Abstract
Most of the ongoing decline in biodiversity, worldwide, is due to human activities, in particular, the expansion of agriculture. In this context, we highlight the need for studies of the taxonomic groups that can provide insights into the dynamics of the ecological communities facing anthropogenic impacts. For this, we evaluated the effects of the environmental changes caused by cattle ranching on five phytophysiognomies (Cerrado Savanna, Amazon Forest, Palm Forest, Marshland, and Mangrove) in the state of Maranhão, Brazil. We tested the hypothesis that the species composition, abundance, and richness of the families Calliphoridae, Mesembrinellidae, and Sarcophagidae (Diptera) are affected by ranching in each of the phytophysiognomies. Specimens were collected at 90 sites, including 45 anthropic sites (cattle ranches) and 45 preserved habitats, using traps baited with bovine lung. We collected 15,023 calliphorids (11 species), 10,772 sarcophagids (52 species), and 241 mesembrinellids (one species). The results indicated significant differences between anthropic and preserved habitats in the species composition, abundance, and richness of sarcophagids, in particular in the Amazon Forest, where the highest species richness was recorded in the anthropic environments. In the case of the calliphorids and mesembrinellids, by contrast, significant differences were found in species composition and abundance in only in four of the five phytophysiognomies analyzed (excluding the Cerrado Savanna), while species richness only varied in the palm forest and marshland. In all cases, lower values were recorded in the anthropic environments. These results indicate that the insects of the families Sarcophagidae, Calliphoridae, and Mesembrinellidae respond differentially to the anthropic activity (cattle ranching) and can be used to evaluate this type of anthropogenic impact systematically. In addition, the Amazon Forest was the phytophysiognomy most impacted by this activity in the Brazilian state of Maranhão.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The homogenized vegetation and high levels of habitat disturbance found in anthropogenic environments can limit the survival of many species (Forister et al. 2019; Sánchez-Bayo and Wyckhuys 2019). The homogenization of the vegetation, in particular, is known to have negative effects on biodiversity (Benton et al. 2003; Tavares et al. 2019). There is convincing evidence that the progressive intensification of agricultural activities is the principal factor determining the population decline observed in a wide variety of taxa, including birds, insectivorous mammals, and insects (Sánchez-Bayo and Wyckhuys 2019). In areas used to raise livestock, the changes in spatial heterogeneity caused by grazing have negative effects on the species composition, richness, and abundance of many types of organism, ranging from invertebrates to mammals (Dennis et al. 1998; Wallis-de-Vries et al. 2007). In the case of arthropod communities, environments with more structurally complex habitats tend to have higher species richness, which is probably due to both the variety of habitats and the greater availability of food resources (Tews et al. 2004). For insects, changes in the habitat or plant community can affect the distribution of species, especially of the more sensitive or specialized taxa (Brown 1997).
The flies (Diptera) of the families Calliphoridae, Mesembrinellidae, and Sarcophagidae are widely distributed and can be found in an ample range of environments (Guimaraes 1977; Shewell 1987a, b; Erzinçlioglu 1996; Pape 1996; Whitworth and Yusseff-Vanegas 2019). These flies have a high ecological value, given the role of their larvae in the decomposition of organic material (Guimarães and Papavero 1999; Furusawa and Cassino 2006). Given this, they are potential bioindicators for the assessment of impacts (Sousa et al. 2015), and can be used to monitor forest restoration programs, given their abundance, the diversity of the niches they occupy, and their multiple trophic interactions (Majer 1987). The anthropophilic habits of the sarcosaprophagous dipterans (Galante and Marcos-Garcia 2004) also make them ideal candidates for use as bioindicators (Majer 1987) and for the the assessment of short-and long-term environmental changes (Sousa et al. 2014).
Studies of calliphorids have detected considerable variation in their ecological requirements and tolerance of environmental impacts, with some species being adapted to anthropogenic environments, while others require preserved habitats (Gomes et al. 2000; Esposito et al. 2009, 2010; Ferraz et al. 2009, 2010; Sousa et al. 2010; Gonçalves et al. 2011; Koller 2011, Cabrini et al. 2013). Mesembrinellids are typical of humid habitats and are exclusive to the tropical forests of the Neotropical region (Guimarães 1977; Wolff and Kosmann 2016). These flies also tend to be associated with primary or preserved forest environments, and are classified as asynanthropic (Mello et al. 2007). The greatest concentrations of mesembrinellid species has been found in the most preserved areas of the Amazon (Esposito et al. 2010; Sousa et al. 2010) and Atlantic Forests (Gadelha et al. 2009; Ferraz et al. 2010; Cabrini et al 2013; Figueiredo et al. 2018). These dipterans may thus be potential bioindicators of environmental quality, responding to different types of environmental impact (Gadelha et al. 2009).
Sarcophagids, by contrast, appear to be more plastic, and tend to be more abundant in anthropogenic environments (Mulieri et al. 2008; Sousa et al. 2011a, b). In this context, a number of studies have focused on the variation in the degree of adaption of these flies to different levels of anthropogenic disturbance, including deforestation, farming, and urban development (Valverde-Castro et al. 2017; Dufek et al. 2019). However, few studies have evaluated the impact of grazing land on these flies. The few studies that do exist report a greater abundance of sarcophagids in areas of pasture in comparison with forest and changes in the characteristics of sarcophagid communities that may reflect the effects of the proximity of these environments to urban areas (Mulieri et al. 2008). In the case of the Calliphoridae and Mesembrinellidae, the available studies have focused on changes in species composition related to human activities (Ferraz et al. 2010; Cabrini et al. 2013; Dufek et al. 2019), but have not investigated specifically the effects of cattle ranching on these flies.
