Abstract
The objective of this research was to develop active packaging that used a vapor-phase antimicrobial agent incorporated into chitosan capsules to extend the shelf life of dry cakes. The clove essential oil (CEO) was encapsulated into chitosan capsules using an emulsion-ionic gelation crosslinking technique. CEO loading in chitosan was taken in different ratios (0.0:1, 0.25:1, 0.50:1, 0.75:1, 1:1, 1.25:1, 1.50:1) and validated using Fourier Transform-Infrared (FT-IR) spectra and a Field-Emission Scanning Electron Microscope (FE-SEM). The thermal stability of the capsules was evaluated using thermogravimetric analysis (TGA). Differential Scanning Calorimetry (DSC) was used to assess the oxidative thermal stability of the compounds. The encapsulation efficiency and loading capacity were 12.01 and 8.01 for 1.50:1 (CEO: Chitosan) loaded samples. The antimicrobial activity of active capsules was performed in the vapor phase. It completely prevented the development of E. coli and S. aureus when CEO was used with chitosan in more than a 1:1 ratio. Finally, the dry cakes were packed with active capsules, and bacterial growth was reduced until the 10th day of packing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial contamination is one of the food industry’s most critical challenges, causing a decrease in shelf-life and posing potential health risks to customers (Al-Naamani et al., 2016). Modern consumers want healthier, more natural products and conveniences like ready-to-eat meals and extended shelf-life under ambient circumstances. Packaging technology has improved, leading to Modified Atmosphere Packaging (MAP) and Active Packaging (AP) to increase the shelf life. Active packaging has gained much interest because it can keep food fresh and extend its shelf life.
Active packaging, which incorporates antioxidant and antimicrobial compounds into packaging materials, is a promising technology. It can enhance packaged foods’ safety and shelf life by preventing microbial growth and lowering lipid oxidation (Muriel-Galet et al., 2015). Several research on antimicrobial and antioxidant packaging have been published in recent years (Hadidi et al., 2020a; Hasheminejad et al., 2019a; Kardam et al., 2021; Priyadarshi et al., 2018b). Antimicrobials provide better protection by releasing active compounds in a controlled manner all over the food surface (Al-Naamani et al., 2016), enhancing the product’s shelf life. Natural antimicrobials, such as essential oils (EOs), are preferred over synthetic antimicrobials for health reasons (Kardam et al., 2021).
Chitosan, the deacetylation product of chitin (Cai & Wang, 2021), is a polysaccharide made up of a random mixture of -(14)-linked D-glucosamine and N-acetyl-D-glucosamine with an amino group (Hasanin & Al Kiey, 2021; Islam et al., 2017; Priyadarshi et al., 2018a; Varma et al., 2004). It is obtained from chitin, a naturally occurring polymer obtained from biological sources such as crab shells, arthropod cuticles, and fungal cell walls, and is used to make chitosan. It is widely used in the pharmaceutical and biological areas (Islam et al., 2017; Lord et al., 2011). Chitosan can be used in food packaging because it is biodegradable and eco-friendly (El-Naggar et al., 2022).
Bakery products have a short life span of 2–4 days (Sharma et al., 2022c). In particular, dry cakes deteriorate with time due to physical, chemical, or microbial spoilage. Microbiological spoilage, in particular, is the primary reason for shelf life (Saranraj & Geetha, 2012). Microbial spoilage can be prevented by adding natural antimicrobial agents such as Essential Oils (EOs). In particular, Clove Essential Oil (CEO) has shown good antimicrobial properties and is made by distilling the dried flower buds of the Syzygium aromaticum tree (Passone et al., 2012). The main component of CEO is eugenol which has inherent antimicrobial activity (K.), and the presence of other phenolic compounds shows antioxidants (Elsayed et al., 2022) and bactericidal effects against Escherichia coli, Staphylococcus aureus, Salmonella typhimurium and Listeria monocytogenes (Radünz et al., 2019). The inherent antibacterial, antifungal, antioxidant, insecticidal, and antiviral activity of CEO helps to preserve food and extend its shelf life (Chaieb et al., 2007; Sebaaly et al., 2015). Therefore, the CEO can be utilized as an antimicrobial agent to increase the shelf life of bakery products.
Many in vitro antimicrobial investigations of EOs in the liquid-phase medium have been documented. Using the vapor-phase activity of EOs, encouraging results have already been produced for fresh vegetables. On the other hand, a high concentration of EOs in close contact with food may affect the sensory quality (taste and flavor) (Gutierrez et al., 2008). To address this issue, an alternate non-direct way for imparting antimicrobial action in the vapor phase without affecting a product’s sensory quality should be implemented (Nadjib et al., 2014). Encapsulation has recently been established as an effective method of safeguarding EOs against evaporation and oxidation (Beyki et al., 2014), offering prolonged activity for encapsulated compounds through controlled release (Yoksan et al., 2010). One of the most promising encapsulation techniques for EOs is an emulsion–ionic gelation crosslinking (Ezhilarasi et al., 2012). It’s a simple procedure that produces stable nanosized particles without the need of heat or hazardous crosslinking agents (Keawchaoon & Yoksan, 2011). Chitosan, a cation polysaccharide, may crosslink with other molecules with various negative charges. Chitosan is frequently used to encapsulate active substances as a wall/shell material. Chitosan has been used as an outer encapsulant material for a range of medications, vitamins, proteins, minerals, and phenolic compounds due to its non-toxicity, biocompatibility, biodegradability, and ability to form films, gels, beads, and particles (Hu et al., 2008; Kowalska et al., 2015; Woranuch & Yoksan, 2013a).
