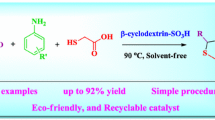
Abstract
Green and economical method has been reported for the synthesis of benzylpyrazolyl naphthoquinone and pyrazolo pyranopyrimidines in water at room temperature by using β-CD-SO3H. β-Cyclodextrin supported sulfonic acid was prepared by simple one step procedure and characterized by FT-IR spectrum, 1H NMR, 13C NMR spectra, TGA, EDAX, XRD, BET surface area analysis and acid–base titration. The present protocol is environmental benign due to heterogeneous reusable catalyst and green reaction medium. This methodology provides excellent yield of the desired product with short reaction time at room temperature, easy workup procedure and no need of column chromatographic separation. Pyrazolyl derivatives are of much importance because this fragment is a key moiety in numerous biologically active compounds.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, the chemical industry has received considerable attention towards the development of new processes due to growing environmental concerns [1]. In most of the industries the major pollution sources are volatile solvents and hazardous catalysts [2, 3]. To avoid the environmental impacts, there is need to design safer, reusable reaction medium and catalysts [4]. In this direction aqueous phase organic synthesis has attracted more attention of chemists as it overcomes the harmful effects associated with the organic solvents and is environmentally benign [5]. In catalysis era, homogeneous catalysts are more efficient than heterogeneous catalysts [6], however, they have shortcomings such as no recovery, high cost and tedious workup which increases the interest towards heterogeneous catalysts which are cost effective [7], eassy recoverable, reusable and involve simple procedure to form the product [8]. Due to this, heterogeneous catalysis has emerged as a useful tool for organic transformation [9, 10].
In modern synthetic organic chemistry, the development of efficient recyclable catalyst systems is one of the most important topics [11]. For this purpose, some catalytically active centres have been often immobilized on inorganic materials, synthetic organic polymers or hybrid materials [12, 13]. Biopolymers such as starch, cellulose, chitosan or wool are emerging as green and sustainable supports in heterogeneous catalysis [14, 15]. Among these, cyclodextrins (CDs) has also drawn much attention due to its water-solubility and special hydrophobic cavity [16, 17]. Cyclodextrins (CDs) are macrocyclic oligosaccharides possessing hydrophobic cavities that bind substrates selectively via non-covalent interactions and this outstanding property enable them to be used in various applications [18,19,20]. Native β-CD and chemically modified cyclodextrins have been employed as a phase transfer catalyst [21, 22] for organic reactions, such as Azide-alkyne cyclo-addition reaction and aza-Michael addition reaction [23,24,25]. Herein we have reported β-cyclodextrin supported sulfonic acid (β-CD-SO3H) as a heterogeneous catalyst for the one pot multicomponent synthesis of benzylpyrazolyl naphthoquinone and pyrazolo pyranopyrimidine derivatives.
Over the past decades, the synthesis of complex biologically active scaffolds via the one-pot multicomponent reactions (MCRs) has attracted considerable attention [26]. The synthetic utility of such protocols can be improved significantly by using green solvents and an efficient heterogeneous catalyst [27]. Benzylpyrazolyl naphthoquinone derivatives are of much importance because they exist in many natural products such as atovaquone, lapachol, parvaquone and buparvaquone [28] (Fig. 1). It has been exhibited various biological activities such as antibacterial [29], anti-HIV [30], antiviral, anticoagulant, antioxidant and anticancer etc [31, 32].
Pyrazolo pyranopyrimidine derivatives are important core structure because of their wide applications in pharmaceuticals and in organic synthesis as essential intermediates [33, 34]. Heterocyclic nucleus containing pyrazolo pyranopyrimidine are useful as antipyretic, analgesic [35] anti-tubercular, antibacterial, anti-cancer, anti-inflammatory, anti-microbial, fungicidal, insecticidal and molluscicidal (Fig. 2) [36, 37]
As a consequence, the synthesis of both the nuclei will be beneficial from the biological point of view. Due to such wide range of applications of these scaffolds, number of chemists have been attracted towards to synthesis such molecules. Recently very few reports are available for the synthesis of benzylpyrazolyl naphthoquinone and pyrazolo pyranopyrimidine derivatives. The reported methods for the synthesis of benzylpyrazolyl naphthoquinones are MgCl2 in ethylene glycol [38], microwave assisted synthesis in water [39], Er(OTf)3 in ethanol [40], p-TSA in water [41] and for pyrazolo pyranopyrimidine meglumine in water [42], heteropolyacid supported catalyst in water [43], magnetized water [44], Cu-immobilized mesoporous silica nanoparticals [45], ChCl: Urea [46], SBA-Pr-SO3H [47], DABCO [48], titanium dioxide nanowires [49], oleic acid [50] etc. Knowing the chemical and pharmacological importance of the benzylpyrazolyl naphthoquinones and as a part of our continuing efforts towards the development of green and sustainable routes for preparation of biologically active compounds [51,52,53,54], herein, we have reported highly efficient, heterogeneous β-CD-SO3H catalyzed one pot synthesis of benzylpyrazolyl naphthoquinones and pyrazolo pyranopyrimidine derivatives in water.