In the Brazilian state of Maranhão, the suppression of the native forest cover is related primarily to the expansion of areas of farmland, principally for cattle pasture and monocultures, which has profound impacts on the local fauna and flora (Lemos 2001). Although cattle ranching is concentrated primarily on the northern lowland plain (Baixada Maranhense), the Cerrado savanna in the south of the state, and the palm forest (Cocais) of the central region, it is found throughout Maranhão, including the Amazon Forest, in the west (Barreto et al. 2008). This provides an opportunity to study the effects of cattle ranching on fly communities in the full range of natural environments found in the state, in order to detect and analyze the influence of this type of impact on community structure and ecosystem services.
The present study assesses the community structure of the necrophagous dipterans of the families Calliphoridae, Mesembrinellidae, and Sarcophagidae in well-preserved and anthropic (cattle ranches) environments in five natural phytophysiognomies (Amazon Forest, Cerrado Savanna, Palm Forest, marshland, and mangrove) in Maranhão state, northern Brazil. We tested the hypothesis that the species composition, richness, and abundance of the three dipteran families, in particular, the Calliphoridae + Mesembrinellidae (C + M) group, are impacted by the presence of grazing land in all five phytophysiognomies, especially in the more complex vegetation types (Amazon Forest, Cerrado Savanna, and Palm Forest), which have more habitable niches.
Material and methods
Study area
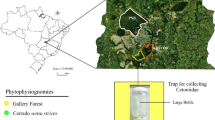
The present study was conducted at 90 sites, 45 located in preserved areas and 45 in neighboring anthropic environments, on cattle ranches, in 17 municipalities in the state of Maranhão, Brazil (Fig. 1, S1 Table). The 90 sites (sampling units) were distributed evenly among the five natural phytophysiognomies found in the state, that is, the Cerrado Savanna, Amazon Forest, Palm Forest, Marshland, and Mangrove.
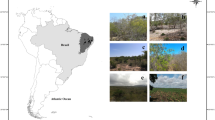
The Cerrado Savanna can be divided into four habitat subgroups based on the stature and density of its trees and shrubs—savanna woodland (Cerradão), arboreal savanna, park savanna, and grassy-shrubby savanna (IBGE 2012). Areas of Cerradão were selected for the present study. This forest formation has a non-grassy herbaceous stratum dominated by seedlings and shrubs, with relatively well-developed tree cover and taller trees than those found in the other savanna subtypes (Marinho-Filho et al. 2010) (S2 photographs of the five phytophysiognomies).
The Amazon Forest is dominated by vegetation of the dense rainforest type, with trees reaching a height of 20 m (IBGE 2012). Tropical forests are known for their high plant diversity (Svenning 1999), which is sustained in large part by niche differentiation (Svenning 2001), and is closely related to the spatial heterogeneity of the forest (S2 Photographs).
The Palm Forest (Cocais) is located between the Cerrado savanna and the Amazon Forest, and is characterized by a mixture of the plant species found in these two types of vegetation. The Cocais are evergreen forests dominated by the babassu palm (Orbignya phalerata), and trees of relatively reduced height in comparison with the typical dense Amazon broadleaf forest (S2 Photographs).
The marshland zone is a large area formed by a series of lakes surrounded by extensive marshes, which are dry for approximately seven months of the year. These floodplains are dominated by open vegetation, with some taller trees and palms. The sites selected for the present study were open fields dominated by herbaceous vegetation, interspersed with a few trees and lianas (S2 Photographs).
The mangrove is a microphanerophyte community found in brackish environments, generally in estuaries and coastal channels, where the muddy substrates support this specialized vegetation adapted to highly saline conditions, including Rhizophora mangle L., Avicennia spp. (the local species depends on the latitude), and Laguncularia racemosa L., which grows on the highest terrain, which is only flooded at high tide (S2 Photographs).
We considered anthropic areas to be those in where the original vegetation had been impacted by cattle ranching, such as deforestation for the planting of pasture or the use of natural pastures as grazing land, a phenomenon typical of the marshland and mangrove. Sousa et al. (2016) provide a more detailed description of the five phytophysiognomies surveyed in the present study.
Data collection
Nine replicates of each phytophysiognomy were sampled, with a minimum distance of 2 km between each. In each area, two sites were sampled, one representing the natural vegetation and the other, representing the anthropic environment. Each site was sampled twice, with five traps per sample, and a total of 180 traps per phytophysiognomy, and 900 traps overall. The field campaigns for the collection of the dipteran specimens were conducted during the dry season in 2010 (August–October, 2011 (April–October), and 2012 (May–November).
The specimens were collected using traps designed specifically to capture saprophagous dipterans (Ferreira 1978) and used successfully by Sousa et al. (2010). The traps were baited with 50 g of cow lung (decomposed for 24 h) hung on tree branches 40 cm above the ground in shaded locations at 200-m intervals along a 1 km transect of 200 m, where they were left for 48 h.
The calliphorid and mesembrinellid specimens were identified using the taxonomic keys of Mello (2003), Carvalho and Mello-Patiu (2008), Kosmann et al. (2013), and Whitworth and Yusseff-Vanegas (2019). The sarcophagids were identified using the species keys available for the genera Oxysarcodexia Townsend (Lopes and Tibana 1987) and Peckia Robineau-Desvoidy (Buenaventura and Pape 2013) and other references, including Lopes (Lopes 1939, 1958, 1989), Tibana (1976, 1981), Tibana and Xerez (1985), and Guimarães (2004). As the taxonomic identification of the sarcophagids is based primarily on the male genitalia, only the males were identified in the present study. Part of the material collected was prepared in a dry medium and deposited in the Entomology Collection at the Goeldi Museum (Museu Paraense Emílio Goeldi: MPEG) and the Museum of Zoology at the Biological Sciences Institute of the Federal University of Pará (UFPA), which are both located in the city of Belém, Pará (Brazil). The remaining specimens were preserved in a liquid medium (70% ethanol) and included in the teaching collection of the Environmental Sciences and Biodiversity Laboratory at the São Luis campus of Maranhão State University (UEMA).