The objective of this work was to develop an active packaging based on chitosan encapsulated CEO with vapor phase antimicrobial characteristics to increase the shelf life of the dry cake. CEO was encapsulated in chitosan with varying amounts of CEO and characterized for the morphological, chemical, thermal, encapsulation efficiency, antioxidant, and antimicrobial properties. Capsules were first tested for antioxidant activities and then antibacterial activity tested against E. coli and S. aureus via vapor phase. Finally, the capsules were packed in sachets with dry cakes to study the shelf-life extension based on antimicrobial tests.
Material and Methods
Materials
Clove oil (purity 85%), Chitosan (degree of deacetylation > 75%, molecular weight 2.9 × 105 gmol−1), Acetic Acid (purity 99%), Tween-80, Tripolyphosphate (TPP), and Ethanol were purchased from Himedia Laboratories Pvt. Ltd., India. The bacterial culture of E. coli (MTCC number 1698) and S. aureus (MTCC number 1789) were supplied by ‘Microbial Type Culture Collection and Gene Bank’ (MTCC), Chandigarh, India.
Preparation of CEO-Loaded Chitosan Capsules
Chitosan antimicrobial capsules were formed in two steps: oil-in-water (O/W) type emulsion and ionic gelation of chitosan with TPP, as previously described by Woranuch and Yoksan (2013a) with minor modifications. Briefly, chitosan solution (2% w/v) was prepared by dissolving chitosan flakes in a 1% v/v aqueous acetic acid solution at ambient temperature overnight. Tween 80 (0.3 g/40 ml) was added to the chitosan solution and agitated for 30 min at 40 °C until homogeneity. During the stirring, CEO was added to the solution dropwise, and the agitation was continued for another 30 min. Chitosan to CEO of varying ratios was mixed (1:0, 1:0.25, 1:0.50, 1:0.75, 1:1, 1:1.25, 1:1.50). A 1% w/v TPP solution was prepared, and chitosan solution was added dropwise into it with the aid of a syringe while stirring at room temperature for 30 min. The resulting capsules were freeze-dried and stored at 4 °C for further analysis.
Determination of Encapsulation Efficiency (EE) and Loading Capacity (LC)
UV–Vis spectroscopy was used to assess the quantity of loaded CEO in chitosan microcapsules. CEO-loaded chitosan capsules were crushed in 200 µl of 2 M HCL solution, mixed with 2 mL of ethanol, and further centrifuged at 10,000 RPM for 10 min. The supernatant was collected and analyzed in UV–Vis Spectroscopy (UV-1800, Shimadzu) at a wavelength of 282 nm for CEO concentration (Hadidi et al., 2020a; Hasheminejad et al., 2019b; Woranuch & Yoksan, 2013a). Equations (1) and (2) were used to compute the EE and LC, respectively.
Encapsulation and Color Variation Analysis of Capsules
For the capsules’ color analysis, the CIE L*a* b* values were determined using Spectro Eye Spectrophotometer (Gretag Macbeth, Switzerland). The parameter L* specifies lightness, with values ranging from 0 to 100 representing black to white. The value of a* varies from green (negative) to red (positive). Finally, the parameter b* indicates positive and negative values for yellow and blue (Sharma et al., 2021, 2022b; Singh et al., 2018). The results of l*, a*, b*, and total color difference (ΔE) were reported individually. Equation (4) was used to calculate the ΔE:
Fourier Transform-Infrared (FT-IR)
The FTIR spectra of CEO, chitosan, and CEO-loaded chitosan capsules were studied using a Perkin Elmer FT-IR C91158 spectrophotometer equipped with an Attenuated Total Reflectance (ATR) sample accessory and a diamond crystal plate. Spectra were acquired at a resolution of 4 cm−1 between 4000 and 400 cm−1. Before analysis, the baseline of each spectrum was modified.
Differential Scanning Calorimetry (DSC)
DSC (TA Instruments, USA) was used to determine the glass transition temperature (Tg) of chitosan and CEO-loaded chitosan capsules. The samples were tested at temperatures ranging from 25 to 600 °C at a heating rate of 10 °C/min.
Thermo-gravimetric Analysis (TGA)
Thermal stabilities of chitosan, CEO, and CEO-loaded chitosan capsules were measured using a thermo-gravimetric analyzer (TA Q50 system, USA). Nearly 10 mg of each sample was weighed and heated from 25 to 600 °C at a heating rate of 10 °C/min under a nitrogen environment.
Morphology of the Microcapsules
The surface morphology of capsules was examined using a field emission scanning electron microscope (TESCAN MIRA3 LMH, USA) operating at acceleration voltage (10–20 kV). The capsules were cut into small pieces before being sputter-coated with gold and placed on the sample holder.
