Abstract
In this study, an inclusion complex of benzoic acid with β-cyclodextrin (BA-βCD) was obtained from water–ethanol solvents. The yield of complex synthesis in binary mixtures is greater than in water and reaches maximum value at 0.10 mol fraction of ethanol. Results of FTIR spectroscopy analysis showed that the main difference in the spectra of the acid and inclusion complex was observed in the frequency ranging from 2500 to 3100 cm−1, corresponding to aromatic hydrogen vibrations. These vibrations are highly attenuated in complex. Phase solubility and differential scanning calorimetry studies revealed that the inclusion complex was obtained with 1:1 stoichiometric ratio and the solubility of benzoic acid increased with an increase in β-cyclodextrin concentrations in water. The logarithm of stability constant in water was found to be lgK = 1.99. The thermodynamic parameters for the reaction of (BA-βCD) complex formation in H2O–EtOH solvents were determined from calorimetric experiments carried out by means of the calorimetric titration system TAM III (TA Instruments) at T = 25 °C. The heat effects of mixing β-cyclodextrin solutions with benzoic acid were obtained from water–ethanol mixed solvents containing X(EtOH) = (0.00, 0.10, 0.20 and 0.30) mole fraction at pH = 3.6 and T = 25 °C. However, at X(EtOH) = 0.30 mol fraction, according to the calorimetric titration data, no complex formation occurs. When transferring from H2O to H2O–EtOH solvents, complex stability decreases from lgK = 2.4 to lgK = 0.7, wherein the reaction exothermicity increases from − 12.2 kJ mol−1 to − 44.3 kJ mol−1. An increase in the exothermicity of complexation is accompanied by a decrease in the entropic contribution to the change in the reaction Gibbs energy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benzoic acid (BA) is the simplest aromatic carboxylic acid that inhibits the growth of mold, yeast and some bacteria. Thus, BA and its salts are usually used as food preservatives, beverages and cosmetics. However, the high doses of BA can affect the liver and kidneys and irritate skin and eyes [1, 2]. It has been shown that BA can combine with ascorbic acid to obtain benzene that is highly dangerous for living organisms [2]. However, the solubility of BA in water is poor, which significantly decreases its bioavailability. Currently, there are several methods to improve solubility of poorly soluble drugs, which include pH adjustment, micronization, solid dispersion [3], cosolvent addition [4], and surfactant addition [5]. One of the simplest and most effective ways is encapsulation of cyclodextrins. β-cyclodextrin (βCD) is composed of seven glucopyranose units forming a cyclic, cone-shaped cavity with a hydrophilic outer surface and a relatively hydrophobic inner surface [6]. The ability of cyclodextrins to form inclusion complexes with a guest molecule depends on two main factors. The first critical factor is the compatibility of the size of the guest molecule with the diameter of host cavity. If the guest has the wrong size, it will not fit properly into the cyclodextrins cavity. The second critical factor is the thermodynamics of complexation between reagents, products of reaction and solvents [7, 8]. For a complex to form, there must be a favorable net energetic driving force that pulls the guest into the cyclodextrins [9].
In addition, water also forms stable hydrates with cyclodextrins, complicating the complexation. Most studies of intermolecular interactions focused on the study of selective recognition processes in aqueous solutions. The molecular complexation of cyclodextrins with aromatic carboxylic acids compounds in water is usually characterized by low stability of complexes and low exothermicity of complexation, making it difficult to obtain molecular complexes, their study and further practical use. Previous studies indicated that adding a small amount of cosolvent to water facilitates the complexation of βCD with hydrophobic guest [10]. Recently, cosolvents have been widely used in the synthesis of organic substances and they seem to be promising factors for creating supramolecular pharmacologically active structures. They work by reducing the hydrogen bond density of water and consequently its ability to “squeeze out” nonpolar solutes [11]. A combination of both cosolvent and cyclodextrin additions has a particular interest. Some authors observed synergistic effects of cosolvency and complexation [12, 13]. For example, in the water-organic solvents, the complexation constants of pyrene/cyclodextrins were found to be greater than in water [12]. The authors suggested that the organic solvents play the role of a space-regulating molecule and therefore the drug molecule can better fit into the cavity of cyclodextrin. However, in other studies, it was found that the antagonistic effect of cosolvents decreases the complexation constant when compared with that in water [14, 15].