In the present study, we follow the arrangements of Marinho et al. (2017) and Whitworth and Yusseff-Vanegas (2019), who consider the Mesembrinellidae to be a valid family, rather than a subfamily of the Calliphoridae. Even so, Rognes (1997) aligns the Mesembrinellidae closely with some other calliphorid subfamilies, such as the Auchmeromyinae, Bengaliinae, and Phumosiinae. Given the taxonomic proximity of these two families, and the identification of only one mesembrinellid species in the study area, the analyses of species richness, composition, and abundance conducted in the present study considered a single group containing the calliphorids and mesembrinellids (referred to here as the C + M group).
Data analysis
Species richness was estimated for each type of environment (preserved vs. anthropic) in each phytophysiognomy using the first-order jackknife estimator, with 1000 randomizations, considering the number of traps as the samples (Colwell et al. 2004). This analysis was run in EstimateS, the Statistical Estimation of Species Richness and Shared Species from Samples,version 9.0 (Colwell 2013).
Each occurrence of a species considered to be rare (Colwell 2013) increases the heterogeneity of the data set and the probability of encountering a new species. In this case, the first-order jackknife estimator is less strict than other estimators, with species occurring in only one sample (“unique species”) being considered rare (Santos 2009). For this reason, we selected this estimator as the most appropriate for the present study. The sampling efficiency of the species of the C + M group and the family Sarcophagidae was calculated using the following formula: (observed richness/estimated richness) × 100. A confidence interval-based inference approach was used to test the hypothesis that the species richness of the two groups (C + M and Sarcophagidae) is affected by the anthropogenic impact of cattle ranching on each of the phytophysiognomies. This approach was also based on first-order jackknife estimates (Colwell et al. 2004), with the two types of environment (anthropic and preserved) being considered significantly different when the confidence interval of one environment did not overlap with the mean value of the other.
Species composition was analyzed using non-metric multidimensional scaling (NMDS) based on a Bray–Curtis dissimilarity matrix (Legendre and Legendre 1998; Clarke and Warwick, 2001). Prior to this, the abundance data were log (x + 1) transformed to reduce the effect of discrepant values. The present study tested only a single factor, that is, the presence/absence of cattle ranching. To test the hypothesis that cattle ranching has differential effects on the species composition and abundance of the two dipteran groups (C + M and Sarcophagidae) a nonparametric permutational analysis of variance (PERMANOVA), for models with multiple factors, was applied based on a Bray–Curtis similarity index, with 9999 permutations (Andersen 2005). When significant results were obtained by the PERMANOVA, multiple pairwise a posteriori tests were applied to compare the two environments in each phytophysiognomy. All analyses were run in the R program of the R Development Core Team (2018), using the Vegan (Oksanen et al. 2007) and Mass packages (Ripley et al. 2013).
Results
A total of 15,023 calliphorid specimens were collected during the present study, representing seven genera and 11 species (S3 Table). Two species, Chrysomya albiceps and Cochliomyia macellaria, together accounted for nearly 71% of the total number of specimens collected in the two environments, although both species were more abundant at the anthropic sites. Chloroprocta idioidea and Paralucilia paraensis were more abundant in the preserved environments, by contrast (S3 Table).
The family Mesembrinellidae was represented by a single species, Mesembrinella bicolor, which was present only at one preserved Amazon forest site, where 241 specimens were collected (S3 Table). In the case of the sarcophagids, 10,772 specimens were collected in 15 genera and 52 species (S4 Table). Tricharaea (Sarcophagula) occidua and Peckia (Sarcodexia) lambens represented approximately 73% of the specimens, and while they were collected in the two environments, they were more abundant at the anthropic sites (S4 Table). Oxysarcodexia intona (Curran & Walley, 1934), Peckia (Euboettcheria) collusor (Curran & Walley, 1934) and Peckia (Squamatodes) ingens (Walker, 1849) were more abundant in the preserved environments.
Species richness
Sampling efficiency varied between 62 and 100% for the Calliphoridae and between 70 and 88% for the Sarcophagidae (Table 1), which indicates that the sampling effort employed in the present study was adequate to determine the diversity of these dipterans found in the different study areas.
The highest estimated species richness values for the C + M group were recorded in both environments (preserved and anthropic) of the Cerrado Savanna and Amazon Forest, and in the preserved Palm Forest (Table 1). When comparing this dipteran group between environments in each phytophysiognomy, significant differences were found only in the palm forest and marshland areas, where species richness was greater in the preserved environments. These differences are significant because the confidence intervals recorded for the two environments do not overlap (Fig. 2).
Estimated species richness of the Calliphoridae + Mesembrinellidae (C + M) group (Jackknife 1; mean ± confidence interval) per environment (anthropic and preserved) sampled in each phytophysiognomy in Maranhão state, Brazil (2010–2012). C Cerrado Savanna, P Palm Forest, A Amazon Forest, M Marshland, ME Mangrove, a anthropic, p preserved. Observation: the dotted rectangles highlight the phytophysiognomies with significant differences between environments
In the family Sarcophagidae, the highest estimated species richness values were recorded in both environments (preserved and anthropic) in the Cerrado Savanna and Palm Forest, and at the anthropic sites in the Amazon Forest (Table 1). Significant differences (non-overlapping confidence intervals) were only found in the Amazon Forest, however, where species richness was higher in the anthropic environments (Fig. 3).