2 Experimental
2.1 Materials and Methods
The different substrates used for the synthesis of pyrazolyl naphthalenedione and pyrazolo pyranopyrimidine derivatives were purchased from Sigma Aldrich and Alfa Aesar and used without any further purification. TLC was carried out using silica gel G60 F254 plates (Merck). The melting points of products were determined in open capillary tubes and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker-Avance 300 and 400 MHz and 75 and 100 MHz spectrometer using TMS as an internal standard and CDCl3/DMSO-d6 as a solvent.
2.2 Preparation of β-Cyclodextrin-sulfonic Acid
To a magnetically stirred mixture of β-cyclodextrin (5.107 g, 4.5 mmol) in CH2Cl2 (20 mL), chlorosulfonic acid (1.048 g, 9 mmol) was added slowly drop by drop at 0 °C during 3 h. After completion of addition, the mixture was stirred for 2 h at room temperature (25–28 °C) to remove HCl from reaction vessel. Then, the mixture was filtered and washed with methanol (30 mL) and dried at room temperature to obtain sulfonated β-cyclodextrin as white powder (5.28 g). The prepared catalyst was characterized by FT-IR, 1H NMR, 13C NMR spectra, TGA, EDAX, XRD, BET surface area analysis and acid–base titration etc. which confirm the presence of –SO3H group.
2.3 General Procedure for the Synthesis of Dihydro-1H-pyrazolyl Naphthalene-1, 4-dione
In a round bottom flask, stirred a mixture 3-methyl-1-phenyl-1H-pyrazol-5-ol (1 mmol), substituted aldehyde (1 mmol) and 2- hydroxy naphthoquinone (1 mmol) in 5 mL of water and 10 mol% β-CD-SO3H catalyst at room temperature (25–28 °C). The reaction mixture was stirred for stipulated time and reaction progress was monitored by thin layer chromatography (TLC), after completion of the reaction the solid product was filtered and washed with water. The synthesized compounds were identified by comparing physical and spectral data (FT-IR, 1H NMR, 13C NMR and MS) with reported one.
2.4 General Procedure for the Synthesis of Pyrazolo Pyranopyrimidines
In a round bottom flask, stirred a mixture of ethyl acetoacetate (1 mmol) and hydrazine hydrate (1 mmol) in 5 mL of water at room temperature. To this solution substituted aldehyde (1 mmol), thiobarbituric acid (1 mmol) and catalyst β-CD-SO3H (10 mol%) were added. The reaction mixture was stirred for stipulated time and the reaction progress was monitored by thin layer chromatography (pet ether: ethyl acetate 7:3) (TLC), after completion of the reaction the solid product was filtered and washed with water. The synthesized compounds were identified by comparing physical and spectral data (FT-IR, 1H NMR, 13C NMR and MS) with reported one.
3 Results and Discussion
3.1 Preparation and Characterization of β-Cyclodextrin-sulfonic Acid
The β-CD-SO3H was synthesized by recently reported method [23]. The synthesized catalyst was characterised by FT-IR spectrum (ALPHA 100508, Bruker), 1H NMR, 13C NMR (Bruker 400 MHz and 100 MHz, D2O) spectra, TGA (SDT Q600 V20.9 Build 20), Elemental analysis from EDAX, XRD, BET surface area (Quantachrome Instruments v11.02) analysis and acid–base titration.
3.2 FT-IR Analysis
The FT-IR spectra of β-CD and β-CD-SO3H have been shown in (Fig. 3). The compound showed O–H stretching vibration at 3288.83 cm−1, the C–H stretching vibration at 2922.03 cm−1 and the C–OH stretching vibration at 1023.71 cm−1. The characteristic adsorption peaks of sulfonate groups S=O stretching vibration at 1151.94 and 1076.34 cm−1 were observed in the spectrum of β-CD-SO3H, confirms the successful grafting of sulfonic acid functionality onto β-CD.