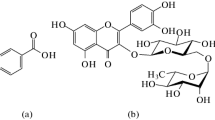
Some studies on the interaction between BA and βCD have already been reported [1, 2, 16, 17]. It has been demonstrated that BA forms 1:1 inclusion complex with βCD in water as follows:
No literature data about the complexation between BA and βCD in H2O–EtOH mixed solvent were found. Therefore, it is necessary to consider the influence of water–ethanol solvent compositions on the complexation process of BA with βCD, and its thermodynamics in water–ethanol solvents. In addition, we reported the effects of cosolvency and complexation in this case and analyzed the solvation-thermodynamic contributions of reagents to the change in the thermodynamic characteristics of complex formation.
Materials and methods
Materials
BA was purchased from Sigma-Aldrich and βCD from Fluka, and both reagents were used as received without further purification. The water content in βCD was determined by thermogravimetry and considered during calculation of the concentration. Thus, βCD contained 7.5 mass% of water. Ethanol of Xilong Scientific Co. (China) was distilled under atmospheric pressure. The residual water was determined densimetrically to be 1.81 mass%. Dimethyl sulfoxide of Xilong Scientific Co. (China) was used as received without further purification. All experiments were carried out in distilled water.
Preparation of inclusion complex
A solid-state complex between BA and βCD in 1:1 molar ratio was prepared. BA (0.41 g, 3.33 10–3 mol) and βCD (3.78 g, 3.33 10–3 mol) were accurately weighed and dissolved in 100 and 300 cm3 water–ethanol solvents, respectively, and composition of ethanol in mixture solvent is 0.00; 0.05; 0.10; 0.20; and 0.24 mol fraction. The solution of βCD was added into the BA solution, 10 cm3 each time, and was stirred by a magnetic stirrer for 24 h at 25 °C. The reaction solution was settled for 48 h at 4 °C to obtain a fine white precipitate. The precipitate was washed several times with dimethyl sulfoxide (DMSO) and dried in vacuum oven.
The complexation yield was calculated as the ratio of the dried complex mass to the sum of BA and βCD:
where m(BA-βCD), m(BA) and m(βCD) are the masses of the obtained complex, BA and βCD, respectively.
Methods
Fourier transforms infrared (FTIR) spectroscopy
Fourier transform IR spectra were recorded on a Nicolet iS10 (Thermo Scientific-USA) spectrophotometer. The spectra for BA, βCD and their complexes were recorded. Samples were prepared in KBr disks with a hydrostatic press at a force of 5.2 T cm−2 for 3 min. The scanning range was 450–4000 cm−1 and the resolution was 1 cm−1.
Differential scanning calorimetry (DSC)
BA, βCD and inclusion complex (BA-βCD) were studied by a differential scanning calorimeter (DSC; DSC204F1 (NETZSCH-Germany). Accurately weighed solid samples (approximately 3–4 mg) were placed in aluminum pans and scanned from 25 to 300 °C at a constant heating rate of 10 °C min−1, under the air atmosphere. The temperature for the onset of melting (Tonset) was obtained from curves by taking the slope of the melting curve at the inflection point and extrapolating to the baseline.
The differential scanning calorimeter was preliminarily calibrated with a pure indium standard. Obtained values Tonset for indium (Tonset = 155.8 ± 0.03 °C) and heat of fusion (Hf = 28.20 ± 0.20 J g−1) are in agreement with values recommended in [18, 19].