Estimated species richness of the Sarcophagidae (Jackknife 1; mean ± confidence interval) per environment (anthropic and preserved) sampled in each phytophysiognomy in Maranhão state, Brazil (2010–2012). C Cerrado Savanna, P Palm Forest, A Amazon Forest, M Marshland, ME Mangrove, a anthropic, p preserved. Observation: the dotted rectangles highlight the phytophysiognomies with significant differences between environments
Species composition and abundance
Dipteran species composition and abundance varied significant between environments (anthropic and preserved) in both the C + M group (pseudo-F = 43.87; df = 1; p < 0.001) and the Sarcophagidae, pseudo-F = 6.88; df = 1; p < 0.001 (Table 2). Multiple a posteriori comparisons indicate significant differences between environments (p < 0.001) in the C + M group in four of the five phytophysiognomies evaluated, i.e., Palm Forest, Amazon Forest, Marshland, and Mangrove (Table 3). In the sarcophagids, however, a significant difference (t = 2.16; p = 0.003) was found only in the case of the Amazon Forest (Table 3). The variation in the composition of the dipteran communities did not vary significantly between environments in the other phytophysiognomies analyzed (Table 3).
The NMDS ordination segregated the C + M communities into four groups (Fig. 4). One of these groups encompassed the dipteran communities recorded in the preserved Amazon Forest (Ap), while a second group included the community of both environments in the Cerrado Savanna (Ca and Cp) and Palm Forest, Pa and Pp (Fig. 4, Axis 1). A third group was formed by both Mangrove environments (MEa and MEp) and the fourth, by the two Marshland environments, Ma and Mp (Fig. 4, Axis 1).
Non-metric multidimensional scaling (NMDS) ordination of the data on the Calliphoridae + Mesembrinellidae community recorded at the 90 study sites surveyed in the present study in Maranhão state, Brazil, from 2010 to 2012. The sites are coded by phytophysiognomy (C Cerrado Savanna, P Palm Forest, A Amazon Forest, M Marshland, ME Mangrove) and environment (a anthropic, p preserved). Observations: the arrows indicate a species association, while the dotted lines delimit specific groups
With the exception of the Cerrado Savanna, the species composition of the C + M communities varied between the anthropic and preserved environments of each phytophysiognomy. A considerable difference was found between environments in the Amazon Forest, for example, which indicates that anthropogenic impacts determined a major shift in species composition in this phytophysiognomy (Fig. 4, Axis 1). Some C + M species were more associated with preserved environments, for example, in the Amazon Forest (C. idioidea, M. bicolor, and Lucilia eximia) and Palm Forest (L. eximia) (Fig. 4). The species C. albiceps was associated with the anthropic environments of the Amazon Forest and Palm Forest, as well as both environments in the Cerrado Savanna, while C. macellaria and Chrysomya megacephala were strongly associated with the two Mangrove environments (Fig. 4).
In the analysis of the sarcophagid, two groups were formed (Fig. 5), one encompassing the Cerrado Savanna, Palm Forest, and Amazon Forest sites, and the other, the Mangrove and Marshland sites (Fig. 5; Axis 1). In this analysis, the anthropic and preserved environments of the Amazon Forest were well-spaced, which indicates that their sarcophagid faunas were quite distinct (Fig. 5; Axis 2). There was no clear evidence of divergence between the communities in the anthropic and preserved environments in the other phytophysiognomies, however (Fig. 5). In this analysis, P. (S.) lambens was associated more with the anthropic environments of the Cerrado Savanna and Amazon Forest, whereas T. (S.) occidua was associated only with the anthropic environment of the Amazon Forest. One species, O. intona, was associated with both environments in the Marshland, while P. (P.) chrysostoma was associated with the two environments in the Mangrove.
Non-metric multidimensional scaling (NMDS) ordination of the data on the Sarcophagidae community recorded at the 90 study sites surveyed in the present study in Maranhão state, Brazil, from 2010 to 2012. The sites are coded by phytophysiognomy (C Cerrado Savanna, P Palm Forest, A Amazon Forest, M Marshland, ME Mangrove) and environment (a anthropic, p preserved). Observations: the arrows indicate a species association, while the dotted lines delimit specific groups
Discussion
The results of the present study indicate that the changes in land use associated with cattle ranching in the Brazilian state of Maranhão have systematic impacts on the patterns of species richness, abundance, and composition of necrophagous dipteran communities (Calliphoridae + Mesembrinellidae (C + M) group, and Sarcophagidae) in the state’s principal phytophysiognomies. In the Palm Forest and Marshland, cattle ranching provoked a decline in the species richness of the Calliphoridae + Mesembrinellidae (C + M) group. A similar pattern of decreasing species richness in impacted environments has been observed in the C + M in a number of previous studies (Centeno et al. 2004; Sousa et al. 2010; Battan-Horenstein et al. 2016; Dufek et al. 2019), although some research has found a relatively high diversity in impacted habitats. Cabrini et al. (2013), for example, recorded a higher calliphorid species richness and diversity in areas with greater anthropogenic impact (grazing land and plantations) in the Brazilian Atlantic Forest in comparison with more preserved environments, such as forest fragments and secondary forest. In this study, Mesembrinella bellardiana, which is typical of preserved environments, was the most abundant species in all environments, with more than half of all the individuals collected.
