2%).
This statement agrees with the generally accepted specification for the DDS, asking for an intermetallic phases content of less than 1%, or lower, to maintain the toughness value of 40–50 J.
Generally, this deterioration is attributed to σ-phase, but this statement would not seem to be very precise. It is true that, if the σ-phase is present, the toughness is lowered, but our results indicate that the toughness of 2205 steel is already lowered before significant σ content appears. The main drop in toughness occurs at the early stages of precipitation, when the only phase detected is χ-phase, as small and rare particles. Surely, σ is a dangerous phase for the toughness, but it does not seem to be the phase which determines the embrittlement of the DDS steels, especially at very low intermetallic phases content, when the σ could be still virtually absent.
These results about the precipitation of secondary phases support Nilsson [15] and Gunn [2] suggestions about the drop of toughness before any intermetallic phase could be detected by ordinary metallographic techniques.
However, the effect of extremely low precipitation content, about 0.5%, on the toughness cannot be analyzed by metallographic techniques, but studying fracture mechanisms, in order to clarify if such small and rare intermetallic particles could induce the embrittlement.
Hardness
The hardness is quite constant (250 ± 10 HV) until 8% of secondary phases, with a moderate increase (290 HV) in correspondence of 10% volume fraction. This trend is comprehensible considering that the mean hardness of sigma is 800 HV and the effect on steel hardness becomes detectable only at about 10%. It confirms that the hardness is not a sensitive parameter for low amounts of secondary phases, as indicated by Chen [11] and Nilsson [16].
Conclusions
The effects of isothermal treatments in the temperature range 780–900 °C on the microstructure and mechanical properties of SAF 2205 duplex stainless steel can be so summarized:
-
During the isothermal heat treatments, χ-phase is the first intermetallic phase to precipitate, always at the α/γ boundaries: σ-phase appears later on, and gradually substitutes χ-phase;
-
In the early stages, the kinetic of intermetallic phases formation follows an Avrami-type law, suggesting that the growth is favoured by the grain boundaries diffusion;
-
A drastic drop of the toughness is evident at very low intermetallic phases content, about 0.5–1%, even before σ-phase formation, when only few small χ-phase particles can be detected at high magnification (2500×) by metallographic techniques;
-
The low intermetallic phase content, even though it induces the drop in toughness, scarcely affects hardness values.
References
Nilsson JO (1997) in Proceedings of the 5th world conference on duplex stainless steel. KCI Publishing, p 73
Gunn RN (2000) in Proceedings of duplex America. KCI Publishing, p 299
Carlson L, Bengtsson L, Rolander U, Pak S (992) in Proceedings of the stainless steel ‘92 Conference. Institute of Metals, Stockholm, Sweden, p 335
Duprez L., Cooman B.C., Akdut N (2000) in Proceedings of duplex America. Italian Association of Metallurgy, AIM, Milano, p 355
Kim SB, Paik KW, Kim YG (1998) Mater Sci Eng A247:67
Jiang ZL, Chen XY, Huang H, Liu XY (2003) Mater Sci Eng A363:263
Johnson E, Kim YJ, Scott Chumbley L, Gleeson B (2004) Scr Mater 50:1351
Ahn YS, Kang JP (2000) Mater Sci Technol 16:382
Chen TH, Weng KL, Yang JR (2002) Mater Sci Eng A338:259
Shek CH, Shen GJ, Lai JKL, Duggan BJ (1994) Mater Sci Technol 10:306
Lee KM, Cho HS, Choi DC (1999) J Alloys Comp 285:156
Chen TH, Yang JR (2001) Mater Sci Eng A311:28
Calliari I., Magrini M., Ramous E. (2005) Praktische Metallographie 42:74
Duprez L, Cooman BC, Akdut N (2000) Mater Technol Steel Res 10:417
Nilsson JO (1992) Mater Sci Technol 8:685
Nilsson JO, Kangas P, Karlsson T, Wilson A (2000) Metall Mater Trans 31A:35
Acknowledgements
Acciaierie Valbruna (Vicenza) are thanked for alloy supplying, prof. A. Molinari and G. Straffelini (University of Trento) are gratefully acknowledged for the Charpy impact test.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calliari, I., Zanesco, M. & Ramous, E. Influence of isothermal aging on secondary phases precipitation and toughness of a duplex stainless steel SAF 2205. J Mater Sci 41, 7643–7649 (2006). https://doi.org/10.1007/s10853-006-0857-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0857-2