Antioxidant Activity Measurement
The antioxidant activities of chitosan, CEO, and CEO-loaded chitosan capsules were evaluated using DPPH free radical scavenging (Hashem et al., 2022). Each sample was combined with 100 mL of 0.1 M ethanolic DPPH solution and kept in the dark for 1 h at room temperature. The absorbance at 517 nm was measured using UV–Vis spectroscopy (UV-1800, Shimadzu). A control sample was prepared without CEO, and the baseline was adjusted with ethanol. The antioxidant capacity was determined as a percentage of the DPPH radical scavenging capability using Eq. (4) (Kadam et al., 2021).
Determination of Antimicrobial Properties
The vapor phase method examined the inhibitory activity of CEO-loaded chitosan capsules. Nutrient agar solution was prepared and autoclaved before making agar plates. 20 mL of nutrient agar solution was placed in bottom particulates and kept until solidification in laminar flow. After the agar solidification, 200 μL of bacterial suspension was spread with the help of a L-shaped glass spreader. Simultaneously, the 300 mg CEO encapsulated chitosan capsules were placed in sachets and heat sealed. One sachet was pasted on the top of the Petri plate with the help of double-sided tape. This top plate was used to cover the bottom inoculated agar plate to ensure the release of CEO in the vapor phase. These plates were incubated at 37 °C to test for antibacterial characteristics.
Shelf-Life Study and Color of Cake
Preparation of Cake Sample and Packaging
The cake was prepared by mixing the following ingredients: 1 cup of flour, ½ cup of sugar, ¼ cup of oil, ¾ cup of milk, ¼ tsp of baking soda, 1 tsp baking powder, and no preservatives were used. The ingredients were mixed and baked in the microwave for 5 min at high power. The cakes were packed in LDPE Ziplock bags. On the other hand, 300 mg of CEO encapsulated chitosan capsules were sealed in a sachet of 25 mm × 42 mm size, as shown in Fig. 1a. These sachets were also packed along with cakes in LDPE pouches and stored at 25 °C for further microbial analysis Fig. 1b.
Sensory Evaluation
The effect of clove oil released from the chitosan capsule may change the aroma of the bread. Therefore, sensory evaluation in terms of the dry cakes’ aroma and textures was done for 10 days.
Microbial Analysis of Cake Samples
For microbial analysis, the total plate count was determined. Briefly, the nutrient agar solution was prepared and autoclaved before making the agar plate. 25 mL of agar was poured on petri plates under laminar conditions and rested until the agar gelation. Approximately 1 g of the cake was homogenized with 10 mL of distilled water and centrifuged for 3 min at 5000 rpm at room temperature; then, the supernatant was taken and serially diluted three times. The prepared agar plates were then inoculated with 200 µL of the diluted solution. These plates were stored at 37 °C, and bacterial growth was observed. The same procedure was applied for all the packed bread with and without CEO-encapsulate chitosan every 5th day.
Results and Discussion
Encapsulation Efficiency (EE), Loading Capacity (LC)
The absorbance at 282 nm was used to calculate the amount of CEO loaded into the capsule. Table 1 shows that the LC of CEO in chitosan capsules made with a TPP crosslinking agent was 0.55–8.01% and increased with increasing CEO concentration. This meant that 100 g of chitosan capsule contains between 0.55 and 8.01 g of CEO. This LC was higher when compared to previous works using a 1% TPP crosslinker (Woranuch & Yoksan, 2013a). This might be due to the shrinkage of nanoparticles (Ajun et al., 2009) in previous works, but our work involved macro capsules, in which shrinkage by crosslinker was not elevated. Encapsulation efficiency (EE) of particles prepared using 1% (w/v) of TPP was in the range of 1.275–12.01% (Table 1). The EE tended to increase with increasing initial eugenol content; the maximum EE value was obtained for the sample prepared using a weight ratio of chitosan to eugenol of 1:1.50 (12.01%).
Encapsulation and Color Variation
CEO-loaded chitosan capsules were formed in two steps: droplet production and solidification. The droplet-creation process is an oil-in-water emulsion, while the solidification method is ionic gelation. The protonated amino group of the chitosan molecule surrounded the oil droplet with polyphosphate groups of the TPP molecule, resulting in the creation of capsules in the chitosan solution. The color difference was easily noticeable when the concentration of CEO in chitosan increased. Table 1 displays the capsules’ L*, a*, b*, and E values. The unloaded capsules were light in color, whereas CEO-loaded capsules had dark yellow–brown color. The loading of CEO into the capsules decreased the L* value from 58.56 (unloaded) to 49.78 (loaded 1:1.5), indicating a loss of luminance. This was in resemblance with the actual color as the capsules became darker. The* value, once in red and on the other hand, increased from 7.9 (unloaded) to 14.54 (loaded 1:1.5), indicating an increase in green. The capsules with high CEO were brownish, and this can be represented by a high value of a* showing redness. Further, the b* value decreased from 29.45 (Unloaded) to 22.78 (loaded 1:1.5); this was not a significant decrease, showing less change in yellow color. Finally, the ΔE values were calculated using a 1:0 unloaded sample as a reference. The ΔE value of 1:0.25 and 1:0.5 loaded capsules were less than 5, showing that there were nominal color changes in CEO-loaded sample when the concentration was 1:0.5. ΔE further increased as CEO concentration increased, and a significant increase was observed above the 1:1 ratio (Table 2).