Sarcophagid species richness was highest in the anthropic areas of the Amazon Forest. Sousa et al. (2011a) compared clearings opened for oil prospecting within the continuous forest in western Amazonia, and found that the sarcophagid fauna was richest in the clearings. The loss of forest cover may favor some sarcophagids due to their ecological characteristics, including their heliophilous behavior (Willmer 1982) and their ability to exploit the ephemeral resources that are typical of these environments (Sousa et al. 2011b). Some dipterans are able to colonize anthropogenic environments successfully, in particular, the more generalist species and those with less specific habitat preferences, which are more able to adapt to novel environments and niches, resulting in an increase in their abundance and species richness (Mulieri et al. 2011). Some sarcophagids are considered to be biological invaders, that are even able to colonize different continents, as in the case of Peckia (Sarcodexia) lambens and Peckia (Peckia) chrysostoma, both native to the New World and introduced in Australia and the species Sarcophaga ruficornis which is Native to the Paleártica region and has been introduced in almost all continents (Pape 1996). Despite not being a consensus, some studies, e.g., Mulieri et al. (2008), Sousa et al. (2011a), Yepes-Gaurisas et al. (2013), and Valverde-Castro et al. (2017), have identified an association with anthropic environments in the subfamily Sarcophaginae.
Due to the distribution patterns of the calliphorids and mesembrinellids (Sousa et al. 2010, 2011a, b, 2014), the species richness of these families was expected to have been affected by the anthropogenic impacts in the more heterogeneous phytogeographic environments (Cerrado Savanna, Palm Forest, and Amazon Forest). These phytophysiognomies have a greater tree density and a taller canopy than the Marshland and Mangrove, which implies the loss of a greater amount of substrates and microhabitats following deforestation for cattle ranching. By contrast, the sarcophagid species richness varied significantly between environments only in the Amazon Forest, where it increased in the anthropic environment. Otherwise, little variation was found between environments (preserved and anthropic) in the more heterogeneous phytophysiognomies, although species richness did tend to be higher, in general, than in the Marshland and Mangrove. This pattern may also be related, at least in part, to spatial effects, given that most species tend to occur within a given phytophysiognomy (Blackburn and Gaston 1996; Rahbek 1997). In particular, the Cerrado, Palm Forest and Amazon Forest together cover more than 70% of the study area, which may also determine higher overall levels of biodiversity in these phytophysiognomies.
The hypothesis that the anthropogenic impacts on the phytophysiognomies affect the composition and abundance patterns of the C + M group and the sarcophagids was corroborated. The anthropogenic process affected the species composition and abundance of the C + M group in most of the phytophysiognomies, although the Amazon Forest was the most affected. The greater abundance of C. albiceps and C. macellaria in the anthropic Amazon Forest environment (Aa), and of M. bicolor in the preserved Amazon Forest (Ap), was fundamental to the differentiation of the assemblages between these environments. Mesembrinella bicolor is asynanthropic and, like other mesembrinellids, is found in forest (Guimarães 1977; Whitworth and Yusseff-Vanegas 2019).
In the sarcophagids, species composition and abundance only varied significantly between the anthropic and preserved environments in the Amazon Forest. Even so, the patterns observed in the Cerrado Savanna, Palm Forest, and Amazon Forest were distinct from those recorded in the Mangrove and Marshland (considering both environments). The species T. (S.) occidua, P. (S.) lambens, O. intona, and P. (P.) chrysostoma contributed most to this dissimilarity. Anthropogenic impacts resulted in an increase in the abundance of T. (S.) occidua, P. (S.) lambens, and P. (P.) chrysostoma. Tricharaea (S.) occidua presents a strong preference for areas of human settlement and has high synanthropic indices (Yepes-Gaurisas et al. 2013), and in rural environments, it has been collected in pastures (Mulieri et al. 2008). Peckia (S.) lambens and P. (P.) chrysostoma also present affinities for habitats modified by humans, and are seen as species with positive synanthropic indices (Yepes-Gaurisas et al. 2013; Valverde-Castro et al. 2017).
Studies along an anthropogenic gradient (urban–rural-forest) have demonstrated systematic shifts in the composition of the calliphorid, mesembrinellid, and sarcophagid communities as a result of environmental change, in Colombia (Beltran et al. 2012; Yepes-Gaurisas et al. 2013; Valverde-Castro et al. 2017), Argentina (Mulieri et al. 2011; Patitucci et al. 2011), England (Hwang and Turner 2005) and Brazil (Linhares 1981; Gadelha et al. 2015). Clearly, the species composition and relative abundance of the dipterans of these three families are influenced strongly by human impacts on natural environments, as in the case of cattle ranching in the present study. The contradicting responses found among the different dipteran families in the present study reinforces the potential of this group as indicators of environmental conditions. The general lack of a clear relationship between species richness and anthropogenic impacts can be accounted for by the tradeoff between the local extinction of more specialized species and the arrival of more generalist taxa, which tends to balance out the number of species in the community. The increase in species richness observed at moderate levels of anthropogenic impact may be related to the intermediate disturbance hypothesis (Connell 1978), which states that moderate levels of human disturbance promote the coexistence of several different types of species, including native pioneer species, as well as introduced taxa (McKinney 2008).
