Abstract
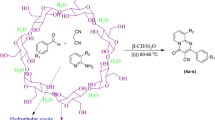
The inclusion complex of isatoic anhydride with β-cyclodextrin was formed as a result of intermolecular interaction between isatoic anhydride with β-CD. The inclusion complex was confirmed by IR spectroscopy, X-ray diffraction and DSC studies. From application of complex, herein we have described a simple and efficient protocol for synthesis of 2, 3-dihydroquinazoline-4(1H)-one derivatives by one pot condensation of isatoic anhydride, ammonium acetate or amine and aldehyde using β-CD as a supramolecular catalyst in aqueous media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins are cyclic oligosaccharides possessing hydrophobic cavity, which binds substrate selectively and catalyze chemical reactions with high selectivity. They catalyze reaction by supramolecular catalysis involving reversible formation of host–guest complexes by non-covalent bonding as seen in enzymes [1, 2]. β-cyclodextrin is a cyclic heptamer composed of seven glucose units jointed head to tail by α 1,4-links. It is widely accepted that the binding forces involved in the inclusion complex formation are Vander Waals interactions, hydrophobic interactions between guest molecules and β-CD [3]. Because of such behavior β-CD is used as catalyst for varieties of organic reactions [4].
Isatoic anhydride is used for synthesis of racemic paraensine [5], its reaction with amine and amides [6] and in synthesis of diverse heterocycles [7]. 2,3-dihydroquinazolinone derivatives are an important class of heterocycles which shows a wide range of biological, antibiotic, antipyretic, analgesic, diuretic, antihistamine, antidepressant and vascodialating activities [8, 9]. The quinazolinone moiety is a building block for various naturally occurring alkaloids [10] such as glycosamine [11], luotonin [12] and deoxyvasicinone [13]. In addition 2,3-dihydroquinazolinones have been shown to act as potent tubulin inhibitors with the impressive antiproliferatrive activity against several human cancer cell lines [14].
Recently a number of classical methods for synthesis of 2,3-dihydroquinazolin-4(1H)-one have been reported in literature involving use of Ethylene diamine diacetate [15], silica sulfuric acid [16], ionic liquid/water [17], p-TsOH [18], KAl(SO4)2·12H2O [19], gallium triflate [20], [bimm]BF4 [21], Montmorillonite K-10 [22], Al(H2PO4)3 [23], condensation of 2-aminobenzamides with aldehyde in presence of p-TsOH/DDQ [24], CuCl2 [25], chiral phosphoric acid [26, 27], iodine [28], nano Fe3O4 [29], use of p-toluenesulphonic acid-paraformaldehyde copolymer [30], silica bonded N-propyl sulfamic acid [31], etc. However most of the reported methods involve the use of organic solvents, metal catalyst with tedious procedure and low yields of product. Therefore the development of simple, environmentally benign, high yielding and clean synthesis of 2,3-dihydroquinozolin-4(1H)-ones is demand. Water is safe, economical and environmentally benign solvent [32]. In connection with our previous work with cyclodextrin [33] and considering the application of inclusion complex of isatoic anhydride with β-CD [34], herein we have developed a simple methodology for formation of 2, 3-dihydroquinazolin-4(1H)-ones in aqueous media.
Experimental
Material and chemicals
β-CD purchased from Aldrich, isatoic anhydride, ammonium acetate, amines and aldehydes (analytical grade) purchased from Spectrochem and were used without purification.
Methods and instruments
IR spectra were recorded in frequency range from 4000 to 400 cm-1 with IR affinity model-I (Schimadzu) using KBr. Powder X-ray diffraction patterns were obtained using a Philips X’pert MPD System. Differential scanning calorimetry (DSC) analyses were carried out in the temperature range from 30 to 500 °C in a stream of nitrogen atmosphere on DSC-50 thermal analyzer (Shimadzu, Japan). During experiments, aluminium crucibles were used. The heating rate was 10 °C/min, and the flow rate of nitrogen atmosphere was 50 ml/min. Both 1H NMR and 13C NMR were recorded on a Bruker Avance- II spectrophotometer operating at 500 MHz.
