Abstract
Urbanization is increasing globally and causing rapid taxonomic and functional changes in biological communities. Its effects through time in the same communities have not been addressed properly. Here, we evaluate the temporal changes in taxonomic and functional diversity of dung beetles between greenspace (Cerrado stricto sensu) and residential sites in an urban landscape in the Brazilian Cerrado. We sampled dung beetles across 3 years (2013–2015) during the rainy season in the same sites. We evaluated these temporal changes using Temporal Beta Index and beta diversity partitioning into its components of replacement and gain/loss. We expected that residential sites will be taxonomically and functionally poorer compared to greenspace sites over the years. We found a general loss of species and abundance-per species from 2013 to 2015, which was more pronounced in residential sites than greenspace sites, since greenspace sites showed some gain of species from 2014 to 2015. Functional richness, functional evenness, and functional divergence did not change over the years, but were always lower in residential sites than greenspace sites. Functional β-diversity did not change over the years, but was always higher in residential sites than greenspace sites, with similar contribution of functional replacement and nestedness-resultant components. We demonstrate that greenspace sites are taxonomically and functionally more stable, temporally, than residential sites.
Implications for insect conservation
We advocate that public policies aimed at conservation and management of greenspace sites are important tools for maintaining dung beetle diversity and their ecosystem functions in urbanized landscapes in the Brazilian Cerrado.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization is spreading throughout the world due to the increased human population, causing a rapid anthropogenically-driven environmental change (Merckx et al. 2018). This change threatens biodiversity and affects ecosystem functioning from local to global scales (Seto et al. 2012). For instance, species body size can be driven by urbanization effects associated to warming in urban environments and urban habitat fragmentation (Merckx et al. 2018). This filtering of species may affect the ecosystem functioning, since body size is a determinant factor for the structure and dynamics of ecological communities (Woodward et al. 2005) and an important species trait to determine the performance of some ecosystem functions of species (Slade et al. 2007). On the other hand, the maintenance of biodiversity in urban landscapes is essential for humankind well-fare, through the offering of ecosystem services (Bolund and Hunhammar 1999). Therefore, there is a need to develop sustainable initiatives for the conservation of biodiversity in urban landscapes, such as the maintenance of urban forest fragments that can maintain biodiversity within cities (MacGregor-Fors et al. 2016). Furthermore, we need to evaluate these greenspace areas over time to verify their conservation effectiveness.
One way to inform about conservation effectiveness is unveiling changes of biological communities through time via beta diversity metrics (Legendre 2019; Magurran et al. 2019). Beta diversity is the change in a community feature (e.g., composition, structure, trait-based diversity) from one site to another or from a time 1 to a time 2. It has been widely described via incidence- or abundance-based dissimilarity indexes based on species identities and species abundance (Anderson et al. 2011). New analytical developments allowed the expansion of beta diversity calculation to functional diversity (Villéger et al. 2011, 2013). Also, beta diversity can be partitioned into its two main processes: replacement (species or traits substitution) and gain/loss (increase or decrease of species number or functional space) components (Baselga 2012; Villéger et al. 2013). Specifically in assessments through time, the processes causing community changes are directional and can be fully interpreted as gain or loss of species (Legendre 2019) or functional traits. The information resulting from in-depth beta diversity assessments can be helpful not only to evaluate the conservation status of biodiversity through time but also to inform decision-makers on suitable management actions to be taken.
In Brazil, the Cerrado (a neotropical savanna and biodiversity hotspot; Myers et al. 2000; Klink and Machado 2005) is facing increased land use changes and urbanization in recent decades (Brannstrom et al. 2008; Carvalho et al. 2009). This increased urban development transformed natural ecosystems into landscapes characterized by greenspace areas (e.g., urban parks) mixed with constructed areas (e.g., residential sites), varying in terms of size, human occupation (McDonald et al. 2008) and connectivity to natural source areas (Cheptou et al. 2017). In this sense, different urbanized landscape features can affect biological communities differently and a better understanding of this phenomenon is needed if we want to conserve biodiversity within cities (Taylor and Hochuli 2017) to provide the necessary ecosystem services.
Dung beetles (Coleoptera: Scarabaeinae) can be useful as study models to evaluate the impact of urbanization on biodiversity (Halffter and Favila 1993; Salomão et al. 2019; Correa et al. 2021). These insects exhibit wide variation in life history strategies that are reflected in easily measureable functional traits (Halffter and Edmonds 1982; Hanski and Cambefort 1991), such as body size, food relocation behavior (e.g.. rollers, tunnelers and dwellers), and trophic preference (coprophagous, necrophagous or generalists) (Slade et al. 2007; Barragan et al. 2011; Braga et al. 2013; Audino et al. 2017). Therefore, they are good models to taxonomic and functional diversity studies in order to understand the effects of anthropic actions on ecosystem processes (Barrágan et al. 2011; Audino et al. 2017; Correa et al. 2021).
Urbanization effects have also been evaluated on dung beetles (e.g., Jiménez-Ferbans et al. 2008; Radtke et al. 2008; Korasaki et al. 2013; Ramírez-Restrepo and Halffter 2016; Salomão et al. 2019; Frizzas et al. 2020). Most of urbanization-related studies using dung beetles as study models are temporally limited, evaluating spatial patterns from a single sampling period (e.g., Salomão et al. 2019) or sampling only one urban site (e.g., Radtke et al. 2008; Frizzas et al. 2020). A few studies have studied intra-annual patterns of taxonomic diversity (e.g., Korasaki et al. 2013; Frizzas et al. 2020), but we found none dealing with diversity other than taxonomic (i.e., functional or phylogenetic) over more than a year. Therefore, there is a need to understand the temporal effects of urbanization on dung beetles using a multifaceted approach of diversity patterns.