Phase solubility diagrams
The phase solubility diagram was obtained according to the Higuchi and Connors method [20]. An excess amount of BA was added to 25 cm3 of deionized water containing increasing amounts of βCD, and the initial concentration of βCD is changed in the range of 0 ÷ 7 mM. The corresponding thermodynamic equilibrium conditions were reached by shaking the tubes for 72 h at 25 ± 1 °C. UV–Vis spectrophotometer (S80, Biochrom, UK) was used to determine concentrations of the dissolved BA at 273 nm. Samples were filtered through a membrane with a 0.45 µm pore diameter. The binding stability constant (Ks) of the complex was calculated from the phase solubility diagram according to Eq. (3):
where So is the solubility of BA at 25 °C in the absence of βCD and slope means the corresponding slope of the phase solubility diagrams, i.e., the slope of the BA molar concentration versus βCD molar concentration graph.
Isothermal titration calorimetry (ITC)
The thermodynamic parameters for the reaction between BA and βCD in water–ethanol solvents were obtained from the calorimetric experiments carried out by means of the TAM III (TA Instruments, USA) calorimetric titration system at a temperature of 25 °C, equipped with a 20 cm3 titration cell. The microcalorimeter was electrically calibrated, and the calibration was verified with a binding reaction between Ba2+ and 18-crown-6 in water at T = 25 °C [21]. Obtained values (lgK = 3.24 ± 0.40 and ΔrH = − (30.9 ± 0.8) kJ mol−1) correspond with values recommended in [19].
The application of the traditional calorimetric titration with sequential addition of a number(s) of injections into a calorimetric cell is limited by low solubility of BA in H2O–EtOH mixtures. This prevented us from obtaining the necessary concentration ratio of the reagents during one titration experiment. A single addition of titrant portions into a cell solution in each calorimetric titration experiment allows the creation of the optimum concentration conditions for such systems. This experimental procedure was successfully adopted earlier by us for a calorimetric investigation of a “host–guest” complex formation of 18-crown-6 and cryptand [2.2.2] with some amino acids and peptides, as well as for complexes of Cu(II) with glycyl-glycyl-glycine [22,23,24,25]. In a typical calorimetric experiment, the initial concentrations of BA and βCD were changed in the range of 1.4 × 10–3 ÷ 5.92 × 10–1 mol dm−3 and 1.8 × 10–2 ÷ 2.65 × 10–2 mol dm−3, respectively. Calorimetric measurements were carried out in water–ethanol solvents containing 0.00, 0.10, 0.20 and 0.30 mol fraction of EtOH. The primary experimental values of solute and solvent mass have been used. Solutions were freshly prepared just before the measurements in a phosphate buffer, at pH = 3.6, which correspond to the pH of BA solutions with concentrations used in experiments. The range of concentrations of used water–ethanol mixtures is restricted by the low solubility of βCD in water–ethanol mixtures, [26, 27], which limits the set of BA: βCD concentration ratios were required for simultaneous calculation of the stability constants of BA-βCD complex and the enthalpy of its formation reaction from the calorimetric data. However, at X(EtOH) = 0.30 mol fraction, according to the calorimetric titration data, no complex formation occurs. The example of primary experimental data is given in Table 1.
The fraction distribution diagrams of particles in water–ethanol solutions containing BA are calculated by the software KEV [28]. The results show that in water at pH 3.6, the molecular and anion forms of BA are 79.81% and 20.19%, respectively. In water–ethanol solvents at pH 3.6, the molecular form was predominated (94.06% and 98.66% at 0.10 and 0.20 mol fraction of ethanol, respectively. The acid–base equilibrium constants of BA in water–ethanol solvents were from the literature [29] and were used at KEV mathematic treatment. The examples of the fraction distribution diagrams of particles of BA are observed in Fig. 1. Thus, it can be confirmed that the obtained thermodynamic parameters of complex formation in water–ethanol solvents are referred to as the association between molecular forms of BA and βCD.
Results and discussion
Determination of yield of complexation
Complex formation between BA and βCD depends on the composition of binary solvents. Complex was obtained in water–ethanol solvents with different concentrations of ethanol (XEtOH = 0.00, 0.10, 0.20 and 0.24 mol fraction). Yield of complexation (Y) is shown in Table 2.
