Abstract
The present work revealed there was a conceptual difference in the thermal decomposition behaviors between the complexed β-cyclodextrin (CD) in an inclusion system and the β-CD complex of guest. The thermal decomposition behaviors of the solid inclusion complexes of β-CD with ethylenediamine (Eda), diethylenetriamine (Dta) and triethylamine (Tea) were investigated using nonisothermal thermogravimetry (TG) analysis based on weight loss as a function of temperature. In view of TG profiles, a consecutive mechanism describing the formation and thermal decomposition of the three solid supermolecules of β-CD was presented. Heating rate has very different effects on the thermal decomposition behaviors of these complexes. The faster the heating rate is, the higher the melting-decomposition point of the complexed β-CD in an inclusion system is, and on the whole the bigger the rate constant (k) of the thermal decomposition reaction of the complexed β-CD is. The thermal decomposition process of the complexed β-CD for each inclusion system is determined to be simple first-order reaction using Ozawa method. The apparent activation energies (E a) and frequency factors (A) of the thermal decomposition reactions of the complexed β-CD molecules have been also calculated. It is found that when the decomposition reaction of the complexed β-CD encountered a large value of E a, such as that in Dta–β-CD system, an apparent compensation effect of A on E a can provide enough energy to conquer the reaction barrier in prompting the k value of thermal decomposition reaction of the complexed β-CD according to Arrhenius equation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-Cyclodextrin (β-CD) is a cyclic oligosaccharide composed of seven α-d-glucopyranosyl units. It can be visualized as a toroidal, hollow, truncated cone having highly hydrophilic surface and relatively hydrophobic cavity [1–3]. Because of the hydrophobic environment within intramolecular cavity, β-CD can form various supramolecular inclusion complexes with a wide variety of guest molecules [4–6].
Amines take part in many kinds of chemical reactions, and in particular, they can react with an acyl chloride, acid anhydride or ester to form an amide bond. All simple reactions of amines involve bonding of an electron-deficient atom to the amino nitrogen through its unshared electron pair. Ethylenediamine (Eda), diethylenetriamine (Dta) and triethylamine (Tea) are all very typical primary, secondary and tertiary amino-functional organic compounds, respectively. They have been widely applied to areas of synthetic chemistry, coordination chemistry and supramolecular chemistry.
So far, much less attention has been paid to the preparation and characterization of solid β-CD inclusion complexes of organic guests especially short acyclic compounds such as small aliphatic amines, relative to large numbers of reports on complexation interactions between β-CD and alkyl or aryl guests in solution [7–9]. Toxicity experiments show that β-CD as a host molecule can be absorbed as carbohydrate by animal and human bodies [10], and its inclusion complexes of many organic guests have been successfully utilized in perfume, pharmaceutical, food additives and other fields [11]. To improve the thermal stability of guest or to control the release of guest is one of the most interesting problems concerning the studies of solid CD inclusion complexes [12–14].
It is clear that the investigations on the kinetic mechanism of thermal decomposition and the stability of CD inclusion complexes will help us further reveal the nature of inclusion phenomena, and can allow us to estimate the potential practical application of CDs in many industries [15, 16]. It stands to reason that if the thermal decomposition behavior of complexed CD is regarded as that of its inclusion complex of guest, a serious mistake will certainly occur [17–19]. The present work revealed a conceptual difference in the thermal decomposition between the complexed β-CD (without guest) and the complex of β-CD (with guest). In view of the low release temperatures of organic guests compared to the melting-decomposition points of free and complexed β-CD, thermal decomposition behaviors of β-CD complexes occur only before guests disappear from their complexes of β-CD. After entirely releasing guest molecules, thermal decomposition behaviors of β-CD complexes should be regarded as thermal decomposition behaviors of the complexed β-CD without guest. Therefore, it really becomes absolutely necessary to make clear the relationships among the thermal decomposition behaviors of free β-CD, the complexed β-CD without guest and the complex of β-CD with guest. For these reasons, three aliphatic amines: Eda, Dta and Tea were chosen as guest molecules, and β-CD was selected as the host molecule in this study. Their molecular structures are shown in Fig. 1.
In the present work, the inclusion complexes of β-CD with the three simple alkyl amines were prepared and characterized by elemental analysis and 1H NMR spectroscopy. The thermal decomposition behaviors of free β-CD, the complexed β-CD without guest and the β-CD complexes: Eda–β-CD, Dta–β-CD and Tea–β-CD, were investigated using non-isothermal thermogravimetry analysis.