Here, we evaluate the temporal changes in taxonomic and functional diversity of dung beetles between greenspace (Cerrado stricto sensu) and residential sites in an urban landscape in the Brazilian Cerrado. To do so, we sampled dung beetles across 3 years during the rainy season in the same sites. We aimed to answer the following questions: (i) Are the changes in taxonomic and functional diversity of dung beetle assemblages different in greenspace and residential sites over the years? (ii) Are these changes characterized by species and trait loss or gain? We hypothesize that residential sites will be taxonomically and functionally poorer compared to greenspace sites, with increased loss of taxonomic and functional diversity over the years.
Material and methods
Study species
Dung beetles, a highly cost-effective bioindicator group (Spector 2006; Gardner et al. 2008), are a detritus-feeding group of insects highly diverse taxonomically and functionally, which perform some important ecosystem functions, such as nutrient recycling, secondary seed dispersal, improvement of soil physico-chemical characteristics that enhance plant growth (Nichols et al. 2008).
Study area
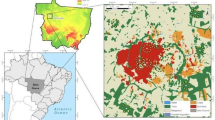
We sampled greenspaces and residential sites in Aquidauana, Mato Grosso do Sul, Brazil (20° 28′ 04″ S, 55° 47′ 14″ W, 154 m a.s.l.) (Fig. 1). This city has vegetation belonging to the Brazilian Cerrado biome. According to the Köppen classification, the climate is highly seasonal, with dry winter (Aw) and annual precipitation from 1300 to 1600 mm and an average annual temperature of 24–26 °C (Alvares et al. 2013).
Aquidauana has a population of 47,784 (2.69 inhabitants/km2) and an area of 17,087.021 km2, represented by both urban (28.2%) and rural zones (71.8%) (IBGE 2019). As a national trend (Carvalho 2004), there has been an increase in the population and urban households in Aquidauana over the last decades. From early 1990s to early 2000s, the population growth rate increased from 0.5 to 2% (Barros et al. 2015), resulting in an increase of housing areas (e.g. 16,910 houses; IBGE 2019). The migration of the population from countryside to urban areas has been acknowledged as one of the main factors responsible for this increase (Barros et al. 2015). As a main strategy, the municipal government transformed large areas of native vegetation into housing areas to host these migrants, which resulted in small fragments of native vegetation in the urban matrix (Souza and Martins 2010).
In the southern part of the Brazilian Cerrado urbanization rates reached ca. 85%, becoming the region that has undergone the most urban sprawl in Brazil (Sano et al. 2010). With Caatinga and Chaco, the Cerrado is also part of the South American ‘dry diagonal’ of open vegetation (Collevatti et al. 2013), harboring high levels of environmental heterogeneity, biodiversity and endemic species (Myers et al. 2000).
Sampling design
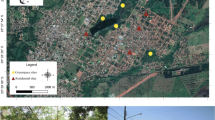
We sampled four fragments of Brazilian Cerrado (hereafter “greenspace sites”) and four residential sites (Fig. 1). Greenspace sites harbor plants of two distinct strata: a woody layer of trees and large shrubs, such as Lafoensia pacari St. Hill, Magonia pubescens St. Hill, and Qualea grandiflora Mart., often 3–8 m in height and with canopy covering ca. 50–90%, which characterizes the phytophysiognomy of Cerrado sensu stricto; and a ground layer composed of grasses, herbs, and small shrubs (Fina and Monteiro 2013), which harbors typical wild animals of the Cerrado biome, such as anteaters, capybaras and peccaries. These sites ranged 3–8 ha in size and often suffer extraction of trees and deposition of waste materials (e.g. food scraps, glasses, plastics and metals) by surrounding households (Souza and Martins 2010). Residential sites had their native vegetation cut and civil construction took place approximately 12 years (Souza and Martins 2010) before the samplings. All greenspace sites were inside the urban matrix. The residential sites are characterized by residential buildings (housing and commercial sites), unpaved roads with tree-lined streets, mainly with large trees, such as Licania tomentosa (Benth) Fritsch and Mangifera indica L. People live in and around the sampling sites, which range from 2 to 5 ha in size, with presence of domestic cats and dogs.
The distance between all greenspace and residential sites was at a minimum of 300 m from each other to insure independence among samples (da Silva and Hernández 2015). Because three greenspace sites sampled come from one large patch, we performed a Mantel test to evaluate the spatial autocorrelation between greenspace sites and the dung beetle assemblage, using the package “vegan” in the R software (R Core Team 2020). We found no clear spatial autocorrelation (rho = 0.73; p = 0.12), ensuring a sampling independence in our sample design. We considered each site as a replicate (i.e., greenspace sites, N = 4; residential sites, N = 4). Despite the spatial limitation of our study, the sample design represents the reality of Aquidauana, a small city with a low number of native vegetation fragments in its urban matrix, which is representative of many other cities in Brazil. Thus, we used a combination of repeated samplings in space and time (3 years of sampling), aiming to carry out a sampling design robust enough to describe dung beetle assemblage patterns in our study system.