As reported in Table 2, the yield of the obtained complex in binary media was greater than in pure water. There are some differences in the complexation between BA and βCD in water: The part of the interior of the βCD cavity in which BA molecule is located can be more hydrophobic than in the presence of EtOH in solvent, and this can be the reason for the higher yield of complexation in binary solvents. In order to clarify this result, we took into account the difference in the structure of the pure solvents as well as in the thermodynamic properties of the solvent mixtures. The highest Y value at 0.1 mol fraction EtOH is probably caused by the reagent solvation changes at the initial additions of EtOH to water. Extremes in the thermodynamic parameters of reagent solvation and complex formation reactions at high concentrations of water in H2O–EtOH solvents have been observed previously [30]. Such effects could be explained by strengthening of the three-dimensional spatial network as water hydrogen bonds occur at the first addition of an organic solution to the solvent [31].
Solid-state studies
The results of Fourier transform infrared spectroscopy analysis
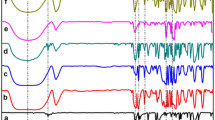
In the IR spectrum of BA (Fig. 2), the valence vibrations of the N–H bonds in the primary amino group and the C–H bonds in the aromatic ring with maxima at 3424 cm−1 and 3068, 2836, 2681, 2558 cm−1, respectively, are registered.
The absorption bands with maxima at 1605, 1581 and 1495 cm−1 belong to the valence vibrations of the C=C bonds in the benzene ring. The band of valence vibrations of the C=O bond in the carboxyl group (COOH) is observed at 1682 cm−1. The valence vibrations of the C–N bond in the amino group connected with benzene ring are observed at 1321 cm−1. The bands of the deformation vibrations of the N–H bonds in the amino group and the C-H bonds in the benzene ring are registered at 927 cm−1 and 1178, 1124, 808 cm−1, respectively [32].
In the FTIR spectrum of βCD, the wide band is registered with the absorption at 3407 cm−1, which is caused by the valence vibrations of the O–H bonds in the primary hydroxyl groups [33]. Also, the absorption band with maximum at 2925 cm−1 is observed. It belongs to the valence vibrations of the C–H bonds in the CH- and CH2- groups. Absorption maxima at 1641 cm−1 and 1417, 1365, 1246, 1158 cm−1 belong to the deformation vibrations of the O–H in the COH and C-H in the CH2OH, CHOH groups, respectively. In the interval of 1200–1030 cm−1, the absorption bands of the valence vibrations of the C-O bonds in the ether and hydroxyl groups of βCD (1081 and 1029 cm−1) are registered. The absorption bands in the region 950–700 cm−1 (940, 857, 754 and 709 cm−1) belong to the deformation vibrations of the C–H bonds and the pulsation vibrations in glucopyranose cycle. The main differences in the spectra of BA and inclusion complex were observed in the frequency range from 2500 to 3100 cm−1, corresponding to aromatic hydrogen vibrations [34]. These vibrations are highly attenuated in mixtures, which is probably due to the inclusion of the acid aromatic rings in the cavity. The band of the valence vibrations of the C=O bond in the carboxyl group of BA is shifted to higher wave number in the spectral pattern of the inclusion complex and registered at 1699 cm−1. The absorption bands of the valence vibrations of the C–O bonds in the ether and hydroxyl groups of βCD in the interval of 1200–1030 cm−1 are slightly broadened for the inclusion complex. Moreover, the absorption bands of the valence vibrations of the C=C bonds in the benzene ring are shifted to 1650, 1599 and 1583 cm−1. The peak at 1246 cm−1 in the spectrum of βCD which belongs to the deformation vibrations of the C–H bonds in the hydroxyl groups is shifted to the 1255 cm−1 and greatly broadened.
The results of DSC analysis
The experiments have been carried out on the BA, βCD, as well as on the inclusion complex. The peak temperature is the temperature at the maximum of the thermal event. This temperature is highly dependent on the sample crystallinity, crystal size, sample preparation and heating rate, which makes this value unreliable for comparison. On the contrary, the onset temperature remains unchanged. The melting temperature, Tonset, is defined by the extrapolated beginning of the curve, being determined by the point of intersection of the tangent with the point of maximum slope, on the principal side of the peak with the extrapolated baseline.