FT-IR
Figure 2a–b illustrates FTIR spectra of CEO, chitosan, and CEO-loaded capsules. CEO shows a characteristic broad peak near 3500 cm−1 indicating -OH stretching (alcohol), 3074 cm−1 and 3000 cm−1 denoting C-H stretching of alkane, 2938 cm−1 and 2842 cm−1 denoting C-H stretching of alkane. Further, peaks were seen at 1637 cm−1 and 1611 cm−1, indicating C = C elongation and 1511 cm−1 denoting C-H stretching (aromatic), 1430 cm−1 and 1367 cm−1 denoting -OH bending, 1264 cm−1 and 1232 cm−1 denoting C-O stretching (aromatic ester), 1149 cm−1 and 1124 cm−1 denoting C-O stretching (tertiary alcohol), 1032 cm−1 denoting C-O stretching (alcohol), 992 cm−1 denoting C = C bending (monosubstituted alkene), 816 cm−1 denoting C = C bending (trisubstituted alkene), 793 cm−1 denoting C-H bending (disubstituted alkene) which were similar to those in literature (Hadidi et al., 2020a; Wang et al., 2021).
In the case of chitosan, the characteristics peaks were observed at 3356 cm−1 denoting -NH and -OH stretching, 2928 cm−1 denoting C-H stretching (alkane), 1651 cm−1 denoting C = O (amide I), 1589 cm−1 denoting -NH (amide II), 1421 cm−1 denoting HN-CO (amide III). Other peaks were observed at 1152 cm−1 denoting C-N stretching (amine) and 989 cm−1 denoting C = C bending (monosubstituted alkene), similar to the previous studies (Hadidi et al., 2020a; Priyadarshi et al., 2018b; Tavares et al., 2019; Wang et al., 2021; Woranuch & Yoksan, 2013b).
To ensure the encapsulation of CEO in chitosan capsules, the FTIR of capsules was also performed. The peaks of chitosan matched with CEO-loaded chitosan capsules as the surface is made of chitosan itself (Fig. 2b). However, some distinct peaks were also observed, which were due to the presence of CEO. This also suggests that CEO might be migrating from inside the capsule toward the environment. The distinct peaks of CEO were observed at 1615 cm−1, indicating C = C stretching, 1512 cm−1 denoting C-H stretching (aromatic), 1430 cm−1 denoting -OH bending, 1270 cm−1 and 1233 cm−1, denoting C-O stretching (aromatic ester), 819 cm−1 denoting C = C bending (trisubstituted alkene), 793 cm−1 denoting C = C bending (monosubstituted alkene).
CEO-Loaded Chitosan Capsules Oxidative Stability
The oxidative stability was examined using a DSC thermograph. In the instance of the CEO, a peak at 258 °C was noted, indicating auto-oxidation of the oil. The neat chitosan didn’t have any oxidation peak, but after the crosslinking with TPP to form a chitosan capsule, the oxidation reaction peak was observed at 248 °C, which might be due to the matrix formation that provides the structural stability, thus preventing the capsule from deformation or disintegration when exposed to heat or external stresses. Further, when CEO was incorporated in chitosan capsules, the peak was observed at 249.5 °C for a 1:0.25 ratio, which shifted to 254 °C for 1:1.5. This increase in oxidation peak with increasing CEO concentration might be attributed to CEO’s antioxidant properties; similar results have been obtained in prior investigations (Woranuch & Yoksan, 2013a). This also indicates the CEO-encapsulated chitosan capsule’s high thermal stability and the CEO’s existence at elevated temperatures. The higher thermal stability of CEO encapsulation results in better antioxidant capacity (Ling et al., 2022). (Fig. 3)
TGA
Thermogravimetric analysis (TGA) is a technique for measuring the mass of a substance as a function of temperature or time when the sample is subjected to a controlled temperature program in a controlled environment. Figure 4 shows the weight loss of clove oil, chitosan, chitosan capsules, and CEO-loaded capsules. TGA of CEO suggests that the onset of degradation temperature was 87 °C, and the maximal degradation peak was achieved at 146 °C, which is near 177 °C in previous studies (Hadidi et al., 2020b). The maximum degradation peak of CEO was less than that of pure eugenol (203 °C) (Figueroa-Lopez et al., 2020) as CEO contains other components which might degrade earlier.
The unloaded chitosan capsules showed two-step mass loss, first at 92 °C, which might be due to moisture loss, and 2nd step at 256 °C representing denaturization of the chitosan matrix (Hadidi et al., 2020a; Kadam et al., 2021; Keawchaoon & Yoksan, 2011; Woranuch & Yoksan, 2013a) and degradation of CH D-glucosamine and N-acetyl-D-glucosamine units, respectively (Tavares & Noreña, 2020). In CEO-loaded chitosan capsules, the degradation occurred in three steps. Temperature-dependent mass loss was detected between 90–100 °C, 220–260 °C, and 300–340 °C. This third transition was due to the release of encapsulated or trapped CEO in chitosan capsules, as seen in previous studies (Keawchaoon & Yoksan, 2011; Woranuch & Yoksan, 2013c). This also concludes that the CEO was successfully encapsulated in the chitosan capsules. Furthermore, CEO-loaded capsules disintegrated at a temperature greater than clove oil, indicating that encapsulation inside chitosan microcapsules increased the thermal stability of clove oil.