Preparation of inclusion complex of isatoic anhydride with β-CD
The complex between isatoic anhydride and β-CD is prepared by coprecipitation method [35] from aqueous ethanol solution. To the clear solution of β-CD (1 mmol) in 25 mL of H2O, isatoic anhydride (1 mmol) previously dissolved in ethanol added dropwise with stirring at room temperature and the resulting mixture allow to stir for 12 h. The white precipited obtained was filtered, washed gently with water and dried.
General procedure for synthesis of 2,3-dihydroquinazolin-4(1H)-one
To a stir solution of β-CD (1 mmol) in 10 mL of H2O, isatoic anhydride (5 mmol) previously dissolved in ethanol added dropwise and the resulting mixture was allowed to stir for 15 min. Then ammonium acetate (6 mmol) or amine (5 mmol) and aldehyde (5 mmol) (in case of solid previously dissolved in ethanol) added dropwise and reaction refluxed with stirring for appropriate time till reaction complete. The progress of reaction is monitored with TLC by extracting reaction mass in ethyl acetate. After completion of reaction, the reaction was cool and extracted with dichloromethane (10 mL). The aqueous layer washed thrice with dichloromethane (3 × 10 mL). The collected organic layer dried with anhydrous Na2SO4, evaporated in rotaevaporator leads to crude product which purified by recrystalization in ethanol. To the aqueous layer acetone added dropwise with stirring till white precipited formed, cool at lower temperature, filtered, washed with cold water and acetone, dried and recycled. All synthesized compounds have been characterized by IR, 1H NMR, 13C NMR and mass spectroscopy and compare with literature data.
Result and discussion
The complex of isatoic anhydride and β-CD is studied by various physical methods. The evidence for association of isatoic anhydride and cyclodextrin provided by A. Kumar and et al. [34] by 1H NMR spectroscopy in which there is upfield shift of H-3 (0.034 ppm) and H-5 (0.058 ppm) of cyclodextrin in complex as compared to uncomplex β-CD indicating the formation of complex. Herein, we have studied IR, XRD and DSC of complex.
Complex formation studied by IR spectroscopy
The formation of inclusion complex of β-CD and guest substances is accompanied by changes in their IR spectra as compared with individual components [35, 36]. Figure 1 shows the IR spectra of β-CD, isatoic anhydride, inclusion complex and physical mixture in solid state. Significance differences in CH and CO vibration modes were found. Peaks are not only shifted after complex formation but the shapes of peaks are also changed. The aliphatic CH of cyclodextrin 2,926 cm−1 and in individual isatoic anhydride 1,728.22 cm−1 shifted to 2,929.87 cm−1 and 1,730.15 cm−1 respectively. This is not observed in physical mixture suggesting an interaction between isatoic anhydride and β-CD. The absorption band at 767.61 cm−1 of disubstituted benzene ring in individual isatoic anhydride shifted to 765.74 cm−1 in inclusion complex, which was unaffected in physical mixture indicating phenyl ring interaction with β-CD.
Complex formation studied by powder X-ray analysis
True inclusion complexes have its diffraction pattern altered from those of pure components [37]. The powder X-ray pattern for individual components, complex and physical mixture is shown in Fig. 2. The diffraction pattern of complex was found to be different than diffraction pattern of pure β-CD and isatoic anhydride. Comparing the pattern for β-CD-isatoic anhydride complex with that of physical mixture reveals mark differences. In complex the new peaks were found and shift in peak position also found where as the physical mixture has the peaks which were superimposition of two individuals. Besides, it is also important to remarks that the peak intensities in complex were decreased with respect to the spectrum of β-CD, indicating the lower degree of crystallinity for the complex.