Dung beetle sampling
Dung beetles were sampled in February of 2013, 2014 and 2015, the middle of the rainy season (Teodoro et al. 2015). The rainy season is the period of highest activity and diversity of dung beetles in tropical ecosystems (Halffter 1991) and most suitable to sample dung beetles in Cerrado fragments (Puker et al. 2014; Correa et al. 2019). Each site received 10 baited pitfall traps (15 cm diameter and 9 cm height) installed across a linear transect (180 m) located ca. 50 m from the edge (in greenspace sites). Traps were 20 m apart and contained 250 ml of a 1.5% liquid detergent solution. Traps were alternately baited with carrion (40 g of decaying beef) and human feces (40 g), placed in plastic containers (50 ml) at the center of each trap using a wire. Two types of baits were used to ensure an accurate representation of the local dung beetle functional and trophic groups (Correa et al. 2016). Besides, these baits consider the two main trophic groups of Neotropical Scarabaeinae: coprophagy and necrophagy (Halffter and Matthews 1966). Plastic lids supported by three wooden sticks (25 cm) were used to cover the traps, in order to reduce bait desiccation and trap overflow caused by rainfall. Traps remained in the field for 48 h per sampling year. The total sampling effort was represented by 240 pitfall traps (i.e., 80 traps/year), with 120 pitfall traps installed in greenspace sites and 120 pitfall traps in residential sites.
After sampling, dung beetles were sorted, counted and identified at genus level using dichotomous key (Vaz-de-Mello et al. 2011) and then sent to the Universidade Federal de Mato Grosso (UFMT, Cuiabá, Mato Grosso, Brazil) where they were identified at species level by an expert (Dr. Fernando Z. Vaz-de-Mello) and deposited.
Functional traits
We used three functional traits that are related to the ecological functions performed by dung beetles to calculate functional metrics (Table S1): body size, food relocation behavior, and trophic preference (Slade et al. 2007; Barragan et al. 2011; Braga et al. 2013; Audino et al. 2017). We measured body size (clypeus to the pygidium) using a digital caliper. Twenty individuals per species were randomly selected as a sample. For species with < 20 individuals, all individuals available were measured. Dung beetle body size is highly correlated with biomass (Lobo 1993), which can determine the performance of ecological functions (Slade et al. 2007). We assigned food relocation behavior following the literature (Halffter and Edmonds 1982; Hanski and Cambefort 1991). The species were categorized as rollers (species that form balls from the food resource, roll them horizontally and bury them), tunnellers (species that construct tunnels below or beneath the food resource and bury food into the bottom of these galleries), and dwellers (species that feed and nest within the food resource). The trophic preference was assigned to species according to the proportion of individuals attracted to certain bait. A species was categorized as coprophagous or necrophagous when its abundance was ≥ 80% in one baited trap (human feces or carrion). Species that did not follow this criterion was considered trophic generalist (Halffter and Arellano 2002).
Data analysis
Since we had repeated samplings through time, we first tested for the interaction between space (S; sites) and time (T; years). A significant S × T interaction can indicate that the spatial structure of the community composition data has changed through time, and conversely, that the temporal changes differed significantly among sites (Legendre and Condit 2019). We tested the space–time interaction on multivariate data (Legendre et al. 2010) using the function “stimodels” of the R package “adespatial” (Dray et al. 2020). We used a Hellinger-transformation on community data before the analysis (Legendre and Gallagher 2001).
After, the dissimilarity in community composition was measured for each greenspace and residential site between T1 and T2 (i.e., between 2013 and 2014, 2014 and 2015, 2013 and 2015). We used the percentage difference (% difference; or Bray–Curtis coefficient) for abundance data to calculate the temporal changes of community composition data (Legendre 2019). For comparisons through time, this dissimilarity coefficient is called Temporal Beta Indices (TBI) (Legendre 2019). The ‘% difference’ index allows the calculation of both gains and losses of individuals-per-species and species. When comparing data from a site at T1 and T2, this index is composed of two parts: B = abundance-per-species (Bray–Curtis) losses and C = abundance-per-species gains (Legendre and Condit 2019). B is the sum of abundances that are higher in T1 than in T2 for all species, while C is the sum of abundances that are higher in T2 than in T1 for all species. B and C are then scaled dividing them by “(2A + B + C)”, where A is the sum of abundances of species that are common at both times. The B and C components are tested for significance via 9999 permutations (Legendre 2019). The B and C statistics were used to produce B–C plots, with B (losses per site) in the abscissa and C (gains per site) in the ordinate (Legendre 2019). B–C plots display visually the relative importance of the species and abundance-per-species losses and gains in a study region (Legendre and Condit 2019). The mean of the differences between the B and C statistics is computed across all sites using a paired t-test implemented in the “TBI” and “plot.TBI” functions, available in the R package “adespatial” (Dray et al. 2020). We also evaluated individual demographic changes between times using paired t-tests (Legendre and Condit 2019), using 9999 random permutations of the values. A Holm correction for multiple testing was applied to the computed p-values. The calculations were carried out using the function “tpaired.krandtest” of the R package “adespatial” (Dray et al. 2020).