Figure 3 shows that the BA sample presents onset temperatures for melting and boiling of 118 °C and 242 °C, respectively. Literature values for this transition are 115.4 °C [35]; 121.4 °C [36] and 122.35 °C [37]. Thus, the data deviate by 2.6 ÷ 4.35 °C from the available literature data. Possible reasons for the discrepancies may be related to the differences in the purity levels of compounds analyzed or to the accuracy of the method used to determine literature values.
In the case of βCD, the endothermic peak with the onset temperature of 84 °C was revealed (Fig. 3, curve c). At this temperature, endothermic effect belongs to the release of water molecules from the inner cavity of βCD. Curve b displays the DSC trace of complex BA-βCD. The endothermic peak (Tonset = 45 °C) is related to the water loss and more importantly, therefore, indicates the presence of a fraction of βCD, which does not interact with the guest molecule. The complete disappearance of the BA endothermic peak was observed for complex instead. This phenomenon can be assumed as proof of interactions between the components of the respective binary systems [38]. This can be considered as indicative of BA amorphization and inclusion complex formation.
Solubility studies: phase solubility diagram
The calibration equation of BA in water was set up to build phase solubility diagram by UV–Vis spectroscopies. Calibration equation of BA in water is y = − 0.01877 + 835.6 × with R2 = 0.998, where y is optical densities and x is concentration of BA in the solution. This equation was used for calculating BA concentration in the solution containing βCD. Results indicated that in water, BA concentration increases linearly with increasing βCD (Fig. 4). The slope of the line is 0.0367 with R2 = 0.995. The linear profile of the diagram points at a 1:1 stoichiometric ratio as for the formation of BA-βCD complex.
Stability constant of the complex connects the concentration of the complexes formed with the concentrations of the reagents in Eq. (3) and was found to be lgK = 1.99 that falls within the literature values (lgK = 1.94 [1], lgK = 2.6 [2]).
Thermodynamics of complex formation in water–ethanol solvents
The thermodynamic parameters (lgK, ΔrH, ΔrG, TΔrS) of complex formation have been calculated by the program HEAT [39]. The analysis of the experimental data treatments by HEAT was reported earlier in detail [22,23,24]. The thermodynamic parameters of reaction (1) in H2O–EtOH solvents are presented in Table 3.
The thermodynamic parameters of BA-βCD complex formation in water were determined using a calorimetric method and were in agreement with the data in [2, 17, 40]. The absence of any information about the activity coefficients of reagents leads to the evaluation of association parameters which are not exactly defined thermodynamically. Only an apparent constant can be determined, and consequently, the standard Gibbs energy, enthalpy and entropy suffer from the same limitations.
The increase in EtOH concentrations in the mixed solvent leads to the decrease in the stability of BA-βCD complex and enhancement in the exothermicity of its formation reaction.
An increase in the exothermicity of complexation is accompanied by a decrease in the entropic contribution to the change in the Gibbs energy.
The thermodynamics of a complex formation (1) was discussed by the analysis of the reagent solvation contributions to thermodynamic parameters of the reaction on the basis of the solvation-thermodynamic approach [42]. According to the solvation-thermodynamic approach, the influence of the change in solvent composition on the reaction (1) (ΔrY(solvent), ΔrY(water)) and on the thermodynamic parameters of solvation of each reagent and product (Y(Z)(solvent), Y(Z)(water)) can be quantitatively described by the following equations:
where ΔtrYr and ΔtrY(Z) are the thermodynamic function (ΔtrH, ΔtrG, TΔtrS) of transfer for the reaction and the reagents, respectively.
The transfer thermodynamic functions of Gibbs energy (ΔtrGr) for reactions (1) were calculated as follows:
where lgК(BA-βCD)s and lgК(BA-βCD)w are stability constants of BA-βCD complex in water–ethanol solvents and in water, respectively, which are presented in Table 3.