Complex formation studied by DSC
The DSC thermogram for isatoic anhydride, β-CD-isatoic anhydride complex and physical mixture is represented in Fig. 3. The thermogram of isatoic anhydride shows characteristic endothermic peak at 237.75 °C corresponding to its fussion peak. As regards with the analysis of β-CD-isatoic anhydride complex, the peak of isatoic anhydride found at 211.62 °C. Whereas in physical mixture, the peak of isatoic anhydride was found at 221.35 °C with less effect as compare to true inclusion complex clearly indicating an interaction between β-CD and isatoic anhydride. This lowering of melting of isatoic anhydride in complex state is due to variation of intermolecular forces amongst isatoic anhydride by β-CD. The second endothermic peak in isatoic anhydride occurs at 374.41 °C related to its decomposition. It disappears in ‘c’ and ‘d’ may be due to alteration with β-CD. The endothermic peak of β-CD-isatoic anhydride complex around 105-113 °C is associated to crystal water losses from β-CD. Owing to the association between isatoic anhydride and β-CD, the exothermic peak of β-CD in complex shifted to higher temperature of 319.72 °C than in physical mixture occurs at 309.43 °C. Alteration in these thermal properties due to intermolecular interactions supports the formation of inclusion complex.
Water is safe, economical and environmentally benign solvent; however the fundamental problem in with performing reactions in water is that many organic substrates are hydrophobic and insoluble in water. This difficulty is solved by inclusion formation characteristic of β-CD that enhances the solubility of guest molecule in water. By taking the advantage of inclusion complex ability of β-CD with isatoic anhydride we report the synthesis of 2,3-dihydroquinazolin-4(1H)-ones from reaction of isatoic anhydride, ammonium acetate or amine and aldehyde in aqueous media (Scheme 1, 2). For optimization the reaction has been carried out under various conditions; the results are mentioned in Table 1. The better result was obtained when 0.2 mmol of β-CD used with short reaction time of 2 h and yield of 86 % (Table 1, entry 3). The other organic solvents like ethanol, DMF, DMSO, acetonitrile and DCM does not show significant results. Increase in amount of catalyst also not show improvement in results. The reaction also performed using other ammonium salts like ammonium carbonate gives 73 % of yield in 3 h where as with ammonium chloride affords only 46 % and with long reaction time of 8 h.
To explore generality and scope of β-CD as a supramolecular catalyst, additional reaction of isatoic anhydride, ammonium acetate or amines and benzaldehyde were attempted. The result obtain are listed in Table 2. The array of aldehyde bearing either electron donating or electron withdrawing groups on aromatic ring was investigated. The reaction time was found to be short for aliphatic amines with good to moderate yield of product (Table 2, entry 14, 15). The plausible general mechanistic pathway is shown in Scheme 3. First the isatoic anhydride is activated with microenvironment of β-CD cavity by the formation of hydrogen bonding with carbonyl group followed by nucleophilic attack of amine on the carbonyl group to give intermediate-I. Then decarboxylation resulting into the formation of intermediate-II followed by subsequent cyclisation with aldehyde. β-CD was recovered in good quantity with 95, 92 and 88 % for first, second and third recycle respectively.
Conclusion
In conclusion, the complex of isatoic anhydride and β-CD was prepared by co-precipitation method. The inclusion phenomena of isatoic anhydride with β-CD were successfully characterized by FTIR, XRD and DSC methods. Based on this, we have developed a simple and ecofriendly protocol for one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones in aqueous media using β-CD as a catalyst. The advantage of procedure includes simplicity of operation, good yields, low cost and recyclability of catalyst.