In addition to varying responses between the groups, we also detected variation in species associations, with some species being more closely associated with preserved environments, others with anthropic environments, and some with both types of environment. The calliphorids C. idioidea and L. eximia and the mesembrinellid, M. bicolor, were all associated with natural environments, as verified in previous studies (Esposito et al. 2009, 2010; Gadelha et al. 2009, 2015; Sousa et al. 2010). By contrast, whereas species such as C. albiceps, C. macellaria and C. megacephala, are more abundant in anthropic environments in some areas, they have also been recorded in more pristine areas (Gomes et al. 2000; Ferraz et al. 2010; Gonçalves et al. 2011, Koller 2011). A greater abundance of C. albiceps and C. macellaria in anthropized environments has already been found (Nuorteva 1963; Ferreira and Barbola 1998; Gomes et al. 2000; Ferraz et al. 2010; Koller 2011). These studies indicate that these species prefer areas inhabited by humans or those that have suffered some degree of anthropogenic change, such as forest margins and pastures.
The sarcophagids P. (S.) lambens and T. (S.) occidua, associated in this study with anthropic environments in the Cerrado Savanna and Amazon Forest, have also been recorded in open areas, such as pastures, in other studies (Almeida 1984; Mulieri et al. 2008). The species O. intona and P. (P.) chrysostoma, associated with both types of environment, are capable of adapting to different environments (Ferraz 1995; Sousa et al. 2011b; Vasconcelos et al. 2013), given that they have been recorded in both forest and open areas.
The results of the present study indicate that cattle ranching in natural phytophysiognomies affects the species richness, composition, and abundance of the calliphorids, mesembrinellids, and sarcophagids, in particular in the Amazon Forest, but also with differential responses. Reliable data on biodiversity are important to define public policies and ensure the economic development of a region or biome, such as criteria for regulating the use of an Ecological Economic Zone, and the integration of its economic, social, and environmental relationships. The results of the present study highlight the negative impacts of cattle ranching on the more forested areas such as the Amazon Forest and the Palm Forest, where it would be recommendable to avoid this activity in favor of more sustainable types of land use, such as the production of babassu oil or the extraction of other natural forest products that do not require shifts in land use. The findings of the present study thus provide important insights for the regulation and planning of cattle ranching in Maranhão. If the conversion of natural habitats to cattle pasture continues, special attention should be given to the Amazon Forest, Palm Forest, Marshland, and Mangrove phytophysiognomies, where anthropogenic impacts cause significant shifts in the dipteran communities.
It is important to note here that, while the Cerrado is defined as a biodiversity hotspot (Myers 2000; Martinelli et al. 2014), the other phytophysiognomies surveyed in the present study may suffer similar or even greater pressures than the Cerrado Savanna, given their much smaller areas (except in the case of the Amazon Forest). The Palm Forest is especially interesting in this context, given that this natural system is used as a source of subsistence by many extractive communities, for the production of babassu oil, a practise that is being lost progressively with the advance of cattle ranching in the region. The Palm Forest is also an important transition zone between the Amazon Forest and the Cerrado Savanna, which contains a unique biodiversity that includes representatives of the Amazonian, Atlantic Forest, and Cerrado biomes, as shown in studies of other taxonomic groups (Martins et al. 2009). Furthermore, C. idioidea, M. bicolor and Lucilia eximia were associated strongly with preserved environments. If natural areas continue to be impacted on a large scale, then, these species should be monitored or included in management programs to prevent their extinction.
Conflict of interest
All the authors participated in (a) the conception and design of the study, or the analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content, and (c) the approval of the final version; This manuscript has not been submitted to, nor is under review at, at any other journal or other publishing venue. The authors have no affiliation with any organization that has a direct or indirect financial interest in the subject matter discussed in the manuscript.
Ethical approval
Please be advised that the specimens analyzed in the present study were collected according to Brazilian law. The permits for the collection and transportation of zoological specimens (necrophagous Diptera) were provided by the Chico Mendes Institute for the Conservation of Biodiversity (ICMBio)/Sisbio, in accordance with federal law and the regulations of the Brazilian Environmental Ministry, through process number 1403-1 (for private properties) and 29342-1 (for the Gurupi Biological Reserve, a federal conservation unit in the state of Maranhão). At private properties, permission from the landowner or property manager was obtained prior to sampling. None of the study species are protected by Brazilian law or red-listed.
References
Almeida JM (1984) Sinantropia de Sarcophagidae (Diptera) na Região Metropolitana do Rio de Janeiro. Arq Univ Fed Rural Rio de Janeiro 7:101–110
Anderson M (2005) PERMANOVA: a Fortran computer program for permutational multivariate analysis of variance. Department of Statistics University of Auckland, New Zealand
Barreto P, Pereira R, Arima E (2008) A pecuária e o Desmatamento na Amazônia na Era das Mudanças Climáticas. Imazon, Belém
Battan-Horenstein M, Bellis LM, Gleiser RM (2016) Diversity of necrophagous blowfly (Diptera: Calliphoridae) of medical and veterinary importance in urban environments in Córdoba (Argentina). Caldasia 38:183–195. https://doi.org/10.15446/caldasia.v38n1.57837.