The influence of the composition of H2O–EtOH solvents on the change in Gibbs energy of reactions (1) and desolvation of reagents is shown in Fig. 5.
The values ΔtrG(βCD) are nearly zero at the X(EtOH) = 0.0 ÷ 0.2 mol fraction concentration. Thus, the decrease in the stability of the complex is determined by solvation of BA (ΔtrG(BA)) and desolvation of complex.
When adding a small EtOH amount to the solvent (XEtOH < 0.05 mol fraction), the increase in exothermicity of complex formation reaction (ΔtrHr) is determined by desolvation of βCD (Fig. 6).
Conclusions
Previously, we found that solvents H2O–EtOH, H2O-DMSO and H2O-Me2CO have similar effect on the thermodynamic parameters of formation of the host–guest complex formations between macrocyclic molecules 18-crown-6 (18C6) and glycyl–glycyl–glycine, glycine, d,l-alanine and l-phenylalanine [22, 25, 44]. These molecular complexes became more stable when adding the organic component to the solvent. In contrast, the increase in EtOH concentration led to the decrease in the BA-βCD complex stability, as in the case of the complex formation of hydroxypropyl-β-cyclodextrin with quercetin [45]. Changes in the Gibbs energy of molecular complexes formed by the “host” molecules 18C6 and βCD, however, are due to the resolvation of “guest” molecules. In cases of complexes with 18C6, the desolvation of “guests” is observed, and in the BA-βCD complexes, the increase in BA solvation occurs.
Furthermore, an increase in exothermicity of both types of complex formation reactions in H2O–EtOH mixed solvents is observed. An increase in the concentration of ethanol leads to an increase in the entropy contribution of BA-βCD complex formation reaction, which decreases the stability of BA-βCD.
In conclusion, addition of small amounts of ethanol to water has put impact on the complex formation and therefore thermodynamic studies are important for improving bioavailability of “guest” in nonaqueous media.
References
Mendez SG, Espinar FJ, Alvarez AL, Longhi MR, Quevedo MA, Zoppi A. Ternary complexation of benzoic acid with β-cyclodextrin and aminoacids. Experimental and theoretical studies. J Incl Phenom Macrocycl Chem. 2016;85:33–48.
Terekhova I, Koz’biał M, Kumeev R, Gierycz P. Complex formation of native and hydroxypropylated cyclodextrins with benzoic acid in aqueous solution: volumetric and 1H NMR study. Chem Phys Lett. 2011;514:341–6.
Brewster ME, Vandecruys R, Peeters J, Neeskens P, Verreck G, Loftsson T. Comparative interaction of 2-hydroxypropyl-beta-cyclodextrin and sulfobutylether-beta-cyclodextrin with itraconazole: phase-solubility behavior and stabilization of supersaturated drug solutions. Eur J Pharm Sci. 2008;34(2–3):94–103.
Yan H, Ping L, Samuel HY. Solubilization of Fluasterone in cosolvent/cyclodextrin combinations. Int J Pharm. 2003;264:25–34.
Soares-Sobrinho JL, Santos FL, Lyra MA, Alves LD, Rolim LA, Lima AA, Nunes LC, Soares MF, Rolim-Neto PJ, Torres-Labandeira JJ. Benznidazole drug delivery by binary and multicomponent inclusion complexes using cyclodextrins and polymers. Carbohydr Polym. 2012;89(2):323–30.
Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS Pharm Sci Tech. 2005;6:329–57.
Kustov AV, Smirnova NL, Neueder R, Kunz W. Amino acid solvation in aqueous kosmotrope solutions: temperature dependence of the L-Histidine-Glycerol interaction. J Phys Chem B. 2012;116:2325–9.
Kustov AV. The aromatic amino acid behaviour in aqueous amide solutions—the temperature dependence of the L-phenylalanine-urea interaction. J Thermal Anal Calorim. 2007;89:841–6.