References
Breslowong, R., Dong, S.D.: Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 98, 1997–2011 (1998)
Desper, J.M., Breslow, R.: Catalysis of intramolecular aldol condensation by imidazole bearing cyclodextrin. J. Am. Chem. Soc. 116, 12081–12082 (1994)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Bhosale, S.V., Bhosale, S.V.: Beta cyclodextrin as a catalyst in organic synthesis. Min. Rev. Org. Chem. 4, 143–157 (2007)
Coppola, G.M., Schuster, H.F.: Application of isatoic anhydride chemistry to the synthesis of racemic paraensine. J. Heterocyl. Chem. 21, 1409–1410 (1984)
Clark, R.H., Wagner, E.C.: Isatoic anhydride I reaction with primary and secondary amines and with some amides. J. Org. Chem. 9, 55–67 (1944)
Kappe, T., Stadlbour, W.: Isatoic anhydride and their uses in heterocyclic synthesis. Adv. Heterocly. Chem. 28, 127–182 (1981)
Na, Y.H., Hong, S.H., Lee, J.H., Park, W.K., Baek, D.J., Koh, H.Y., Cho, Y.S., Choo, H., Pae, A.N.: Novel quinazolinone derivatives as 5-HT7 receptor ligands. Bioorg. Med. Chem. 16, 2570–2578 (2008)
Wolfe, J.F., Rathman, T.L., Sleevi, M.C., Campbell, J.A., Greenwood, T.D.: Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J. Med. Chem. 33, 161–166 (1990)
Mhaske, S.B., Argade, P.: The chemistry of recently isolated naturally occurring quinazoline alkaloids. Tetrahedron 62, 9787–9826 (2006)
Kametani, T.: Loc, CV, Higa, T., Koizumi, M., Ihara, M., Fukumoto, K.J.: Iminoketene cycloaddition. 2. Total syntheses of arborine, glycosminine, and rutecarpine by condensation of iminoketene with amides. J. Am. Chem. Soc. 99, 2306–2309 (1977)
Mason, J.J., Bergman, J.: Total synthesis of luotonin A and 14-substituted analogues. Org. Biomol. Chem. 5, 2486–2490 (2007)
Liu, J.-F., Ye, P., Sprague, K., Sarget, K., Yohannes, D., Baldino, C.M., Wilson, C.J., Ng, S.C.: Novel One-pot total syntheses of deoxyvasicinone, mackinazolinone, isaindigotone, and their derivatives promoted by microwave irradiation. Org. Lett. 5, 3363–3366 (2005)
Chinigo, G.M., Paige, M., Grindrod, S., Hamel, E., Dakshanamurthy, S., Chruszcz, M., Minor, W., Brown, M.L.: Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 51, 4620–4631 (2008)
Narsimhulu, M., Lee, Y.R.: Ethylenediamine diacetate-catalyzed three-component reaction for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones and their spirooxindole derivatives. Tetrahedron 67, 9627–9634 (2011)
Salehi, P., Dabiri, M., Zolfigol, M.A., Baghban-zadeh, M.: A novel method for the one-pot three-component synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Syn lett 7, 1155–1157 (2005)
Chen, J., Su, W., Wu, H., Liu, M., Jin, C.: Eco-friendly synthesis of 2,3-dihydroquinazolin-4(1H)-ones in ionic liquids or ionic liquid–water without additional catalyst. Green Chem. 9, 972–975 (2007)
Shi, D., Rong, L., Wang, J., Zhung, Q., Wang, X., Hu, H.: Synthesis of quinazoline-4(3H)-ones and 1,2-dihydroquinazolin-4(3H)-ones with the aid of a low-valent titanium reagent. Tetrahedron Lett. 44, 3199–3201 (2003)
Doheri, M., Salehi, P., Otokesh, S., Baghbanzadeh, M., Kozehgary, G., Mohammadi, A.A.: Efficient synthesis of mono- and disubstituted 2,3-dihydroquinazolin-4(1H)-ones using KAl(SO4)2·12H2O as a reusable catalyst in water and ethanol. Tetrahedron Lett. 46, 6123–6126 (2005)
Chen, J., Wu, D., He, F., Liu, M., Wu, H., Su, W.: Gallium(III) triflate-catalyzed one-pot selective synthesis of 2,3-dihydroquinazolin-4(1H)-ones and quinazoline-4(3H)-ones. Tetrahedron Lett. 49, 3814–3818 (2008)