FE-SEM
The surface morphology of chitosan capsules and CEO-loaded chitosan capsules was examined by FE-SEM. According to the electron microscopy, the unloaded chitosan capsule was free of holes or circular patches (Fig. 4a), while the CEO-loaded capsules had tiny holes (Fig. 4b–g). These holes might represent the small CEO droplets coming out of the capsules. As the CEO concentration increased, the pores on the capsule surface increased, as shown in previous studies (Priyadarshi et al., 2018b; Sharma et al. 2022a). This also denotes that the CEO can be easily released from the chitosan capsules (C. Chen et al., 2018a). (Fig. 5)
Antioxidant Activity of the Capsules
The antioxidant activity of the CEO-loaded and unloaded capsules was investigated using the DPPH scavenging test. The antioxidants in the DPPH analysis convert the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical into a molecule with yellow-colored (2,2-diphenyl-1-picrylhydrazine), and the extent of this reaction is significantly dependent on the antioxidants’ hydrogen-donating capacity. Others have found pure CEO’s free radical scavenging action, which is primarily due to the presence of phenolic and terpenes like eugenol and -caryophyllene, which can provide hydrogen to the DPPH to stabilize it (X. Chen et al., 2017; Donsì et al., 2011). In this approach, the freshly made DPPH solution has a deep purple color, with maximum absorption at 517 nm. Antioxidants can scavenge DPPH free radicals and convert them into a colorless product, resulting in a decrease in absorbance at 517 nm. CEO encapsulated capsules had DPPH radical scavenging activity ranging from 91.82 to 93.05%, while unloaded capsules had 90.89% (Fig. 6), which were much higher than previous studies involving Quercetin-loaded chitosan nanoparticles (Souza et al., 2014).
Antimicrobial Activity of the Capsules
The antibacterial activity of capsules was investigated in this work against E. coli and S. aureus. As the content of clove oil in capsules increases, bacterial growth reduces, which was in line with past studies when clove essential oil with cinnamon was used as an antimicrobial agent against E. coli (Cava-Roda et al., 2012). Antibacterial activity was measured in colony-forming units (CFU/ml). Antimicrobial observation shows that capsules having Chitosan to CEO ratio of 1:0.75, 1:1, 1:1.25, 1:1.50, E. coli growth was completely inhibited in most Petri plates (C. Chen et al., 2018b). When tested against S. aureus, no viable bacterial colony was observed at Chitosan to clove oil ratio 1:1, 1:1.25, 1:1.50.(Fig. 7)
Sensory Analysis
Sensory analysis was done manually by observing the dry cakes for ten days. On day 0, the cakes were soft and yellow in texture. On day 10, the color of both the cakes turned light and dull but had no significant difference when compared. When comparing odor, both dry cakes had no significant changes; this might be due to the sustained release of CEO, thus not affecting the aroma compared to the polymer film release. However, when packed in LDPE, the dry cakes were a little hard compared to those packed with an antimicrobial sachet.
Shelf Life of Dry Cakes
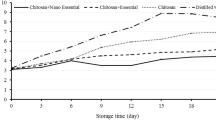
The CEO-loaded chitosan capsules with a loading ratio 1:1 showed complete inhibition against E. coli and S. aureus. Therefore, this was chosen for the antimicrobial study of dry cakes. The dry cakes were packed in LDPE (Low-Density Polyethylene) pouches with and without antimicrobial sachet and studied for a shelf-life extension, Fig. 8. On the initial day, 5.94 log CFU/ml of bacterial growth was observed, as shown in Table 3. When packed in LDPE pouches without capsules and stored at 25 °C, the bacterial growth increased to 6.39 log CFU/ml. At the same time, it decreased to 5.79 log CFU/ml when packed with an antimicrobial sachet. The decrease in bacterial growth might be due to the release of essential oil and its bactericidal effect on dry cakes. On day 10, the dry cakes packed without capsules in LDPE pouches crossed the 7 log CFU/ml compared to 6.24 log CFU/ml with an antimicrobial sachet. 7 log CFU/ml was used as a limited amount of bacteria in literature for white bread (bakery product) (Sharma et al., 2022c); this concludes that the shelf life of dry cakes in LDPE pouches is less than 10 days (5–10 days) and when packed with antimicrobial sachets, the shelf life extended above 10 days.
Conclusion
In this study, the developed antimicrobial microcapsules, without having physical contact with the food, can reduce the growth of the bacteria on the dry cakes and thus extend the shelf life. The CEO was loaded into the chitosan capsule with the help of the ionic gelation crosslinking method. As the clove oil concentration increased, the capsules became darker, and the encapsulation efficiency and loading capacity of about 12.015% and 8.01% for 1.50:1 loaded samples were achieved. FE-SEM and FTIR of the capsule concluded that the pores in the capsule increased with the increase in loading, and the FTIR peaks of CEO were present in the capsule. Thermal degradation of the loaded capsule occurred in three steps rather than two steps compared to chitosan. The antioxidant activity of the CEO was confirmed with the DSC, as an oxidation peak was observed near 248. DPPH radical scavenging, on the other hand, showed high antioxidant activity, more than 90%. To increase the shelf life of dry cakes, the antimicrobial property of different loading was done against S. aureus and E. coli, and the 1:1 loading completely inhibited bacterial growth. Thus, the 1:1 loaded capsules were used to extend the shelf life of dry cakes and increase the shelf life for more than 10 days.