Beltran YTP, Segura NA, Bello FJ (2012) Synanthropy of Calliphoridae and Sarcophagidae (Diptera) in Bogotá, Colombia. Neotrop Entomol 41:237–242
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol Evol 18:182–188
Blackburn TM, Gaston KJ (1996) Spatial patterns in the geographic range sizes of bird species in the New World. Philos Trans R Soc London Ser B 351(897):912
Brown Jr KS (1997) Insetos como rápidos e sensíveis indicadores de uso sustentável de recursos naturais. In: Martos HL, Maia NB (eds) Indicadores Ambientais. PUC-SP, Sorocaba, pp 143–155
Buenaventura E, Pape T (2013) Revision of the New World genus Peckia Robineau Desvoidy (Diptera: Sarcophagidae). Zootaxa 3622:1–87
Cabrini I, Grella MD, Andrade CFS, Thyssen P (2013) Richness and composition of Calliphoridae in an Atlantic Forest fragment: implication for the use of dipteran species as bioindicators. Biodivers Conserv 22:2635–2643
Carvalho CJB, Mello-Patiu CA (2008) Keys to the adults of the most common forensic species of Diptera in South America. Rev Bras Entomol 52:390–406
Centeno N, Almorza D, Arnillas C (2004) Diversity of Calliphoridae (Insecta: Diptera) in Hudson, Argentina. Neotrop Entomol 33:387–390
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Colwell RK (2013) Estimates: statistical estimation of species richness and shared species from samples. Version 9.0. https://purl.oclc.org/estimates. Accessed 23 Apr 2013
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Connell JH (1978) Diversity in Tropical Rain Forests and Coral Reefs. Science 199:1302–1310
Dennis P, Young MR, Gordon IJ (1998) Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecol Entomol 23:253–264
Dufek MI, Oscherov EB, Damborsky MP, Mulieri PR (2019) Calliphoridae (Diptera) in human-transformed and wild habitats: diversity and seasonal fluctuations in the Humid Chaco Ecoregion of South America. J Med Entomol. https://doi.org/10.1093/jme/tjy234
Erzinçlioglu Z (1996) Blowflies. Naturalist’s handbooks. The Richmond Publishing Co. Ltd, Great Britain
Esposito MC, Sousa JRP, Carvalho-Filho FS (2009) Diversidade de Calliphoridae (Insecta, Diptera) em ambientes de matas e próximos de habitações da Estação Científica Ferreira Penna (ECFPn), Melgaço/PA, e da cidade de Portel/PA. In: Lisboa PLB (ed) Caxiuanã: Desafios para a Conservação de uma Floresta na Amazônia. Museu Paraense Emílio Goeldi, Belém, pp 461–469
Esposito MC, Sousa JRP, Carvalho-Filho FS (2010) Diversidade de Calliphoridae (Insecta: Diptera) na base de extração petrolífera da Bacia do Rio Urucu, na Amazônia brasileira. Acta Amaz 40:579–584
Ferraz MV (1995) Larval and pupal periods of Peckia chrysostoma and Adiscochaeta ingens (Diptera: Sarcophagidae) reared under laboratory conditions. Mem Inst Oswaldo Cruz 90:611–614
Ferraz ACP, Gadelha BQ, Aguiar-Coelho VM (2009) Análise faunística de Calliphoridae (Diptera) da Reserva Biológica do Tinguá, Nova Iguaçu, Rio de Janeiro Rev Bras Entomol 53: 620–628.
Ferraz ACP, Gadelha BQ, Aguiar-Coelho VM (2010) Effects of forest fragmentation on dipterofauna (Calliphoridae) at the Reserva Biológica do Tinguá, Nova Iguaçu. RJ Braz J Biol 70:55–63
Ferreira MJM (1978) Sinantropia de dípteros muscóides de Curitiba. Paraná I Calliphoridae Rev Bras Biol 38:445–454
Ferreira MJM, Barbola IF (1998) Sinantropia de califorídeos (Insecta: Diptera) de Curitiba, Paraná, Brasil. Rev Bras Biol 58:203–209
Figueiredo ALD, Carvalho RPD, Azevedo WTDA, Teixeira MLF, Rebello MT, Ramos ACDC, Aguiar VM (2018) Faunistic analysis of the families Calliphoridae and Mesembrinellidae (Diptera) at Jardim Botânico do Rio de Janeiro, Brazil. J Med Entomol. https://doi.org/10.1093/jme/tjy123
Forister ML, Pelton EM, Black SH (2019) Declines in insect abundance and diversity: we know enough to act now. Conserv Sci Pract. https://doi.org/10.1111/csp2.80
Furusawa GP, Cassino PCR (2006) Ocorrência e distribuição da Calliphoridae (Díptera, Oestroidea) em um fragmento de Mata Atlântica secundária no município de Engenheiro Paulo de Frontin, Médio Paraíba. RJ Rev Biol Ciênc Terra 6:152–164
Gadelha BQ, Ferraz ACP, Aguiar VM (2009) A importância dos mesembrinelíneos (Diptera: Calliphoridae) e seu potencial como indicadores de preservação ambiental. Oecol Brasil. https://doi.org/10.4257/oeco.2009.1304.09
Gadelha BQ, Ribeiro AC, Aguiar VM, Mello-Patiu CA (2015) Edge effects on the blowfly fauna (Diptera, Calliphoridae) of the Tijuca National Park. Rio de Janeiro, Brazil. https://doi.org/10.1590/1519-6984.05614
Gomes A, Koller WW, Barros ATM (2000) Sazonalidade da mosca-varejeira, Cochliomyia macellaria (Díptera:Calliphoridae) na região dos cerrados, Campo Grande, MS. Rev Bras Parasitol Vet 9:125–128
Galante E, Marcos-Garcia MA (2004) Decomposer insects. In: Encyclopedia of entomology. Springer, Dordrecht. https://doi.org/10.1007/0-306-48380-7_1171
Gonçalves L, Dias A, Espindola CB, Almeida FS (2011) Inventário de Calliphoridae (Diptera) em manguezal e fragmento de Mata Atlântica na região de Barra de Guaratiba, Rio de Janeiro, Brasil. Rev Bras Biocienc 9:50–55