Dabiri, M., Salehi, P., Baghbanzadeh, M.: Ionic Liquid Promoted Eco-friendly and Efficient Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones. Monatsh. Chem. 138, 1191–1194 (2007)
Salehi, P., Dabiri, M., Baghbanzadeh, M., Bahramnejad, M.: One-Pot, Three-component synthesis of 2, 3-dihydro-4(1H)-quinazolinones by Montmorillonite K-10 as an efficient and reusable catalyst. Synth. Commun. 36, 2287–2292 (2006)
Shaterian, H.R., Oveisi, A.R., Honarmand, M.: synthesis of 2, 3-dihydroquinazoline-4(1H)-ones. Synth. Commun. 40, 1231–1242 (2010)
Shaabani, A., Maleki, A., Mofakham, H.: Click Reaction: highly efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Synth. Commun. 38, 3751–3759 (2008)
Abdel-Jalil, R.J., Voelter, W., Saeed, M.: A novel method for synthesis of 4(3H)- quinazolinones. Tetrahedron Lett. 45, 3475–3476 (2004)
Cheng, X., Vellalath, S., Goddard, R., List, B.: Direct catalytic asymmetric synthesis of cyclic aminals from aldehydes. J. Am. Chem. Soc. 130, 15786–15787 (2008)
Rueping, M., Antonchick, A.P., Sugiono, E., Grenader, K.: Asymmetric brønsted acid catalysis: catalytic enantioselective synthesis of highly biologically active dihydroquinazolinones. Angew. Chem. Int. Ed. 48, 908–910 (2009)
Dabiri, M., Salehi, P., Bahramanejad, M., Alizadeh, M.: A practical and versatile approach toward a one-pot synthesis of 2,3-disubstituted 4(3H)-quinazolinones. Monatsch Chem. 141, 877–881 (2010)
Zhang, Z.H., Lu, H.Y., Yang, S.H., Gao, J.W.: Synthesis of 2, 3-dihydroquinazolin-4(1H)-ones by three-component coupling of isatoic anhydride, amines, and aldehydes catalyzed by magnetic Fe3O4 nanoparticles in water. J. Comb. Chem. 12, 643–646 (2010)
Teuri, A.S., Bolouk, S.: One-pot, three-component synthesis of 2,3-dihydroquinazolin- 4(1H)-ones using p-toluenesulfonic acid–paraformaldehyde copolymer as an efficient and reusable catalyst. Monatsch Chem. 141, 1113–1115 (2010)
Niknam, K., Jafoopour, N., Niknam, E.: Silica-binded N-propylsulfamic acid as a recyclable catalyst for synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Chin. Chem. Lett. 22, 69–72 (2011)
Li, C.J., Chan, T.H.: Organic reactions in aqueous media. Wiley, New York (2007)
Patil, D.R., Dalal, D.S.: One-pot, solvent free synthesis of Hantzsch 1, 4-dihydropyridines using β-cyclodextrin as a supramolecular catalyst. Lett. Org. Chem. 8, 477–483 (2011)
Kumar, A., Tripathi, V.D., Kumar, P.: β-Cyclodextrin catalysed synthesis of tryptanthrin in water. Green Chem. 13, 51–54 (2011)
Kemelbekov, U., Luo, Y., Orynbekova, Z., Rustembekov, Z., Haag, R., Saenger, W., Pralivey, K.: IR, UV and NMR studies of β-cyclodextrin inclusion complexes of kazcaine and prosidol bases. J. Incl. Phenom. Macrocycl. Chem. 69, 181–190 (2011)
Choi, S.H., Kim, S.Y., Ryoo, J.J., Park, J.Y., Lee, K.P.: FT-Raman and FTIR spectra of the nonsteroidal antiinflammatory drug ketoprofen included in cyclodextrin. Anal. Sci. 17, 1785–1788 (2001)
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Intl. Ed. Engl. 19, 344–362 (1980)
Acknowledgments
The authors are thankful to Department of Science and Technology and University Grants Commission, New Delhi, India for financial support of this work. We are also thankful to Dr. K. J. Patil, Emeritus Professor, School of Chemical Sciences for useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, D.R., Ingole, P.G., Singh, K. et al. Inclusion complex of Isatoic anhydride with β-cyclodextrin and supramolecular one-pot synthesis of 2, 3-dihydroquinazolin-4(1H)-ones in aqueous media. J Incl Phenom Macrocycl Chem 76, 327–332 (2013). https://doi.org/10.1007/s10847-012-0203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0203-z