Donze C, Coleman AW. Solvent effects in competition between guest molecules for β-cyclodextrin. J Incl Phenom Mol Recogn Chem. 1995;23:11–21.
Yoshii H, Kometani T, Furuta T, Watanabe Y, Linko YY. Formation of inclusion complexes of cycldextrin with ethanol under anhydrous conditions. Biosci Biotechnol Biochem. 1998;62:2166–70.
He Y, Li P, Yalkowsky SH. Solubilization of Fluasterone in cosolvent/cyclodextrin combinations. Inter J Pharm. 2003;264:25–34.
Zung JB, Munoz de la Pena A, Ndou TT, Warner IM. Influence of alcohol addition on the γ-CD: pyrene complex. J Phys Chem. 1991;95:6701–9.
Reer O, Muller BW. Investigation of the influence of cosolvents and surfactants on the complexation of dexamethasone with hydroxypropyl-β-cyclodextrin by use of a simplex lattice design. Eur J Pharm Biopharm. 1993;39:105–11.
Loftsson T, Ólafsdóttir BJ, Friðriksdóttir H, Jónsdóttir S. Cyclodextrin complexation of NSAIDs: physicochemical characteristics. Eur J Pharm Sci. 1993;1:95–101.
Pitha J, Hoshino T. Effect of ethanol on formation of inclusion complexes of hydroxypropylcyclodextrins with testosterone or with methyl orange. Inter J Pharm. 1992;80:243–51.
Belyakova A, Varvarin AM, Khora OV, Oranskaya EI. The Interaction of β-Cyclodextrin with benzoic acid. Russ J Phys Chem A. 2008;82:228–32.
Terekhova IV. Comparative thermodynamic study on complex formation of native and hydroxypropylated cyclodextrins with benzoic acid. Therm Acta. 2011;526:118–21.
Shimkin A. Optimization of DSC calibration procedure. Thermochim Acta. 2013;566:71–6.
Eysel W, Breuer KH. The calorimetric calibration of differential scanning calorimetry cells. Thermochim Acta. 1982;57:317–29.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–2122.
Wadso I, Goldberg RN. Standard in isothermal microcalorimetry. Pure Appl Chem. 2001;73:1625–39.
Usacheva TR, Sharnin VA, Chernov IV, Matteoli E. Calorimetric investigation of the reaction of molecular complex formation of 18-crown-6 with D, L-alanine in water–ethanol mixtures. J Therm Anal Calorim. 2013;112:983–9.
Usacheva TR, Pham Thi L, Kuzmina KI, Sharnin VA. Thermodynamics of complex formation between Cu(II) and glycyl–glycyl–glycine in water–ethanol and water–dimethylsulfoxide solvent. J Therm Anal Calorim. 2017;130:471–8.
Usacheva TR, Pham Thi L, Terekhova IV, Kumeev RS, Sharnin VA. Application of isothermal titration calorimetry for evaluation of water-acetone and water-dimethylsulfoxide solvents influence on the molecular complex formation between 18-crown-6 and triglycine at 298.15K. J Therm Anal Calorim. 2015;121:975–81.
Usacheva TR, Pham Thi L, Sharnin VA. Calorimetric study of the molecular complex formation of glycyl–glycyl–glycine with 18-crown-6 in aqueous organic solvents. Russ J Gen Chem. 2017;87:591–9.
Chatjigakis AK, Donze C, Coleman AW. Solubility Behavior of β-Cyclodextrin In Water/cosolvent Mixtures. Anal Chem. 1992;64:1632–4.
Coleman AW, Munoz M, Chatjigakis AK. Classification of the solubility behaviour of β-cyclodextrin in aqueous-co-solvent mixtures. J Phys Org Chem. 1993;6:651–9.
Meshkov AN, Gamov GA. KEV: a free software for calculating the equilibrium composition and determining the equilibrium constants using UV–Vis and potentiometric data. Talanta. 2019;198:200–5.
Xingen H, Ruisen L, Hanxing Z. Enthalpies and entropies of dissolution and dissociation of benzoic acid in EtOH-H2O and i-PrOH-EtOH-H2O mixtures. Acta Phys Chim Sinica. 1999;15:838–44.