Experimental
Materials
β-Cyclodextrin was purchased from Shanghai Chemical Reagent Company and recrystallized twice from deionized distilled water before use. Three simple organic amines as guests: Eda, Dta and Tea, were purchased from Shanghai Chemical Reagent Company and used without further purification. All other chemicals were of general-purpose reagent grade unless otherwise stated.
Preparation and characterization of the solid inclusion complexes
The solid inclusion complexes were prepared and purified according to the following method: A guest/host molar ratio of 10:1 was used in the initial reaction mixture with stirring 48 h at 298.2 K. The crude reaction products were, in turn, washed with deionized distilled water (3 × 5 mL), anhydrous ethanol (3 × 3 mL) and anhydrous diethyl ether (3 × 3 mL). After the solvents were removed under vacuum, the solid inclusion complexes were dried over 24 h at 383.2 K in vacuo. The host–guest stoichiometries in the solid complexes were determined to be 2:5 for Eda–β CD (Anal. Calcd. for C94H180N10O70· 4H2O: C, 42.73; H, 7.12; N, 5.30. Found: C, 42.40; H, 7.16; N, 5.53), 1:1 for Dta–β-CD (Anal. Calcd. for C46H83N3O35· H2O: C, 43.98; H, 6.77; N, 3.35. Found: C, 44.16; H, 6.80; N, 3.09) and 3:1 for Tea–β-CD (Anal. Calcd. for C132H225NO105·21H2O: C, 40.81; H, 6.88; N, 0.36. Found: C, 40.95; H, 6.77; N, 0.49), based on elemental analysis. And the stoichiometries were further confirmed by comparing the relative integral areas of the related proton signals between β-CD and the guests. For example, the integral area ratios of the sum of signals of H-2 and H-4 protons of β-CD to CH2 proton signals of Eda at 2.67 ppm, Dta at 2.74 ppm and Tea at 2.81 ppm in their respective inclusion complexes were 1.42, 1.79 and 7.07, respectively, which were in accordance with the results (1.40, 1.75 and 7.00) from elemental analysis.
TG curves were recorded on Shimadzu TGA-50 thermogravimetric analyzer at constant heating rates of 10.0 (ζ1), 15.0 (ζ2) and 20.0 (ζ3) K·min−1 under a nitrogen atmosphere. The solid samples in the study were used as crystallite powders. The sample size was about 5.5 mg for the various TG experiments. Elemental analyses were carried out on an Elementar Vario EL III elemental analyzer. 1H NMR spectra of the solid samples were obtained on Bruker NMR spectrometer at 300 M Hz at 298.2 K, using D2O as solvent and TMS as internal reference.
Results and discussion
TG profiles of β-CD and its inclusion complexes
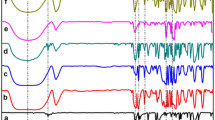
The thermal stabilities of solid inclusion complexes and the kinetic mechanisms of their thermal decomposition reactions can have an important prognostic value not only in academic studies but also in industrial applications [16, 20–22]. The typical TG curves of free β-CD and its three supramolecular inclusion complexes: Eda–β-CD, Dta–β-CD and Tea–β-CD, at three constant heating rates (ζ1, ζ2 and ζ3) were depicted in Fig. 2A–D, respectively.
Thermal decomposition of free β-CD
Figure 2A illustrated the TG curves of free β-CD hydrate, β-CD·8H2O, under three constant heating rates, and the weight loss processes of free β-CD could be divided into three stages. The first weight loss (Stage a) in the range of 298.2–454.4 K was attributed to the release of eight water molecules (cal. 7.71), including complexed water inside the cavity of β-CD and uncomplexed water outside the cavity of β-CD. The second weight loss (Stage b) in the range of 539.3–694.1 K was due to melting-decomposition of β-CD. And finally a nonlinear decline of remains appeared (Stage c). In both a and b stages, the thermal decomposition of free β-CD at a faster heating rate ζ2 or ζ3 compared with ζ1 not only needed a wider range of temperature, but also occurred at a promoted temperature point. And the residual weights (RW, %) at 773.2 K were 12.82% and 13.74% for ζ2 and ζ3, respectively, while RW of 16.80% for ζ1.
Thermal decomposition of the β-CD complexes with guest and the complexed β-CD without guest
In Fig. 2D, it could be seen that the TG curves of Tea– β-CD slightly shifted to high temperature with increasing heating rates especially in the temperature range from 580 to 630 K. The release of water and guest molecules has already finished before 550 K (the boil point of Tea is 386.9 K), and then the residual should be the complexed β-CD. That is to say, the thermal decomposition temperature of the complexed β-CD in Tea–β-CD system was elevated about 9.5–10.5 K along with the increase of heating rates by 5.0 K·min−1. The sharp weight loss resulting from melting-decomposition of the complexed β-CD was much more at a slow heating rate ζ1 than at a fast heating rate ζ2 or ζ3 at the same temperature. For example, the RW values of the complex at 596.3 K with the heating rates of ζ1, ζ2 and ζ3 were 53.24, 69.21 and 77.78%, respectively.