In conclusion, chitosan capsules loaded with CEO can potentially increase dry cake’s shelf life without affecting the sensory properties. This opens new opportunities for other bakery items involving pastries, donuts, muffins, or waffles.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AP:
-
Active packaging
- CEO:
-
Clove essential oil
- CFU:
-
Colony Forming Unit
- CIE:
-
Commission Internationale de l'Elcairage
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DSC:
-
Differential Scanning Calorimetry
- EE:
-
Encapsulation efficiency
- EOs:
-
Essential oils
- FE-SEM:
-
Field-Emission Scanning Electron Microscope
- FT-IR:
-
Fourier Transform-Infrared
- LC:
-
Loading capacity
- LDPE:
-
Low-Density Polyethylene
- MAP:
-
Modified Atmosphere Packaging
- TGA:
-
Thermogravimetric analysis
- TPP:
-
Tripolyphosphate
References
Ajun, W., Yan, S., Li, G., & Huili, L. (2009). Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydrate Polymers, 75(4), 566–574. https://doi.org/10.1016/j.carbpol.2008.08.019
Al-Naamani, L., Dobretsov, S., & Dutta, J. (2016). Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innovative Food Science & Emerging Technologies, 38, 231–237. https://doi.org/10.1016/J.IFSET.2016.10.010
Beyki, M., Zhaveh, S., Khalili, S. T., Rahmani-Cherati, T., Abollahi, A., Bayat, M., et al. (2014). Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Industrial Crops and Products, 54, 310–319. https://doi.org/10.1016/J.INDCROP.2014.01.033
Cai, L., & Wang, Y. (2021). Physicochemical and antioxidant properties based on fish sarcoplasmic protein/chitosan composite films containing ginger essential oil nanoemulsion. Food and Bioprocess Technology, 14(1), 151–163. https://doi.org/10.1007/S11947-020-02564-0/FIGURES/6
Cava-Roda, R. M., Taboada-Rodríguez, A., Valverde-Franco, M. T., & Marín-Iniesta, F. (2012). Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157:H7 in Milk. Food and Bioprocess Technology, 5(6), 2120–2131. https://doi.org/10.1007/S11947-010-0484-4/FIGURES/6
Chaieb, K., Hajlaoui, H., Zmantar, T., Kahla-Nakbi, A. Ben, Rouabhia, M., Mahdouani, K., & Bakhrouf, A. (2007). The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytotherapy Research, 21(6), 501–506. https://doi.org/10.1002/PTR.2124
Chen, C., Xu, Z., Ma, Y., Liu, J., Zhang, Q., Tang, Z., et al. (2018a). Properties, vapour-phase antimicrobial and antioxidant activities of active poly(vinyl alcohol) packaging films incorporated with clove oil. Food Control, 88, 105–112. https://doi.org/10.1016/J.FOODCONT.2017.12.039
Chen, X., Ren, L., Li, M., Qian, J., Fan, J., & Du, B. (2017). Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chemistry, 214, 432–439. https://doi.org/10.1016/J.FOODCHEM.2016.07.101
Donsì, F., Annunziata, M., Sessa, M., & Ferrari, G. (2011). Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Science and Technology, 44(9), 1908–1914. https://doi.org/10.1016/J.LWT.2011.03.003
El-Naggar, M. E., Hasanin, M., & Hashem, A. H. (2022). Eco-friendly synthesis of superhydrophobic antimicrobial film based on cellulose acetate/polycaprolactone loaded with the green biosynthesized copper nanoparticles for food packaging application. Journal of Polymers and the Environment, 30(5), 1820–1832. https://doi.org/10.1007/S10924-021-02318-9/FIGURES/9
Elsayed, N., Hasanin, M. S., & Abdelraof, M. (2022). Utilization of olive leaves extract coating incorporated with zinc/selenium oxide nanocomposite to improve the postharvest quality of green beans pods. Bioactive Carbohydrates and Dietary Fibre, 28, 100333. https://doi.org/10.1016/j.bcdf.2022.100333
Ezhilarasi, P. N., Karthik, P., Chhanwal, N., & Anandharamakrishnan, C. (2012). Nanoencapsulation techniques for food bioactive components: A review. Food and Bioprocess Technology 2012 6:3, 6(3), 628–647. https://doi.org/10.1007/S11947-012-0944-0
Figueroa-Lopez, K. J., Cabedo, L., Lagaron, J. M., & Torres-Giner, S. (2020). Development of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) monolayers containing eugenol and their application in multilayer antimicrobial food packaging. Frontiers in Nutrition, 7. https://doi.org/10.3389/FNUT.2020.00140
Gutierrez, J., Barry-Ryan, C., & Bourke, P. (2008). The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. International Journal of Food Microbiology, 124(1), 91–97. https://doi.org/10.1016/J.IJFOODMICRO.2008.02.028
Hadidi, M., Pouramin, S., Adinepour, F., Haghani, S., & Jafari, S. M. (2020a). Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydrate Polymers, 236, 116075. https://doi.org/10.1016/J.CARBPOL.2020.116075
Hadidi, M., Pouramin, S., Adinepour, F., Haghani, S., & Jafari, S. M. (2020b). Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2020.116075