Based on dung beetle traits (Table S1), we calculated “trait distances” among species using the Gower coefficient, which is adequate for a set of continuous and categorical traits (Podani 1999). We used the function “gowdis” from the R package “FD” for this (Laliberté et al. 2014). After, we ran a principal coordinates analysis (PCoA) based on the Gower distances to obtain trait vectors for subsequent analyses. We used the function “cmdscale” from the R package “stats” for this (R Core Team 2020). These trait vectors are continuous and uncorrelated variables that were used to describe trait differences among species (da Silva et al. 2018). The number of PCoA axes was selected based on the quality of the functional space, quantified by the mean squared-deviation index (mSD) (Maire et al. 2015). Based on this index, the first three PCoA axes were kept, since they provided a high-quality functional space (i.e., mSD < 0.0068) for functional diversity calculations when compared to low and high numbers of axes. To estimate the functional diversity, we used the selected PCoA axes to calculate its three primary components: functional richness, functional evenness, and functional divergence (Villéger et al. 2008). Functional richness represents the multidimensional volume of the functional space occupied by all species traits in an assemblage; functional evenness refers to the regularity of the distribution of abundance in this multidimensional volume; functional divergence quantifies how the species abundance is distributed within the multidimensional volume (Villéger et al. 2008). The use of this set of metrics provides a meaningful framework for a better quantification of the functional diversity of communities (Villéger et al. 2008).
We also evaluated patterns of temporal variation in dung beetle functional diversity by using the β-diversity partitioning method (Baselga 2010; Legendre 2014). The functional diversity among two or more assemblages is equal to the ratio between the functional richness not shared among these assemblages relative to the total functional richness occupied by all assemblages together (Villéger et al. 2013). Therefore, the functional diversity is based on the volume of convex hull intersections between assemblages in a multidimensional functional space, and it can be partitioned into its functional replacement and functional nestedness-resultant components (Villéger et al. 2013). The Sorensen dissimilarity coefficient was calculated and used as a measure of functional β-diversity and partitioned into its replacement and nestedness-related components (Baselga 2010, 2012). Functional replacement means that assemblages host different functional strategies, while functional nestedness means that one assemblage hosts a small subset of the functional strategies present in another assemblage (Villéger et al. 2013). β-diversity partitioning of trait-based diversity was carried out using the R package “betapart” and the function “functional.beta.multi” (Baselga and Orme 2012).
We used Generalized Linear Models (GLMs) to test for differences in taxonomic (abundance and species richness) and functional metrics (FRic, FEve, FDiv, functional Sorensen dissimilarity and its components of replacement and gain/loss) across habitats (greenspace and residential), years (2013, 2014, 2015) and their interaction. We used the Poisson error distribution corrected for overdispersion (quasi-Poisson) for species richness and abundance, and the binomial error distribution corrected for overdispersion (quasi-binomial) for functional metrics (with range 0–1). We checked the error distribution for each model to find the best one by analyzing its residuals (Crawley 2013). All analyses were carried out in R (R Core Team 2020).
Results
We sampled 1573 individuals of 20 species and 13 genera of dung beetles in total, being 1313 individuals of 20 species in greenspace sites and 560 individuals of 17 species in residential sites (Table 1). Over the years, greenspace sites had always more species and individuals than residential sites (Table 1, Fig. 2a, b). However, species richness had a clear decrease from 2013 to 2015, mainly in residential sites. In average, the difference in species richness from greenspace to residential sites increased from 1.47 in 2013 to 2.50 times in 2015.
Type of habitat also determined the metrics of functional richness, functional evenness and functional divergence, with no effect of year and interaction of habitat and year (Table 2, Fig. 2c–e). Functional richness of dung beetles in greenspace sites was 1.95 times higher in 2013, 2.60 times higher in 2014, and 3.58 times higher in 2015 than residential sites on average. Differently from species richness, functional richness did not decrease in both habitats from 2013 to 2015. Functional evenness was 1.30, 1.31, and 1.59 times higher in greenspace sites than residential sites from 2013 to 2015. For functional divergence, greenspace sites had average values 1.47, 1.19, and 1.04 times higher than residential sites.
We found a significant space–time interaction for the whole set sites (R2 = 0.164; F = 2.166; p = 0.008). This interaction means that the spatial structure of the multivariate data (species abundance data) has changed significantly between the years sampled. TBI values ranged between 0.624 (2013) and 0.719 (2014) among greenspace sites over the years, and between 0.506 (2014) and 0.610 (2013) for residential sites. The mean of the differences between loss (B) and gain (C) from 2013 to 2014 was negative, indicating dominance of species loss across all sites (Paired t-test = − 4.069, p = 0.014, mean(C–B) = − 0.461). Only one residential has no loss or gain in this period. In the B–C plot, the green line (B = C) is above the red line (centroid of points), indicating that loss of species dominated the changes in the entire landscape (Fig. 3a). Coprophanaeus cyanescens, Dichotomius bos, Ontherus appendiculatus, and Trichillum externepunctatum showed significant decreases in abundance or disappeared from 2013 to 2014 (Table 3). Between 2014 and 2015, the mean of the differences between loss (B) and gain (C) was positive, suggesting dominance of species gains across all sites (Fig. 3b). However, this general gain was not significant, since half of the sites showed gain or loss (Paired t-test = 0.345, p = 0.739, mean(C–B) = 0.070). Some species, however, had significant decrease or increase; Canthidium sp. decreased in abundance or disappeared from 2014 to 2015, while Trichillum externepunctatum increased in abundance in this period (Table 1). When comparing 2013 to 2015, we also found a significant general loss of species (Paired t-test = − 2.569, p = 0.048, mean(C–B) = − 0.338), with one greenspace site showing some gain (Fig. 3c). Coprophanaeus cyanescens, Coprophanaeus ensifer, Dichotomius bos, Ontherus appendiculatus, and Trichillum externepunctatum showed decreased abundance or disappeared from 2013 to 2015 (Table 3).