However, these phenomena could not be observed in the TG curves of Eda–β-CD and Dta–β-CD shown in Fig. 2B and C respectively. As can be seen in Fig. 2C, the order of the weight loss rates of Dta–β-CD was ζ2 > ζ1 > ζ3 until 503.0 K (the boil point of Dta is 329.1 K), but the rule was changed to ζ3 > ζ1 > ζ2 when it was over 503.0 K. In Fig. 2B, the fastest weight losses of water and guest in Eda–β-CD occurred at the medium heating rate of 15.0 K·min−1 at any temperature, and also the melting-decomposition of the complexed β-CD in Eda–β-CD system was obviously postponed until a higher temperature (TP, 613.2 K) at the heating rate of 20.0 K·min−1(the boil point of Eda is 390.2 K). However, the decomposition point of the complexed β-CD in the three inclusion systems was more or less enhanced with the raise of the heating rate, which was the same as that of free β-CD. These results described above clearly indicated that different heating rates could produce rather different effects on the thermal decomposition of the solid supramolecular inclusion complexes of β-CD.
The decomposition temperature point (TP) with a rapid weight loss, the temperature range (TR) of each stage of the thermal decomposition of β-CD and its inclusion complexes, and the values of RW at 773.2 K are displayed in Table 1.
In Stage a, the temperatures of releasing water molecules in the two complexes: Eda–β-CD and Dta–β-CD were elevated slightly at the heating rate of ζ1, compared with that of free β-CD. However, there was an obvious temperature decrease for Tea–β-CD. The TP value of releasing water molecules of each inclusion complex seemed to change almost nonlinearly with increasing heating rates. It descended firstly from ζ1 to ζ2 and then ascended from ζ2 to ζ3.
It could be easily observed from Fig. 2 that a new stage (Stage a′) appeared in the processes of thermal decomposition of the solid supramolecular complexes because of the release of guest molecules, which did not exist in the TG curves of free β-CD. Furthermore, in Stage a′, Tea–β-CD had a wider temperature range (TR, 383.0–533.5 K; ΔT, 150.5 K) of guest release than Eda–β-CD (TR, 417.1–514.0 K; ΔT, 96.9 K) and Dta–β-CD (TR, 402.6–512.4 K; ΔT, 109.8 K) at the heating rate of ζ1. At the heating rates of ζ2 and ζ3, ΔT of the TR values decreased in the order: Eda–β-CD > Dta–β-CD > Tea–β-CD and Dta–β-CD > Eda–β-CD > Tea–β-CD, respectively. Moreover, in this stage, both TP and ΔT of the TR values had the smallest values at the heating rate of ζ2, and showed the same trend as them in Stage a with increasing heating rates.
However, after all the water and guest molecules has been released, in Stage b, the melting-decomposition points of the complexed β-CD in the inclusion systems all increased to a certain degree in comparison with free β-CD even though three different heating rates were used, suggesting that the thermal decomposition temperature of β-CD as a host could be more or less raised after it formed the supramolecular inclusion complexes with these simple alkyl amines. Moreover, in Stage b, ΔT of the TR values decreased in the order: Eda–β-CD > Dta–β-CD > Tea–β-CD at the heating rates of both ζ1 and ζ3, but in the order: Dta–β-CD > Eda–β-CD > Tea–β-CD at the heating rate of ζ2. And TP of melting-decomposition of the complexed β-CD decreased in the order: ζ3 > ζ2 > ζ1, that is to say, the faster the heating rates were, the higher the decomposition points of the complexed β-CD in these systems were, suggesting that there should exist a close relationship between heating rates and melting-decomposition temperatures of the complexed β-CD molecules.
In Stage c, as shown in Fig. 2, the value of RW for the same complex at ζ1 was very different from that either at ζ2 or at ζ3 even at the same temperature. From Table 1, there was the smallest difference (<3%) of the RW values in Tea–β-CD among three heating rates, and the largest difference (>13%) in Eda–β-CD between ζ1 and ζ2 or ζ3, reflecting that heating rate had quite different effects on the final thermal decomposition of these complexed β-CD molecules in different solid complexes.