Hasanin, M., & Al Kiey, S. A. (2021). Development of ecofriendly high performance anti-corrosive chitosan nanocomposite material for mild steel corrosion in acid medium. Biomass Conversion and Biorefinery, 1, 1–14. https://doi.org/10.1007/S13399-021-02059-8/FIGURES/7
Hashem, A. H., Al Abboud, M. A., Alawlaqi, M. M., Abdelghany, T. M., & Hasanin, M. (2022). Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Starch - Stärke, 74(1–2), 2100165. https://doi.org/10.1002/star.202100165
Hasheminejad, N., Khodaiyan, F., & Safari, M. (2019a). Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chemistry. https://doi.org/10.1016/j.foodchem.2018.09.085
Hasheminejad, N., Khodaiyan, F., & Safari, M. (2019b). Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chemistry, 275, 113–122. https://doi.org/10.1016/J.FOODCHEM.2018.09.085
Hu, B., Pan, C., Sun, Y., Hou, Z., Ye, H., Hu, B., & Zeng, X. (2008). Optimization of fabrication parameters to produce chitosan-tripolyphosphate nanoparticles for delivery of tea catechins. Journal of Agricultural and Food Chemistry, 56(16), 7451–7458. https://doi.org/10.1021/JF801111C/ASSET/IMAGES/LARGE/JF-2008-01111C_0002.JPEG
Islam, S., Bhuiyan, M. A. R., & Islam, M. N. (2017, September 1). Chitin and Chitosan: Structure, properties and applications in biomedical engineering. Journal of Polymers and the Environment. Springer New York LLC. https://doi.org/10.1007/s10924-016-0865-5
Kardam, S. K., Kadam, A. A., & Dutt, D. (2021). Retention of cinnamaldehyde in poly(vinyl alcohol) films intended for preservation of faba beans through vapor-phase antimicrobial effect. Food Packaging and Shelf Life, 29, 100704. https://doi.org/10.1016/J.FPSL.2021.100704
Kadam, A. A., Singh, S., & Gaikwad, K. K. (2021). Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control, 124, 107877. https://doi.org/10.1016/J.FOODCONT.2021.107877
Keawchaoon, L., & Yoksan, R. (2011). Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids and Surfaces b: Biointerfaces, 84(1), 163–171. https://doi.org/10.1016/J.COLSURFB.2010.12.031
Kowalska, J., Szewczyńska, M., & Pośniak, M. (2015). Measurements of chlorinated volatile organic compounds emitted from office printers and photocopiers. Environmental Science and Pollution Research, 22(7), 5241–5252. https://doi.org/10.1007/s11356-014-3672-3
Ling, J. K. U., Sam, J. H., Jeevanandam, J., Chan, Y. S., & Nandong, J. (2022). Thermal degradation of antioxidant compounds: Effects of parameters, thermal degradation kinetics, and formulation strategies. Food and Bioprocess Technology, 15(9), 1919–1935. https://doi.org/10.1007/S11947-022-02797-1/FIGURES/2
Lord, M. S., Cheng, B., McCarthy, S. J., Jung, M. S., & Whitelock, J. M. (2011). The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials, 32(28), 6655–6662. https://doi.org/10.1016/J.BIOMATERIALS.2011.05.062
Muriel-Galet, V., Cran, M. J., Bigger, S. W., Hernández-Muñoz, P., & Gavara, R. (2015). Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. Journal of Food Engineering, 149, 9–16. https://doi.org/10.1016/J.JFOODENG.2014.10.007
Nadjib, B. M., Amine, F. M., Abdelkrim, K., Fairouz, S., & Maamar, M. (2014). Liquid and vapour phase antibacterial activity of Eucalyptus globulus essential oil = Susceptibility of selected respiratory tract pathogens. American Journal of Infectious Diseases, 10(3), 105–117. https://doi.org/10.3844/AJIDSP.2014.105.117
Passone, M. A., Girardi, N. S., & Etcheverry, M. (2012). Evaluation of the control ability of five essential oils against Aspergillus section Nigri growth and ochratoxin A accumulation in peanut meal extract agar conditioned at different water activities levels. International Journal of Food Microbiology, 159(3), 198–206. https://doi.org/10.1016/J.IJFOODMICRO.2012.08.019
Priyadarshi, R., Sauraj, Kumar, B., & Negi, Y. S. (2018a). Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydrate Polymers, 195(February), 329–338. https://doi.org/10.1016/j.carbpol.2018.04.089
Priyadarshi, R., Sauraj, Kumar, B., Deeba, F., Kulshreshtha, A., & Negi, Y. S. (2018b). Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocolloids, 85(March), 158–166. https://doi.org/10.1016/j.foodhyd.2018.07.003
Radünz, M., da Trindade, M. L. M., Camargo, T. M., Radünz, A. L., Borges, C. D., Gandra, E. A., & Helbig, E. (2019). Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chemistry, 276, 180–186. https://doi.org/10.1016/J.FOODCHEM.2018.09.173
Saranraj, P., & Geetha, M. (2012). Microbial spoilage of bakery products and its control by preservatives. International Journal of Pharmaceutical & Biological Archives, 3(1), 38–48. https://www.researchgate.net/publication/259495423_Microbial_Spoilage_of_Bakery_Products_and_Its_Control_by_Preservatives. Accessed 28 May 2023