B–C plots comparing dung beetle composition in greenspace and residential sites showing the losses (B) and gains (C) computed from the abundance data. Green line with slope of 1: line where gain equal loss. The red line was drawn parallel to the green line (i.e., with slope = 1) and passing through the centroid of the points. Its position below the green line indicates that, on average, species loss dominated gain from 1 year to another. Circles indicate dominance of loss, while squares indicate dominance of gain or equal contribution (smaller squares). Circle and square sizes are scaled to their values
Regarding functional beta diversity, type of habitat was also determinant in driving dung beetle functional beta diversity, i.e. Sorensen dissimilarity based on multidimensional volume of species traits (Table 4). Residential sites always had higher values of functional Sorensen dissimilarity than greenspace sites (Fig. 4), ranging from 1.69 (2014–2015) to 2.19 (2013–2014) times higher in residential sites than greenspace sites. The functional beta diversity components of replacement and nestedness were not affected by habitat type, year or their interaction (Table 4).
Discussion
In this study, we evaluated the changes over the years in taxonomic and functional diversity of dung beetles between greenspace and residential sites in an urban landscape in a threatened biome, the Brazilian Cerrado. We hypothesized that residential sites will be taxonomically and functionally poorer compared to greenspace sites over the years, and our hypotheses were confirmed. According to our two questions, we found that greenspace and residential sites changed differently through time in taxonomic and functional terms regarding dung beetle diversity. We found a general loss of species and abundance-per species from 2013 to 2015, which was more pronounced in residential sites than greenspace sites, since greenspace sites showed some gain of species from 2014 to 2015. Moreover, functional richness, functional evenness, and functional divergence did not change between years, but were always lower in residential sites than greenspace sites. Functional β-diversity did not change between years as well, but was always higher in residential sites than greenspace sites, with similar contribution of functional replacement and nestedness-resultant components. Therefore, greenspace sites are possibly taxonomically and functionally more stable than residential sites trough time in the Brazilian Cerrado.
Among our results, the consistent pattern over the years of higher functional β-diversity found in residential than greenspace sites was the most unexpected. For instance, Salomão et al. (2020) found that there is a tendency towards a higher taxonomic change of dung beetles in pastures, despite the lower richness and abundance, than in forest fragments at the Mexican’s Los Tuxtlas Biosphere Reserve, which may be due to the unstable conditions and lower environmental complexity of pastures compared to forests (Filgueiras et al. 2019; Salomão et al. 2020). Here, we also found a low taxonomic and functional richness in residential sites, which resulted in high functional change over the years. These high values of functional β-diversity, over time, found in residential sites must be taken with caution. Undoubtedly, this pattern has emerged because of the species- and individual-poor assemblages sampled in these sites when compared to greenspace sites. Dominant functional groups can maintain a similar functional structure over space and time, causing a pattern of low functional β-diversity despite being species-rich or more taxonomically dissimilar (Villéger et al. 2012). This is the case of greenspace sites, where there are high species richness, abundance, functional richness, functional evenness, and functional divergence but lower changes of the multidimensional functional space compared to species- and individuals-poor sites, such as residential sites.
Over the years, residential sites also presented a higher decline of species richness compared to greenspace sites. In 2014 and 2015, greenspace sites showed similar average values of richness and also similar values of observed species richness (S = 18, 18, and 16 for the consecutive years). On the other hand, both average and observed values of richness decreased over the years in residential sites (S = 15, 10, and 7 for the consecutive years). This implies an increased effect of urbanization on residential sites than greenspace sites in the urbanized landscape of the Brazilian Cerrado and that residential sites are less stable. Thus, the residential sites are possibly more vulnerable to variations in weather conditions from year to year than greenspace sites. The negative effect of urbanization has been found for dung beetles in other ecosystem, such as the Brazilian Atlantic Forest. For instance, Korasaki et al. (2013) found that urbanization negatively affected dung beetle assemblages, decreasing the species richness and number of individuals. Salomão et al. (2019) found that increasing urbanization negatively affected the abundance of coprophagous species, as well as the species richness, abundance, and biomass of roller beetles, indicating that specific functional groups of dung beetles can be affected by urbanization differently. These effects have also been found for other insect groups, such as Carabidae (Martinson and Raupp 2013) and Staphylinidae beetles (Nagy et al. 2018).
Five species (4 tunnellers and 1 dweller) showed decreased abundance or disappeared from 2013 to 2015. Coprophanaeus cyanescens and Coprophanaeus ensifer are large-bodied tunnelers (> 1 cm). Coprophanaeus cyanescens has been recorded in the Brazilian Atlantic forest (Edmonds and Zidek 2010) and exotic pastures (Tissiani et al. 2017), while C. ensifer is widely distributed in the Brazilian Cerrado (Edmonds and Zidek 2010; Maldaner et al. 2020). Dichotomius bos and O. appendiculatus are also large-bodied tunnelers and T. externepunctatum is a small dweller (> 0.5 cm). These three species are widely distributed in introduced Brazilian pastures (Tissiani et al. 2017). Although these five species cited are capable to colonize urban ecosystems (e.g. residential and greenspace sites), the ecological conditions (e.g. microclimate, food resource availability) found in these ecosystems may not be enough to support high populations of these species over the time (Correa et al. 2021).