As discussed above, the thermal decomposition processes of the β-CD inclusion complexes of guest consisted of two stages, the first released water molecules and the second excluded guest molecules. And the thermal decomposition of the complexed β-CD without guest molecules contained two steps, the first corresponded to a rapid melting-decomposition and the second corresponded to a slow carbonization and incineration. Obviously, there is a conceptual difference in the thermal decomposition behaviors between the complexed β-CD and the complexes of β-CD.
It should be noted that the values of PWL in Table 1 corresponding to the release of water and guest molecules were in good agreement with the weight losses of the observed steps in TG profiles of Eda-β-CD at ζ1 or ζ3 and Dta–β-CD at ζ1–ζ3. However, the correlation between the PWL values and the observed weight losses of Eda-β-CD at ζ2 and Dta–β-CD at ζ1–ζ3 was poor. When this phenomenon occurred, it could be attributed to the factors as follows: First, some of guest molecules were released ahead of time (before its boiling point) due to the phase change heat of water, which resulted in that the observed weight loss of water in TG profiles seemed to be bigger than its PWL value. Second, the complexed β-CD were partly decomposed ahead of schedule (before its melting-decomposition) resulting from latent heat of evaporation of guest, which led to that the observed weight loss of the guest in TG profiles seemed to be bigger than its PWL value. Third, heating rates of samples and physical properties of guest such as boiling point might be also related with the phenomenon. Additionally, the number of hydration water molecules of the detected samples in air before and during measurements of TG and elemental analyses could be different from each other.
A proposed consecutive mechanism describing the formation and decomposition of β-CD complexes
According to the findings in Fig. 2 and Table 1, a consecutive mechanism describing the processes (A–D) concerning the formation and thermal decomposition of the solid inclusion complexes of β-CD was depicted in Fig. 3. The formation of hydrated inclusion complexes was regarded as process A; the releases of water and guest in these complexes referred to processes B and C, respectively, and the melting-decomposition of the complexed β-CD was looked on as process D.
Stages a, a′ and b listed in Table 1 corresponded to decomposition processes B, C and D, respectively, in Fig. 3. Stages c shown in Table 1 and Fig. 2 revealed a further weight loss of the complexed β-CD because of being incinerated. In general, four TG curves (2A–2D) in Fig. 2 and four consecutive steps (A–D) in Fig. 3 approximately draw the outline of the formation and decomposition of the β-CD supermolecules of the organic amines. Furthermore, many obvious differences in the four figures, as well as important differences among the TG curves in the same figure such as Fig. 2B, C and D at different heating rates, should be ascribed to structural differences in solid β-CD complexes of these guests. Therefore, the results from TG profile analyses clearly revealed that in the case of the same host, minor structural differences of these alkyl amines as guest molecules, such as different chain lengths and different numbers of amino groups and ethyl groups among the three guests, Eda, Dta and Tea, could lead to the significant differences in the formation (different stoichiometry) and decomposition (different TP, TR and ΔT) of their solid inclusion complexes of β-CD [23–28].
Several sequential chemical or physical processes could describe the consecutive mechanism depicting the formation and decomposition of the three supramolecular inclusion complexes according to Fig. 3 as follows:
-
(1)
x β-CD (l) + nH2O (l) + y guests (l) → (β−CD) x · (guest) y · nH2O (s)
-
(2)
(β-CD) x · (guest) y · nH2O (s) → (β−CD) x · (guest) y (s) + nH2O (g)
-
(3)
(β-CD) x · (guest) y (s) → (β−CD) x (s) + y guest (g)
-
(4)
(β-CD) x (s) → melting, decomposition, boiloff → carbonization, incineration → remains
Different guests produced different n, x and y values (for Eda, n = 4, x = 2, y = 5; for Dta, n = 1, x = 1, y = 1 and for Tea, n = 21, x = 3, y = 1), which resulted in a different molecular stack form of the complexed β-CD molecules, (β-CD) x (s), in their respective supramolecular complexes even after the release of guest molecules from these inclusion complexes. The fourth process described above corresponded to main part of weight loss (WL, %) in the TG profiles shown in Fig. 2. The WL range of TG curves in the β-CD complexes from 30% to 70% should be able to approximately reflect the melting-decomposition process of the complexed β-CD after the complete release of guest molecules.
Nonisothermal thermogravimetry analysis of the complexed β-CD
Continuous distribution kinetics is generally employed to analyze the thermal decomposition of solid samples according to Ozawa theory [29]. Equation 1 can represent the kinetics of the melting-decomposition reaction of the complexed β-CD,
where x is the fraction of WL at the heating time t, k is the rate constant. E a is the apparent activation energy. A, Rand T are the frequency factor, molar gas constant and absolute temperature, respectively. And f(x) only depends on the fraction of WL relating to the reaction mechanism. As regards a simple dissociation reaction, f(x) can be described by Eq. 2,
where n is the apparent reaction order. If heating rate (ζ) is defined by Eq. 3,
then substitute f(x) from Eq. 2 and ζ from Eq. 3 into Eq. 1 affords Eq. 4, which relates dx/dT to A, ζ, E a, x, n and T.