3.3 1H NMR and 13C NMR Spectrum
1H NMR and 13C NMR spectrum β-CD-SO3H (Fig. 4) was recorded in D2O. In 1H NMR spectrum the ring protons were observed at δ 3.48–3.86 ppm as a multiplet and the –CH2 protons appeared at δ 4.9 ppm as a doublet (J = 4 Hz). In 13C NMR spectrum the –CH2 carbon showed peak at δ 60.2 ppm whereas the ring carbons were observed at δ 71.7, 72.0, 73.0, 81.0 and 101.8 ppm.
3.4 TGA Analysis
Thermal gravimetric analysis (TGA) analysis of β-CD-SO3H were performed over the range of 30 to 800 °C, with a temperature increase rate of 10 °C min−1 in a nitrogen atmosphere (Fig. 5). According to TGA diagrams, the weight loss processes of β-CD-SO3H could be divided into three stages. The first weight loss in the range of 27.41–138.33 °C was attributed to the release of water molecules, including complexed water inside the cavity of β-CD-SO3H and uncomplexed water outside the cavity of β-CD-SO3H. The second weight loss in the range of 138.33–313.87 °C was due to the fact that the sulfonate groups were degraded. The third weight loss in the range of 313.87–479.32 °C was attributed to the decomposition of the β-CD framework. Therefore, β-CD-SO3H is stable below about 150 °C, which is enough for constant weight during the catalysis procedure of our experiments.
3.5 EDAX Analysis
The energy dispersive analysis X-ray (EDAX) (Fig. 6) of β-CD-SO3H revealed carbon and oxygen as the major elements are attributed to β-cyclodextrin skeleton whereas peak of sulphur in its respective energy position at 2.2–2.4 keV also supports the formation of desired catalyst. The loading of SO3H group was found to be 0.8625 mmol of functional group per gram of catalyst.
3.6 Acid–Base Titration
Further, the quantity of SO3H group was performed by employing volumetric titration analysis and was found to be 0.87 mmol per gram of catalyst.
3.7 XRD Analysis
The powder X-ray diffraction pattern (Fig. 7) of β-cyclodextrin-SO3H exhibited a broad diffraction peak (2ɵ = 15° to 30°), which can assigned to C (002) planes indicates that amorphous carbon composed of aromatic carbon sheets oriented in considerably random fashion.
3.8 BET Analysis
BET surface areas and pore size was calculated using the standard Brunauer-Emmett-Teller (BET) equation and was found to be 8.32 m2/g and 21.56 Å for β-cyclodextrin. After sulfonation the specific surface areas for β-cyclodextrin-SO3H decreased with respect to β-cyclodextrin and found to be 7.27 m2/g and pore size increases 48.08 Å which indicates the successful linking of sulfonic groups on β-cyclodextrin.
3.9 Optimization of Reaction Conditions
After successful synthesis and characterization of β-CD-SO3H we have checked the catalytic activity of β-CD-SO3H for the synthesis of benzylpyrazolyl naphthoquinone. Initially, we carried out the model reaction of 3-methyl-1-phenyl-1H-pyrazol-5-ol, 4-methoxy benzaldehyde and 2-hydroxy naphthoquinone (Scheme 1) in different solvent at various temperatures. The optimized results are shown in (Table 1).
Initially, we carried out model reaction by using 5 mol% β-CD-SO3H at room temperature and solvent free condition, it was observed that after long time no product formation was observed. After that we have carried out model reaction in different polar solvents such as water, ethanol, methanol, acetonitrile DCM, DMF and THF. It was observed that, in water excellent yield of the product was observed at room temperature than other solvents. It is due to the phase transfer character of β-CD-SO3H, which contain oligosaccharide units are highly soluble in water. In addition, we have performed model reaction at higher temperature but the temperature effect does not observed on the rate of reaction, only slight increase in the yield of the product was obtained.
After optimization of reaction with reference to solvent and temperature, we have optimized the mole proportion of the catalyst to carry out the reaction smoothly. For that purpose we have carried out model reaction at 5, 10, 15, 20 and 25 mol% (Table 2). It was observed that when the amount of catalyst increases from 5 to 10 mol% the yield of the product increases up to 94% but after that the amount of catalyst increases, there was no effect on rate of reaction.
We have compared the obtained results with the reported methods, as compared to reported methods, we got better results with short reaction time and aqueous medium at room temperature (Table 3).
With the optimized reaction conditions in hand, we tended to investigate the generality and limitations of this method. The reaction of various aromatic aldehydes, 3-methyl-1-phenyl-1H-pyrazol-5-ol and 2-hydroxy naphthoquinone were explored under the optimized reaction conditions to produce a series of benzylpyrazolyl naphthoquinone derivatives (Table 4). Most of the reactions precede very efficiently, various aromatic aldehydes containing electron-withdrawing and electron-donating substituents shows equal ease towards the product formation with high yield (Scheme 2).