Here, we were able to show that the decline of species richness and abundance in residential sites has clear effects on the functionality of dung beetles in these habitats. The two dominant genera found in residential sites were Canthidium and Dichotomius, both with two species commonly found in open ecosystems or disturbed habitats (Tissiani et al. 2017; da Silva et al. 2019). These few species alone may not be sufficient to maintain the whole range of ecosystem functions provided by this group, especially in a growing scenario of species loss, as we found.
Our findings support the claim on the importance of conserving greenspace sites in urban cities, because these habitats can provide refuge for biodiversity in an urban matrix (Korasaki et al. 2013; Salomão et al. 2019; Correa et al. 2021). We advocate that public policies aiming to conserve and manage greenspace sites are important tools for maintaining dung beetle diversity and their ecosystem functions in urbanized landscapes in the Brazilian Cerrado. As the study of dung beetles in urbanized landscapes is at an early stage, we highlight the need for broad effort to gather information on the responses of this group to urbanization in different scenarios, using a comprehensive approach of diversity measures, including the role of these beetles in urban ecosystem functionality (Ramírez-Restrepo and Halffter 2016).
References
Alvares CA, Stape JL, Sentelhas PC et al (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Anderson MJ, Crist TO, Chase JM et al (2011) Navigating the multiple meanings of beta diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Audino LD, Murphy SJ, Zambaldi L et al (2017) Drivers of community assembly in tropical forest restoration sites: role of local environment, landscape, and space. Ecol Appl 27:1731–1745. https://doi.org/10.1002/eap.1562
Barragan F, Moreno CE, Escobar F et al (2011) Negative impacts of human land use on dung beetle functional diversity. PLoS ONE 6:e17976. https://doi.org/10.1371/journal.pone.0017976
Barros WM, Gomes RL, Marcato Júnior J (2015) Análise dos contingentes populacional e habitacional da cidade de Aquidauana-MS: atualização e perspectiva. Revista GeoPantanal 19:59–69
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A (2012) The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob Ecol Biogeogr 21:1223–1232. https://doi.org/10.1111/j.1466-8238.2011.00756.x
Baselga A, Orme CDL (2012) betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812. https://doi.org/10.1111/j.2041-210X.2012.00224.x
Bolund P, Hunhammar S (1999) Ecosystem services in urban areas. Ecol Econ 29:293–301. https://doi.org/10.1016/S0921-8009(99)00013-0
Braga RF, Korasaki V, Andresen E et al (2013) Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. PLoS ONE 8:e57786. https://doi.org/10.1371/journal.pone.0057786.g001
Brannstrom C, Jepson W, Filippi AM et al (2008) Land change in the Brazilian Savanna (Cerrado), 1986–2002: comparative analysis and implications for land-use policy. Land Use Policy 25:579–595. https://doi.org/10.1016/j.landusepol.2007.11.008
Carvalho JMA (2004) Crescimento populacional e estrutura demográfica do Brasil. UFMG/Cedeplar, Belo Horizonte
Carvalho FMV, De Marco P, Ferreira LG (2009) The Cerrado into-pieces: habitat fragmentation as a function of landscape use in the savannas of central Brazil. Biol Conserv 142:1392–1403. https://doi.org/10.1016/j.biocon.2009.01.031
Cheptou P-O, Hargreaves AL, Bonte D et al (2017) Adaptation to fragmentation: evolutionary dynamics driven by human influences. Philos Trans R Soc B 372:20160037. https://doi.org/10.1098/rstb.2016.0037
Collevatti RG, Terribile LC, de Oliveira G et al (2013) Drawbacks to palaeodistribution modelling: the case of South American seasonally dry forests. J Biogeogr 40:345–358. https://doi.org/10.1111/jbi.12005
Correa CMA, Puker A, Korasaki V et al (2016) Attractiveness of baits to dung beetles in Brazilian savanna and exotic pasturelands. Entomol Sci 19:112–123. https://doi.org/10.1111/ens.12169
Correa CMA, Braga RF, Puker A et al (2019) Patterns of taxonomic and functional diversity of dung beetles in a human-modified variegated landscape in Brazilian Cerrado. J Insect Conserv 23:89–99. https://doi.org/10.1007/s10841-018-00118-6
Correa CMA, Ferreira KR, Puker A et al (2021) Greenspace sites conserve taxonomic and functional diversity of dung beetles in an urbanized landscape in the Brazilian Cerrado. Urban Ecosyst. https://doi.org/10.1007/s11252-021-01093-8
Crawley MJ (2013) The R Book. Wiley, Chichester
da Silva PG, Hernández MIM (2015) Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS ONE 10:e0126112. https://doi.org/10.1371/journal.pone.0126112
da Silva PG, Hernández MIM, Heino J (2018) Disentangling the correlates of species and site contributions to beta diversity in dung beetle assemblages. Divers Distrib 24:1674–1686. https://doi.org/10.1111/ddi.12785
da Silva PG, Nunes CA, Ferreira LF et al (2019) Patch and landscape effects on forest-dependent dung beetles are masked by matrix-tolerant dung beetles in a mountaintop rainforest archipelago. Sci Total Environ 651:1321–1331. https://doi.org/10.1016/j.scitotenv.2018.09.195