After separating variables in Eq. 4, an integral treatment is given, and then Eq. 5 can be obtained.
If n = 1, the following equation i.e. Eq. 6 is given after integral of Eq. 5 [31].
where C is a constant. The typical linear plots of ln{ln[1/(1–x)]} against 1/T of the complexed β-CD in Dta–β-CD and Tea–β-CD systems, were shown in Fig. 4, corresponding to three different heating rates, respectively. It could be easily seen from Fig. 4A and B, that there existed a very good linear correlation between ln{ln[1/(1–x)]} and 1/T. The correlation coefficients (r) of the six straight lines were all in the range from 0.994 to 0.999. A similar significant linear relationship, not perfect, but still pretty good, between ln{ln[1/(1–x)]} and 1/T of the complexed β-CD in Eda–β-CD system at ζ2 (r = 0.964) or ζ3 (r = 0.983) was also found (see Fig. 5B).
However, as can be seen in Fig. 5A, the r value of the linear relationship between ln{ln[1/(1–x)]} and 1/T of the complexed β-CD in Eda–β-CD system at ζ1 was only 0.897, due to a large relative deviation between the data point at WL of 70% and the other data points. But as for the other four data points, a very good linear relationship (r = 0.999) was also observed (see Fig. 5A). When it was turned to ζ2 and ζ3, as can be seen in Fig. 5B, the r values of the linear relationships of the five data points were not as good as that of the four data points except for the one at WL of 30%.
When the WL value of Eda–β-CD system at ζ1 increased from 30% to 60%, the linear temperature rise was only 36.7 K (from 583.3 to 620.0 K, see Table 3). However, when the temperature rose from 620.0 to 692.2 K, the WL value only increased by 10% (from 60% to 70%), indicating that a higher temperature (>671.0 K, see Table 1) would lead, at most, to a very slight increase of the WL value of Eda–β-CD system at ζ1. The WL values of Eda–β-CD system at ζ2 and ζ3 showed a linear increase from 40% to 70% in the temperature range 596.7–620.4 K and 606.7–627.9 K, respectively. However, when the temperature fell from 596.7 to 573.1 K for Eda–β-CD system at ζ2 or from 602.4 to 596.0 K for Dta–β-CD system at ζ3, the WL value only decreased by 10% (from 40% to 30%), indicating that a lower temperature (see Table 1) would only lead to a very slight increase of the WL values of Eda–β-CD system at ζ2 and ζ3.
The relationship of ln{ln[1/(1–x)]} against 1/T for free β-CD in the three heating rates were also examined in order to compare with complexed β-CD. In Fig. 6A, the data points presented a linear distribution, and the values of r were pretty good for ζ2 and ζ3 (0.998), which is similar to the complexed β-CD. The preceding experimental results permitted us to presume that the melting-decomposition processes of both free β-CD and the complexed β-CD were likely to be simple first-order reactions i.e. n = 1 [31–34].
The E a values of the melting-decomposition reaction of free β-CD and the complexed β-CD can be calculated by Ozawa method [29]. Equation 7 can be obtained after doing an integral treatment to Eq. 4 [30].
Because f(x) does not change under the same fraction of WL, the kinetic parameters of the thermal dissociation of the complexed β-CD can be determined based on the slope and interact of a linear plot of log ζ against 1/T [35]. The calculated values of E a and A were listed in Table 2. The apparent activation energy of the thermal dissociation of the complexed β-CD decreased in the order: Dta–β-CD > Tea–β-CD > Eda–β-CD system, reflecting there was a relative high activation energy barrier against the thermal dissociation of the complexed β-CD in Dta–β-CD system. These average values of E a in both Eda–β-CD (x/y = 2/5, E a = 34.4 kJ·mol−1) system and Tea– β-CD (x/y = 3/1, E a = 38.5 kJ·mol−1) system were very low relative to those in both Dta–β-CD (x/y = 1/1, E a = 84.2 kJ·mol−1) system and vanillin–β-CD (x/y = 1/1, E a = 180.2 kJ·mol−1) system [36], suggesting the host–guest stoichiometries in the solid complexes could produce an effect on the thermal dissociation of the complexed β-CD, possibly because the molecular stack of the complexed β-CD molecules in its solid supermolecules was a close relative of host–guest stoichiometry. As for Dta–β-CD system with a one-to-one chemical stoichiometry, structurally, there probably was a relatively strong interaction of intermolecular hydrogen bonding between two complexed β-CD molecules. The different thermal stability of the complexed β-CD in the three solid complexes should be attributed to the differences in supramolecular conformations of the complexes or in molecular stacks of the complexed β-CD. The frequency factor (A) of the complexed β-CD in these inclusion systems also had the same descendent order as calculated values of E a.