Due to such good results of β-CD-SO3H, we explore the scope of this catalyst for the synthesis of pyrazolopyranopyrimidine derivatives. For this, we have carried out the four component reaction of ethyl acetoacetate, hydrazine hydrate, different aromatic aldehydes and thiobarbituric acid in water at room temperature (25–30 °C). The reactions proceed smoothly with various substituted aromatic aldehydes to afford the desired products in good to excellent yields (Table 5). Variation of the electronic properties and the position of functional groups on the aromatic ring of the aldehyde did not show obviously impact on the yield of the product (Scheme 3).
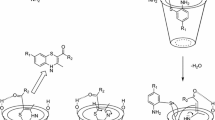
3.10 Plausible Reaction Mechanism
The plausible mechanism that could be accounted for this four component reaction is depicted in Scheme 4. Initially, aryl hydrazine/hydrazine hydrate 1 is reacted with ethyl acetoacetate 2 to generate the pyrazolone ring 5, which is an isomeric form. Simultaneously, the electrophilicity of carbonyl carbon of aldehyde 3 increases due to the hydrogen bonding of β-CD-SO3H. Nucleophilic attack of 2-hydroxy-1,4-naphthoquinone 4 to activated aldehyde leads to form intermediate 6, followed by Knoevenagel condensation to form intermediate 7. Then, Michael addition of intermediate 5 and unsaturated Knoevenagel product 7 to form intermediate 8, which undergoes tautomeric proton shift to generate the desired product 9.
3.11 Reusability
The reusability of catalysts are highly preferable for a green chemistry prospective. Reusability of the catalyst was tested on the synthesis of compound 4e. The catalyst was easily recovered by filtration after the reaction and washed with acetone. The filtrate was reused directly for the next run. The procedure was repeated and the results indicated that the catalyst could be recycled four times with a slight loss of catalyst activity. The reason for the decreased yields may be that the catalyst was partly lost when it was reused (Fig. 8).
4 Conclusions
In conclusion, we have developed an efficient and practical procedure for the synthesis of benzylpyrazolyl coumarin and pyrazolo pyranopyrimidine derivatives catalyzed by β-CD-SO3H in water at room temperature. The attractive features of this protocol are simple work up procedure, short reaction time, high yield and an eco-friendly catalyst which make it a useful and attractive strategy in synthetic organic chemistry. This protocol offers precious importance in view of green chemistry standpoints such as good stability of catalyst, excellent yields and recyclability which leads to good contribution to the heterocyclic chemistry.
References
Ruhul AM, Kalam MA, Masjuki HH, Fattah IMR, Reham SS, Rashed MM (2015) RSC Adv 5:101023–101044
Zhou Y, Chen G, Long Z, Wang J (2014) RSC Adv 4:42092–42113
Nidheesh PV (2015) RSC Adv 5:40552–40577
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su CY (2014) Chem Soc Rev 43:6011–6061
Xue Z, Ma M-G, Li Z, Mu T (2016) RSC Adv 6:98874–98892
Santoro S, Kozhushkov SI, Ackermann L, Vaccaro L (2016) Green Chem 18:3471–3493
Khalafi-Nezhad A, Mohammadi S (2013) RSC Adv 3:4362–4371
Taheri M, Ghiaci M, Shchukarev A (2018) New J Chem 42:587–597
Sun J, Wang J, Cheng W, Zhang J, Li X, Zhang S, She Y (2012) Green Chem 14:654–660
Kaboudin B, Mostafalu R, Yokomatsu T (2013) Green Chem 15:2266–2274
Jean-Marie A, Griboval-Constant A, Khodakov AY, Monflier E, Diehl F (2011) Chem Commun 47:10767–10769
Salamatmanesh A, Miraki MK, Yazdani E, Heydari A (2018) Catal Lett 148:3257–3268