Dray S, Bauman D, Blanchet G et al (2020) adespatial: multivariate multiscale spatial analysis. R package version 0.3-7. https://CRAN.R-project.org/package=adespatial
Edmonds WD, Zídek J (2010) A taxonomic review of the Neotropical genus Coprophanaeus Olsoufieff, 1924 (Coleoptera, Scarabaeidae, Scrabaeinae). Insecta Mundi 274:1–108
Filgueiras BKC, Melo DHA, Andersen AN et al (2019) Cross-taxon congruence in insect responses to fragmentation of Brazilian Atlantic forest. Ecol Indic 98:523–530. https://doi.org/10.1016/j.ecolind.2018.11.036
Fina BG, Monteiro R (2013) Análise da estrutura arbustivo-arbórea de uma área de Cerrado sensu stricto, município de Aquidauana-Mato Grosso do Sul. Rev Arvore 37:577–585. https://doi.org/10.1590/S0100-67622013000400001
Frizzas MR, Batista JB, Rocha MJ et al (2020) Diversity of Scarabaeinae (Coleoptera: Scarabaeidae) in an urban fragment of Cerrado in Central Brazil. Eur J Endocrinol 117:273–281. https://doi.org/10.14411/eje.2020.031
Gardner TA, Barlow J, Araujo IS et al (2008) The cost-effectiveness of biodiversity surveys in tropical forests. Ecol Lett 11:139–150. https://doi.org/10.1111/j.1461-0248.2007.01133.x
Halffter G (1991) Historical and ecological factors determining the geographical distribution of beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Folia Entomol Mex 82:195–238. https://doi.org/10.21426/B615110376
Halffter G, Arellano L (2002) Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica 34:144–154. https://doi.org/10.1111/j.1744-7429.2002.tb00250.x
Halffter G, Edmonds WD (1982) Nesting behavior of dung beetles (Scarabaeinae). Man and Biosphere Program—UNESCO, Mexico, D.F.
Halffter G, Favila ME (1993) The Scarabaeinae (Insecta: Coleoptera) an animal group for analysing, inventorying and monitoring biodiversity in Tropical Rainforest and modified landscapes. Biol Int 27:15–21
Halffter G, Matthews EG (1966) The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomol Mex 12–14:1–312. https://doi.org/10.1002/mmnz.19690450211
Hanski I, Cambefort Y (1991) Dung beetle ecology. Princeton University Press, Princeton
IBGE (2019) Cidades. Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro, Brazil. http://www.ibge.gov.br. Accessed 8 Sept 2020
Jiménez-Ferbans L, Mendieta-Otálora W, García H et al (2008) Notas sobre los escarabajos coprófagos (Coleoptera: Scarabaeinae) en ambientes secos de la región de Santa Marta, Colombia. Acta Biol Colomb 13:203–303
Klink C, Machado RB (2005) A conservação do Cerrado brasileiro. Megadiversidade 1:147–155
Korasaki V, Lopes J, Brown GG et al (2013) Using dung beetles to evaluate the effects of urbanization on Atlantic Forest biodiversity. Insect Sci 20:393–406. https://doi.org/10.1111/j.1744-7917.2012.01509.x
Laliberté E, Legendre P, Shipley B (2014) FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334. https://doi.org/10.1111/geb.12207
Legendre P (2019) A temporal beta-diversity index to identify sites that have changed in exceptional ways in space–time surveys. Ecol Evol 9:3500–3514. https://doi.org/10.1002/ece3.4984
Legendre P, Condit R (2019) Spatial and temporal analysis of beta diversity in the Barro Colorado Island forest dynamics plot, Panama. For Ecosyst 6:7. https://doi.org/10.1186/s40663-019-0164-4
Legendre P, Gallagher E (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. https://doi.org/10.1007/s004420100716
Legendre P, De Cáceres M, Borcard D (2010) Community surveys through space and time: testing the space-time interaction in the absence of replication. Ecology 91:262–272
Lobo JM (1993) Estimation of dung beetle biomass (Coleoptera: Scarabaeoidea). Eur J Entomol 90:235–238
MacGregor-Fors I, Escobar F, Rueda-Hernández R et al (2016) City “green” contributions: the role of urban greenspaces as reservoirs for biodiversity. Forests 7:146. https://doi.org/10.3390/f7070146
Magurran AE, Dornelas M, Moyes F et al (2019) Temporal β diversity—a macroecological perspective. Glob Ecol Biogeogr 28:1949–1960. https://doi.org/10.1111/geb.13026
Maire E, Grenouillet G, Brosse S et al (2015) How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob Ecol Biogeogr 24:728–740. https://doi.org/10.1111/geb.12299
Maldaner ME, Vaz-de-Mello FZ, Takya DM, Ferreira DC (2020) Molecular phylogeny of coprophanaeus (Megaphanaeus) d'Olsoufieff, 1924 (Coleoptera: Scarabaeidae: Scarabaeinae) and the position of C. bellicosus. Insect Syst Evol 51:241–255. https://doi.org/10.1163/1876312X-00002198
Martinson HM, Raupp MJ (2013) A meta-analysis of the effects of urbanization on ground beetle communities. Ecosphere 4:art60. https://doi.org/10.1890/ES12-00262.1
McDonald RI, Kareiva P, Forman RTT (2008) The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol Conserv 141:1695–1703. https://doi.org/10.1016/j.biocon.2008.04.025
Merckx T, Souffreau C, Kaiser A et al (2018) Body-size shifts in aquatic and terrestrial urban communities. Nature 558:113–116. https://doi.org/10.1038/s41586-018-0140-0
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–845. https://doi.org/10.1038/35002501