Usacheva TR, Ledenkov SF, Sharnin VA. Complex formation of Ag+ with polyether 18-crown-6. Calorimetric and potentiometric methods. J Therm Anal Calorim. 2002;70:379–85.
Arnett EM, Bentrude WG, Burke JJ, MccDuggleby P. Solvent effects in organic chemistry. V. Molecules, ions, and transition states in aqueous–ethanol. J Amer Chem Soc. 1965;87:1541–52.
Afanasiev VN, Efremova LS, Volkova TV. Physical-chemical properties of binary solvents. Water-containing systems; Publisher: Academy of Sciences of the Union of Soviet Republics (USSR) Institute of non-aqueous solution chemistry. Russia. 1988; Part I, p. 104 (in Russian).
Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–53.
Sifaoui H, Modarressi A, Magri P, Stachowicz-Kuśnierz A, Korchowiec J, Rogalski M. Formation of β-cyclodextrin complexes in an anhydrous environment. J Mol Model. 2016;22:207–20.
Price DM. Temperature calibration of differential scanning calorimeters. J Therm Anal. 1995;45:1285–96.
Sorum CH, Durand EA. The melting of binary eutectics. J Am Chem Soc. 1952;74:1071–3.
Murray JP, Cavell KJ, Hill JO. A DSC study of benzoic acid. A suggested calibrant compound. Thermochim Acta. 1980;36:97–101.
Kim KH, Frank MJ, Henderson NL. Application of differential scanning calorimetry to the study of solid drug dispersions. J Pharm Sci. 1985;74:283–9.
Borodin VA, Vasilev VP, Kozlovskiy EA. Processing of results of calorimetric measurements on a computer when studying the complex equilibria in solutions. Zh Neorg Khim. 1982;27:2169–72 (in Russian).
Harata K. Induced circular dichroism of cycloamylose complexes with meta-and para-disubstituted benzenes. Bioorg Chem. 1981;10:255–65.
Lewis EA, Hansen LD. Thermodynamics of binding of guest molecules to α and β-cyclodextrins. J Chem Soc Perkin Trans. 1972;2:2081–5.
Krestov GA. Ionic solvation, Ellis Horwood Ed., New York-London-Toronto-Sydney-Tokyo-Singapore, 1994.
Belica S, Sadowska M, Stepniak A, Graca A, Pałecz B. Enthalpy of solution of α- and β-cyclodextrin in water and in some organic solvents. J Chem Therm. 2014;69:112–7.
Usacheva TR, Sharnin VA. A thermodynamic study of reactions of amino acids with crown ethers in nonaqueous media as examples of guest-host molecular complex formation. Russ Chem Bull. 2015;64:2536–44.
Usacheva TR, Kabirov DN, Beregova DA, Gamov GA, Sharnin VA, Marco B, Laura M, Concetta G. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water-ethanol solvents at T = 298.15 K. J Therm Anal Calorim. 2019;138:417–24.
Acknowledgements
This work was funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under the Grant Number 104.06–2017.329 by RFBR and VAST according to the research project No. 19–53-54004 and by Ministry of Foreign Affairs and International Cooperation of Italy [grants in favor of foreign citizens not residing in Italy and Italian citizens living abroad, No. 946–22/10/2018]. ITC measurements presented in this work were carried out at the Institute of Thermodynamics and Kinetics of Chemical Processes of the Ivanovo State University of Chemistry and Technology (ISUCT) using the equipment of the Center for Collective Use of ISUCT. The authors thank the University of Naples Federico II for the financial support of their collaboration which contributed to the preparation of this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Usacheva, T., Pham, T.L., Nguyen, T.D. et al. Host–guest inclusion complex of β-cyclodextrin and benzoic acid in water–ethanol solvents: spectroscopic and thermodynamic characterization of complex formation. J Therm Anal Calorim 142, 2015–2024 (2020). https://doi.org/10.1007/s10973-020-09807-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09807-4