The melting-decomposition time and temperature corresponding to different WL (from 30% to 70%) in the TG curves of the complexed β-CD in the inclusion systems at three different heating rates were listed in Table 3. These values of k were determined based on Arrhenius equation (see Eq. 8) and also listed in Table 3.
Good linear relationships between logA and E a
Based on a simple compensating rule: logA = QE a + P (Q and P are given compensating constants), the linear correlation between logA and E a for the thermal decomposition reaction of free β-CD was given in Fig. 6B with the r value of 0.932 for Eq. 9.
While the linear correlations between logA and E a for the thermal decomposition reaction of the complexed β-CD molecules in the three inclusion systems were shown in Fig. 7A–C. And they could be expressed by Eq. 10 with r of 0.967 for Eda–β-CD system, Eq. 11 with r of 0.967 for Dta– β-CD system and Eq. 12 with r of 0.899 for Tea–β-CD system, respectively.
Equation 13 with r of 0.993 represents the linear correlation between logA and E a for the thermal decomposition reaction of the complexed β-CD molecules of all the three complexes at different WL values.
Then putting the calculated values of A into the Eqs. 9–12, the corresponding fitted values (E ′a ) of apparent activation energies were obtained (see Table 2). From Table 2, it was found that both of the values of E a and E ′a were quite close, and their relative deviation was not higher than 5%. Furthermore, the average value of E a pretty well approximated to that of E ′a . And their relative deviation was lower than 0.5%, indicating that there was a very good compensation relationship between logA and E a.
Despite the four linear relationships, not good enough (0.890 < r < 0.970), between logA and E a of the complexed β-CD in any one of the inclusion systems and the free β-CD at different WL values (5 data points), it showed us the tendency of the increased E a accompanied advanced value of logA. Then a very good linear correlation (r = 0.993) between logA and E a of the complexed β-CD in the three inclusion systems at different WL values (15 data points) has been found in Fig. 7D, indicating the statistical significance of the linear regression concerning the kinetic compensation of logA to E a of thermal decomposition reaction of the complexed β-CD in the inclusion systems of β-CD with the selected alkyl amines.
It is worth stressing that all the values of E a and k especially A were markedly larger in thermal decomposition reaction of the complexed β-CD in Dta–β-CD system than in both Eda–β-CD system and Tea–β-CD system, as shown in Tables 2 and 3, strongly suggesting that a very large frequency factor should play an important compensatory role in getting over the relative high activation energy barrier and in prompting the reaction rate constant of thermal decomposition of the complexed β-CD according to Arrhenius equation. Moreover, the E a values basically kept invariable with increasing fraction of weight loss, further demonstrating that the thermal dissociation of the complexed β-CD in the three inclusion systems was a very simple one-step dissociation reaction [35, 36].
It could be found from Table 3 that, corresponding to the same weight loss, the decomposition temperature became higher in most instances, when the heating rates increased from 10.0 to 20.0 K·min−1 for the three inclusion systems, and the decomposition time (t, min) and temperature (T, K) of the complexed β-CD under the same weight loss conditions tended to be in agreement with each other. Moreover, the value of k declined accompanying the increase of WL under all the three heating rates, and the decomposition reaction were accelerated to a certain degree with the increase of the heating rates while the value of k for free β-CD was found rather smaller compared with that of the complexed β-CD. Furthermore, the k values of these complexed β-CD molecules decreased in the same order as the values of E a and A: Dta–β-CD > Tea–β-CD > Eda–β-CD system, as shown in Table 3. And the faster the heating rate was, on the whole, the bigger the reaction rate constant of thermal decomposition of the complexed β-CD was.
Clearly, loose fluffy powders were not good thermal conductors because they took up a large volume of space. When a sample was heated in the loose state, a temperature gradient might occur in the different parts of the sample. The formation of thermal gradients could influence the differences observed in TG experiments. It should be noted that β-CD and its inclusion complexes were used as fine crystallite powders in the present work. The heating rates of the samples were moderate in the range of 10–20 K·min−1. When the sample inside the furnace was heated at the moderate heating rates, the temperature difference in the furnace should be rather small because the inner diameter of the furnace was less than 5 cm and the detected samples were fine crystal powders spread on the sample pan in the center of furnace. Moreover, the thermal convection caused by the flowing nitrogen of 25 mL·min−1 at the same time could further decrease such temperature difference to a certain extent. Additionally, the sample weight used in TG measurements was not too big (only about 5.5 mg). All of these should be able to effectively reduce the effect of thermal gradients throughout the sample on TG analysis.