Sebaaly, C., Jraij, A., Fessi, H., Charcosset, C., & Greige-Gerges, H. (2015). Preparation and characterization of clove essential oil-loaded liposomes. Food Chemistry, 178, 52–62. https://doi.org/10.1016/J.FOODCHEM.2015.01.067
Sharma, B., Sauraj, S., Kumar, B., Pandey, A., Dutt, D., Negi, Y. S., et al. (2021). Synthesis of waterborne acrylic copolymer resin as a binding agent for the development of water-based inks in the printing application. Polymer Engineering & Science, 61(5), 1569–1580. https://doi.org/10.1002/PEN.25681
Sharma, B., Singh, S., Prakash, B., Sharma, H., Maji, P. K., Dutt, D., & Kulshreshtha, A. (2022a). Effect of cellulose nanocrystal incorporated acrylic copolymer resin on printing properties of waterborne inks. Progress in Organic Coatings, 167, 106842. https://doi.org/10.1016/J.PORGCOAT.2022.106842
Sharma, K., Babaei, A., Oberoi, K., Aayush, K., Sharma, R., & Sharma, S. (2022b). Essential oil nanoemulsion edible coating in food industry: A review. Food and Bioprocess Technology 2022b 15:11, 15(11), 2375–2395. https://doi.org/10.1007/S11947-022-02811-6
Sharma, P., Ahuja, A., Dilsad Izrayeel, A. M., Samyn, P., & Rastogi, V. K. (2022c). Physicochemical and thermal characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) films incorporating thyme essential oil for active packaging of white bread. Food Control, 133, 108688. https://doi.org/10.1016/j.foodcont.2021.108688
Singh, S., Gaikwad, K. K., & Lee, Y. S. (2018). Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. International Journal of Biological Macromolecules, 107, 1879–1887. https://doi.org/10.1016/J.IJBIOMAC.2017.10.057
Souza, M. P., Vaz, A. F. M., Correia, M. T. S., Cerqueira, M. A., Vicente, A. A., & Carneiro-da-Cunha, M. G. (2014). Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food and Bioprocess Technology, 7(4), 1149–1159. https://doi.org/10.1007/S11947-013-1160-2/FIGURES/6
Tavares, L., Barros, H. L. B., Vaghetti, J. C. P., & Noreña, C. P. Z. (2019). Microencapsulation of garlic extract by complex coacervation using whey protein isolate/chitosan and gum Arabic/chitosan as wall materials: Influence of anionic biopolymers on the physicochemical and structural properties of microparticles. Food and Bioprocess Technology, 12(12), 2093–2106. https://doi.org/10.1007/S11947-019-02375-Y/TABLES/4
Tavares, L., & Noreña, C. P. Z. (2020). Encapsulation of ginger essential oil using complex coacervation method: Coacervate formation, rheological property, and physicochemical characterization. Food and Bioprocess Technology, 13(8), 1405–1420. https://doi.org/10.1007/S11947-020-02480-3/FIGURES/8
Varma, A. J., Deshpande, S. V., & Kennedy, J. F. (2004). Metal complexation by chitosan and its derivatives: A review. Carbohydrate Polymers, 55(1), 77–93. https://doi.org/10.1016/J.CARBPOL.2003.08.005
Wang, W., Zhang, Y., Yang, Z., & He, Q. (2021). Effects of incorporation with clove (Eugenia caryophyllata) essential oil (CEO) on overall performance of chitosan as active coating. International Journal of Biological Macromolecules, 166, 578–586. https://doi.org/10.1016/J.IJBIOMAC.2020.10.215
Woranuch, S., & Yoksan, R. (2013a). Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydrate Polymers, 96(2), 578–585. https://doi.org/10.1016/J.CARBPOL.2012.08.117
Woranuch, S., & Yoksan, R. (2013b). Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2012.08.117
Woranuch, S., & Yoksan, R. (2013c). Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydrate Polymers, 96(2), 578–585. https://doi.org/10.1016/j.carbpol.2012.08.117
Yoksan, R., Jirawutthiwongchai, J., & Arpo, K. (2010). Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloids and Surfaces b: Biointerfaces, 76(1), 292–297. https://doi.org/10.1016/J.COLSURFB.2009.11.007
Acknowledgements
The authors wish to thank the Indian Institute of Technology Roorkee, India, for providing sufficient experimental facilities for carrying out this research.
Funding
This study was funded by the Ministry of Education (MoE), Government of India.
Author information
Authors and Affiliations
Contributions
Harish Sharma: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—original draft. Arihant Ahuja: Formal analysis, Writing—review & editing, Bhavna Sharma: Formal analysis, Writing—review & editing. Anurag Kulshreshtha: Formal analysis. Ashish Kadam: Conceptualization, Methodology, Supervision. Dharm Dutt: Conceptualization, Methodology, Supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, H., Ahuja, A., Sharma, B. et al. Vapor Phase Antimicrobial Active Packaging Application of Chitosan Capsules Containing Clove Essential Oil for the Preservation of Dry Cakes. Food Bioprocess Technol 17, 780–790 (2024). https://doi.org/10.1007/s11947-023-03151-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03151-9