Wu J, Xu FZ, Feng SL, Xue W, Wang ZZ (2016) Heterocycles 92:1629–1642
Yadav GD, Kantam ML, Bhanage BM (2017) ACS Sustain Chem Eng 5:3597–3597
Urmode TD, Dawange MA, Shinde VS, Kusurkar RS (2017) Tetrahedron 73:4348–4354
Hapiot F, Monflier E (2017) Catalysts 7:173–184
Asghari S, Tajbakhsh M, Kenari BJ, Khaksar S (2011) Chin Chem Lett 22:127–130
Thombal RS, Jadhav AR, Jadhav VH (2015) RSC Adv 5:12981–12986
Wu J, Du X, Ma J, Zhang Y, Shi Q, Luo L, Song B, Yang S, Deyu H (2014) Green Chem 16:3210–3217
Girish YR, Sharath Kumar KS, Thimmaiah KN, Rangappa KS, Shashikanth S (2015) RSC Adv 5:75533–75546
Sabzi NE, Kiasat AR (2018) Catal Lett 148:2654–2664
Rai P, Srivastava M, Yadav S, Singh J, Singh J (2015) Catal Lett 145:2020–2028
Tayade YA, Patil DR, Wagh YB, Jangle AD, Dalal DS (2015) Tetthedron Lett 56:666–673
Patil DR, Ingole PG, Singh K, Dalal DS (2013) J Incl Phenom Macrocycl Chem 76:327–332
Tayade YA, Dalal DS (2017) Catal Lett 147:1411–1421
Sudhan PN, Ghashang M, Mansoor SS (2016) BJBAS 5:340–349
Gong K, Wang H, Ren X, Wang Y, Chen J (2015) Green Chem 17:3141–3147
Molnar A, Papp A (2014) Catal Sci Technol 4:295–310
Che F, Wang Y, Shen T, An X, Song Q (2015) C R Chimie 18:607–610
Gu Y (2012) Green Chem 14:2091–2128
Brahmachari G (2015) ACS Sustain Chem Eng 3:2058–2066
Shen T, Fu Z, Che F, Dang H, Lin Y, Song Q (2015) Tetrahedron Lett 56:1072–1075
Rigi F, Shaterian HR (2017) Polycycl Aromat Comp 37:314–326
Maleki A, Jafari AA, Yousefi S (2017) Carbohydr Polym 175:409–416
Bakherad M, Doosti R, Mirzaee M, Jadidi K (2017) IJC 7:27–35
Khalafi-Nezhad A, Shahidzadeh ES, Sarikhani S, Panahi F (2013) Tetrahedron Lett 379:1–8
Panda S, Roy A, Deka SJ, Trivedi V, Manna D (2016) ACS Med Chem Lett 7:1167–1172
Fu Z, Qian K, Li S, Shen T, Song Q (2016) Tetrahedron Lett 57:1104–1108
Wang SL, Ding J, Shi F, Liu YP, Jiang B, Ma N, Tu SJ (2012) J Heterocycl Chem 49:521
Kumar M, Sribalan R, Padmini V (2017) ChemistrySelect 2:489–493
Lakshmanan S, Ramalakshmi N (2016) Synth Commun 46:2045–2052
Li XT, Zhao AD, Mo LP, Zhang ZH (2014) RSC Adv 4:51580–51588
Sadjadi S, Heravi MM, Daraie M (2017) Res Chem Intermed 43:2201–2214
Bakherad M, Keivanloo A, Gholizadeh M, Doosti R, Javanmardi M (2017) Res Chem Intermed 43:1013–1029
Nasresfahani Z, Kassaee MZ (2017) ChemistrySelect 2:9642–9646
Tipale MR, Khillare LD, Deshmukh AR, Bhosale MR (2018) J Heterocycl Chem 00:00
Ziarani GM, Aleali F, Lashgari N, Badiei A, Soorkic AA (2018) IJPR 17:525–534
Heravi MM, Mousavizadeh F, Ghobadi N, Tajbakhsh M (2014) Tetrahedron Lett 55:1226–1228
Dastkhoon S, Tavakoli Z, Khodabakhshi S, Baghernejad M, Abbasabadi MK (2015) New J Chem 39:7268–7271
Ganesan A, Kothandapani J, Subramaniapillai SG (2016) RSC Adv 6:20582–20587
Patil A, Salunkhe R (2018) Res Chem Intermed 44:3337–3348
Lohar T, Kumbhar A, Patil A, Kamat S, Salunkhe R (2019) Res Chem Intermed 45:1639–1651
Mane AH, Patil AD, Kamat SR, Salunkhe RS (2018) ChemistrySelect 3:6454–6458
Patil A, Mane A, Kamat S, Lohar T, Salunkhe R (2019) Res Chem Intermed 45:3441–3452
Acknowledgements
The authors would like to thank Department of Chemistry, Shivaji University, Kolhapur for providing research facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, A., Gajare, S., Rashinkar, G. et al. β-CD-SO3H: Synthesis, Characterization and Its Application for the Synthesis of Benzylpyrazolyl Naphthoquinone and Pyrazolo Pyranopyrimidine Derivatives in Water. Catal Lett 150, 127–137 (2020). https://doi.org/10.1007/s10562-019-02928-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02928-y