Nagy DD, Magura T, Horváth R et al (2018) Arthropod assemblages and functional responses along an urbanization gradient: a trait-based multi-taxa approach. Urban For Urban Green 30:157–168. https://doi.org/10.1016/j.ufug.2018.01.002
Nichols E, Spector S, Louzada J et al (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474. https://doi.org/10.1016/j.biocon.2008.04.011
Podani J (1999) Extending Gower’s general coefficient of similarity to ordinal characters. Taxon 48:331–340. https://doi.org/10.2307/1224438
Puker A, Correa CMA, Korasaki V (2014) Deltochilini and Phanaeini dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in introduced and native ecosystems of Brazil. J Nat Hist 48:2105–2116. https://doi.org/10.1080/00222933.2014.908969
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Austria, Vienna
Radtke MG, Carlton CE, Williamson GB (2008) A dung beetle assemblage in an urban park in Louisiana. Southeast Nat 7:101–110. https://doi.org/10.1656/1528-7092(2008)7[101:ADBAIA]2.0.CO;2
Ramírez-Restrepo L, Halffter G (2016) Copro-necrophagous beetles (Coleoptera: Scarabaeinae) in urban areas: a global review. Urban Ecosyst 19:1179–1195. https://doi.org/10.1007/s11252-016-0536-2
Salomão RP, Alvarado F, Baena-Díaz F et al (2019) Urbanization effects on dung beetle assemblages in a tropical city. Ecol Indic 103:665–675. https://doi.org/10.1016/j.ecolind.2019.04.045
Salomão RP, Favila ME, González-Tokman D (2020) Spatial and temporal changes in the dung beetle diversity of a protected, but fragmented, landscape of the northernmost Neotropical rainforest. Ecol Indic 111:105968. https://doi.org/10.1016/j.ecolind.2019.105968
Sano EE, Rosa R, Brito JLS et al (2010) Land cover mapping of the tropical savanna region in Brazil. Environ Monit Assess 166:113–124. https://doi.org/10.1007/s10661-009-0988-4
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA 109:16083–16088. https://doi.org/10.1073/pnas.1211658109
Slade EM, Mann DJ, Villanueva JF et al (2007) Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J Anim Ecol 76:1094–1104. https://doi.org/10.1111/j.1365-2656.2007.01296.x
Souza EP, Martins SRO (2010) Conflitos territoriais em torno do Parque da Lagoa Comprida, Aquidauana (MS). Percurso 1:273–289
Spector S (2006) Scarabaeine dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae): an invertebrate focal taxon for biodiversity research and conservation. Coleopts Bull Monograph Number 5:71–83. https://doi.org/10.1649/0010-065X(2006)60[71:SDBCSS]2.0.CO;2
Taylor L, Hochuli DF (2017) Defining greenspace: multiple uses across multiple disciplines. Landsc Urban Plan 158:25–38. https://doi.org/10.1016/j.landurbplan.2016.09.024
Teodoro PE, Correa CCG, Torres EF et al (2015) Analysis of the occurrence of wet and drought periods using standardized precipitation index in Mato Grosso do Sul state, Brazil. J Agron 14:80–86. https://doi.org/10.3923/ja.2015.80.86
Tissiani ASO, Vaz-de-Mello FZ, Campelo-Júnior JH (2017) Dung beetles of Brazilian pastures and key to genera identification (Coleoptera: Scarabaeidae). Pesqui Agropecu Bras 52:401–418. https://doi.org/10.1590/s0100-204x2017000600004
Vaz-de-Mello FZ, Edmonds WD, Ocampo FC et al (2011) A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2854:1–73. https://doi.org/10.11646/zootaxa.2854.1.1
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. https://doi.org/10.1890/07-1206.1
Villéger S, Novack-Gottshall PM, Mouillot D (2011) The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol Lett 14:561–568. https://doi.org/10.1111/j.1461-0248.2011.01618.x
Villéger S, Miranda JR, Hernandez DF et al (2012) Low functional β-diversity despite high taxonomic β-diversity among tropical estuarine fish communities. PLoS ONE 7:e40679. https://doi.org/10.1371/journal.pone.0040679
Villéger S, Grenouillet G, Brosse S (2013) Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Glob Ecol Biogeogr 22:671–681. https://doi.org/10.1111/geb.12021
Woodward G, Ebenman B, Emmerson M et al (2005) Body size in ecological networks. Trends Ecol Evol 20:402–409. https://doi.org/10.1016/j.tree.2005.04.005
Acknowledgements
We thank Fernando Z. Vaz-de-Mello (UFMT) for dung beetle identification; Alfredo Raúl Abot (Universidade Estadual de Mato Grosso do Sul, Aquidauana, Mato Grosso do Sul, Brazil) for his lab equipment for insect screening, Mateus Alves and Paulo André Silva for the field support. The samplings were issued by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), the Brazilian biodiversity regulation governmental institute. CMAC thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ‘Bolsista CAPES/Brasil’ for postdoctoral grant (88887.603414/2021-00). PGdS thanks CAPES for postdoctoral grant (PNPD 88882.316025/2019-01, Code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Correa, C.M.A., da Silva, P.G., Ferreira, K.R. et al. Residential sites increase species loss and cause high temporal changes in functional diversity of dung beetles in an urbanized Brazilian Cerrado landscape. J Insect Conserv 25, 417–428 (2021). https://doi.org/10.1007/s10841-021-00310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-021-00310-1