Conclusions
There have been different effects of different heating rates on the thermal decomposition of the three solid supramolecular inclusion complexes of β-CD. In the case of the same host, the minor structural differences of these alkyl amines as guest molecules could result in very important differences in the formation and decomposition of the solid inclusion complexes. The thermal decomposition processes of these complexed β-CD molecules are all simple first-order reactions based on Ozawa method. The complexed β-CD in Dta–β-CD system has the largest value of E a, A and k when compared with it in the other complexes under various weight loss and heating rate conditions. A very large frequency factor plays an important compensatory role in getting over the relative high activation energy barrier and in prompting the reaction rate constant of thermal decomposition of the complexed β-CD molecules according to Arrhenius equation. Moreover, the faster the heating rate is, the higher the decomposition point of the complexed β-CD in an inclusion complex is, and on the whole, the bigger the rate constant of the thermal decomposition reaction is. The different thermal stability of the complexed β-CD in the three solid complexes should be attributed to the differences in supramolecular conformations of the complexes or in molecular stacks of the complexed β-CD in an inclusion system.
References
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743 (1998)
Wenz, G.: Cyclodextrins as building-blocks for supramolecular structures and functional units. Angew. Chem., Int. Ed. Engl. 33, 803 (1994)
Song, L.X., Meng, Q.J., You, X.Z.: Cyclodextrins and their inclusion compounds. Chin. J. Inorg. Chem. 13, 368 (1997)
Cao, Y.J., Lu, X.H., Guo, Q.X.: Theoretical study of the inclusion processes of ibuprofen enantiomers with native and modified β-CDs. J. Incl. Phenom. Mol. Recogn. Chem. 46, 195 (2003)
Hapiot, F., Tilloy, S., Monflier, E.: Cyclodextrins as supramolecular hosts for organometallic complexes. Chem. Rev. 106, 767 (2006)
Song, L.X.: Molecular recognition of double cyclodextrin bridged with amino acid derivatives in aqueous solution. Acta Chim. Sin. 59, 1201 (2001)
Grechin, A.G., Buschmann, H.J., Schollmeyer, E.: Complexation of gaseous guests by solid host. I. Quantitative thermodynamic approach for the reactions of β-Cyclodextrin with amines using data in aqueous solution. Thermochim. Acta 449, 67 (2006)
Aki, H., Niiya, T., Iwase, Y., Kawasaki, Y., Kumai, K., Kimura, T.: Multimodal inclusion complexes of ampicillin with β-cyclodextrins in aqueous solution. Thermochim. Acta 416, 87 (2004)
Vega-Rodriguez, A., Pineiro, A., Perez-Casas, S.: Thermodynamics of the interaction between hydroxypropyl-α-cyclodextrin and alkanols in aqueous solutions. Thermochim. Acta 405, 109 (2003)
Zhang, C.P., Zhang, Y.G.: Cyclodextrin-based pharmaceutics: past, present and future. Yaoxue Tongbao 22, 101 (1987)
Anigboga, V.C., Warner, G.M.: Fluorescence studies of the effects of t-butyl functionalities on the formation of ternary β-cyclodextrin complexes with pyrene. Appl. Spectrosc. 50, 995 (1996)
Song, L.X., Teng, C.F., Yang, Y.: Preparation and characterization of the solid inclusion compounds of α-, β-cyclodextrin with phenylalanine (D-, L- and DL-Phe) and tryptophan (D-, L- and DL-Trp). J. Incl. Phenom. Macrocyc. Chem. 54, 221 (2006)
Yang, Y., Song, L.X.: Study on the inclusion compounds of eugenol with α-, β-, γ- and heptakis(2,6-di-O-methyl)-β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 53, 27 (2005)
Song, L.X., Meng, Q.J., You, X.Z.: Study on the inclusion compounds of β-cyclodextrin with diphenyl and its derivates.Acta Chim. Sin. 53, 916 (1995)
Garcia-Zubiri, I.X., Gonzalez-Gaitano, G., Isasi, J.R.: Thermal stability of solid dispersions of naphthalene derivatives with β-cyclodextrin and β-cyclodextrin polymers. Thermochim. Acta 444, 57 (2006)
Giordano, F., Novak, C., Moyano, J.R.: Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 380, 123 (2001)
Feng, G.Z., Huang, X., Lu, K.: The preparation of inclusion compound of β-cyclodextrin with eicosapentaenoic acid (EPA) and it’s property of the thermal decomposition kinetics. J. Zhengzhou Inst. Technol. 3, 44 (2004)
Zheng, Y., Weng, J.B., Chen, R.Y.: Study on the thermal decomposition kinetics of β-cyclodextrin and phenylalanine using thermogravimetry. J. Fujian Teachers Univ. 13, 55 (1997)
Li, G.S., Yong, G.P., Yan, X.Y., Hu, Y.: Comparison to the thermal decomposition kinetics of several inclusion complex of β-cyclodextrin. Chem. Res. Appl. 15, 101 (2003)
Tian, S.J., Cheng, Q.T., Xi, G.X., Lou, X.D., Li, J.H.: Preparation of inclusion complex of benzyl acetate with β-cyclodextrin and studies on its thermal dissociation. Acta Phys. Chim. Sin. 13, 459 (1997)
Luo, L.B., Chen, H.L., Wang, F., Dai, Q.P., Tang, W.X.: Thermolysis of β-cyclodextrin/alkylcobaloxime inclusion complexes in the solid state. Thermochim. Acta 298, 129 (1997)
Song, L.X., Meng, Q.J., You, X.Z.: Study on inclusion compound of β-cyclodextrin and vanillin. Chem. Res. Appl.6, 99 (1994)
Toda, F., Tanaka, K., Sekikawa, A.: Host-guest complex formation by a solid-solid reaction. J. Chem. Soc., Chem. Commun. 279–280 (1987)
Buvarcza, A., Barcza, L.: Changes in the solubility of β-cyclodextrin on complex formation: guest enforced solubility of β-cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocyclic Chem. 36, 355 (2006)
Song, L.X., Meng, Q.J.: Study on inclusion compound of β-cyclodextrin and 4,4′-bipyridyl. Chin. J. Inorg. Chem. 10, 370 (1994)
Song, L.X., Meng, Q.J., You, X.Z.: Study on the inclusion compound of β-cyclodextrin with (η5-cyclopentadienyl)tricarbonylmanganese(0). Chin. J. Chem. 13, 311 (1995)
Li, J., Zhou, C., Wang, G., Tao, Y., Liu, Q., Li, Y.: Isothermal and nonisothermal crystallization kinetics of elastomeric polypropylene. Polym. Test. 21, 583 (2002)
Chen, P.F.: Study on the thermal decomposition kinetics of inclusion complex of β-cyclodextrin and methyl orange. J. Fujian Teachers Univ-Nature Sci. 14, 47 (1998)
Ozawa, T.: A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 38, 1881 (1965)
Liu, Z.H.: Introduction to Thermal Analysis. Chemical Industry Press, 104 (1991)
Hu, B.H.: The synthesis of urushiol titanium chelate polymers and their structural characteristics. J. Polym. Sci. 11, 198 (1993)
Demsar, A., Bukovec, N., Bukovec, P.: Decomposition kinetics of β-cyclodextrin and inclusion complex of β-Cyclodextrin with ibuproxam, 2-(4-isobutylphenyl)propiohydroxamic acid. Thermochim. Acta 178, 75 (1991)
Song, L.X., Meng, Q.J., You, X.Z.: Study on the multiple recognition of β-cyclodextrin dimer bridged with two 1,2-diamioethane. Acta Chim. Sin. 54, 777 (1996)
Santos, J.G., Conceicao, M.M., Trindade, M.F.S., Araujo, A.S., Fernandes, V.J., Souza, A.G.: Kinetic study of dipivaloylmethane by Ozawa method. J. Therm. Anal. Cal. 75, 591 (2004)
Rocco, J.A.F.F., Lima, J.E.S., Frutuoso, A.G., Iha, K., Ionashiro, M., Matos, J.R., Suarez-Iha, M.E.V.: Thermal degradation of a composite solid propellant examined by DSC – Kinetic study. J. Therm. Anal. Cal. 75, 551 (2004)
Li, G.S., Yong, G.P., Yan, X.Y., Lin, H.: Study on the thermal decomposition kinetics of β-cyclodextrin and vanillin inclusion complex. Food Sci. 23, 42 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, L.X., Teng, C.F., Xu, P. et al. Thermal decomposition behaviors of β-cyclodextrin, its inclusion complexes of alkyl amines, and complexed β-cyclodextrin at different heating rates. J Incl Phenom Macrocycl Chem 60, 223–233 (2008). https://doi.org/10.1007/s10847-007-9369-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9369-1