Guerra WD, Oliveira PC, Pujol-Luz JR (2012) Gafanhotos (Orthoptera, Acridoidea) em áreas de cerrados e lavouras na Chapada dos Parecis, Estado de Mato Grosso, Brasil. Rev Bras Entomol 56:228–239
Guimarães JH (1977) A systematic revision of the Mesembrinellidae, stat. nov. (Diptera, Cyclorrapha). Arq Zool 29:1–109
Guimarães JH, Papavero N (1999) Myasis of man and animals in the Neotropical Region. Editora Plêiade, São Paulo
Guimarães JH (2004) Redescrição dos machos de dez espécies Neotropicais de Ravinia Robineau-Desvoidy, 1863 (Diptera, Sarcophagidae). Arq Mus Nac Rio de Janeiro 62:45–66
Hwang C, Turner BD (2005) Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Med Vet Entomol 19(4):379–391
IBGE (2012) Manual técnico da vegetação brasileira: sistema fitogeográfico, inventário das formações florestais e campestres, técnicas e manejo de coleções botânicas, procedimentos para mapeamentos. Gráfica do Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro, p 272
Koller WW, Barros ATM, Corrêa EC (2011) Abundance and seasonality of Cochliomyia macellaria (Diptera: Calliphoridae) in Southern Pantanal, Brazil. Rev Bras Parasitol Vet 20:27–30
Kosmann C, Mello RP, Harterreiten-Souza ÉS, Pujol-Luz JR (2013) A list of current valid blow fly names (Diptera: Calliphoridae) in the Americas South of Mexico with key to the Brazilian species. EntomoBrasilis 6:74–85
Legendre P, Legendre L (1998) Numerical Ecology. Elsevier, Amsterdam
Lemos JJS (2001) Níveis de degradação ambiental no nordeste brasileiro. Rev Econômica do Nordeste 32:406–429
Linhares AX (1981) Synanthropy of Calliphoridae and Sarcophagidae (Diptera) in the city of Campinas, São Paulo, Brasil. Rev Bras Entomol 25:189–215
Lopes HS (1939) Contribuição ao conhecimento do gênero Helicobia Coquillett (Diptera, Sarcophagidae). Rev Entomológica 10:497–517
Lopes HS (1958) Considerações sobre as espécies de Peckia Desvoidy, 1830 e de gêneros afins. (Diptera, Sarcophagidae). An Acad Bras Ciênc 30:211–239
Lopes HS (1989) On american Sarcophagidae (Diptera) with revision of Pekiamyia Dodge. Rev Bras Biol 49:837–845
Lopes HS, Tibana R (1987) On Oxysarcodexia (Diptera, Sarcophagidae), with descriptions of five new species, key, list and geographic distribution of the species. Rev Bras Biol 47:329–347
Majer JD (1987) Invertebrates as indicators for management. In: Saunders DA, Arnold GW, Burbidge AA, Hopkins AJM (eds) Nature conservation: the role of remnants of native vegetation. Surrey Beatty and Sons Pty Limited with CSIRO and CALM, New South Wales, Australia, pp 353–354
Marinho-Filho J, Machado RB, Henriques RPB (2010) Evolução do conhecimento e da conservação do Cerrado Brasileiro (2010). In: Diniz IR, Marinho-Filho J, Machado RB, Cavalcanti RB (eds) Cerrado: conhecimento científico quantitativo como subsídio para ações de conservação. Thesaurus, Brasília, pp 15–34
Marinho MAT, Wolff M, Ramos-Pastrana Y, Azeredo-Espin AML, Amorim DS (2017) The first phylogenetic study of Mesembrinellidae (Diptera: Oestroidea) based on molecular data: clades and congruence with morphological characters. Cladistics 33:134–152
Martinelli G, Messina T, Santos-Filho L (2014) Red Book of the Flora of Brazil-Rare plants of the Cerrado. Andrea Jakobsson, Rio de Janeiro
Martins UR, Galileo MHM, Limeira-de-Oliveira F (2009) Cerambycidae (Coleoptera) do estado do Maranhão. Brasil Pap Avul Zool 49:229–247
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Mello RP (2003) Chave para identificação das formas adultas das espécies da família Calliphoridae (Díptera, Brachycera, Cyclorrhapha) encontradas no Brasil. Entomol Vectores 10:255–268
Mello RS, Queiroz MMC, Aguiar-Coelho VM (2007) Population fluctuations of calliphorid species (Diptera, Calliphoridae) in the Biological Reserve of Tinguá, state of Rio de Janeiro, Brazil, Iheringia. Série Zoologia 97:481–485
Mulieri PR, Schnack JA, Mariluis JC, Torretta JP (2008) Flesh flies species (Diptera: Sarcophagidae) from a grassland and a woodland in a Nature Reserve of Buenos Aires, Argentina. Rev Biol Trop 56:1287–1294
Mulieri PR, Patitucci LD, Schnack JA, Mariluis JC (2011) Diversity and seasonal dynamics of an assemblage of sarcophagid Diptera in a gradient of urbanization. J Insect Sci. https://doi.org/10.1673/031.011.9101
Myers N et al (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772):853–858
Nuorteva P (1963) Synanthropy of blowflies (Dipt., Calliphoridae) in Finland. Ann Entomol Fenn 29:1–49
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests MASS (2007) The vegan package. Commun Ecol Package 10:631–637
Pape T (1996) Catalogue of the Sarcophagidae of the World (Insecta: Diptera). Mem Entomol Int 8:1–558
Patitucci LD, Mulieri PR, Schnack JA, Mariluis JC (2011) Species composition and heterogeneity of blowflies assemblages (Diptera: Calliphoridae) in urban–rural gradients at regional scale in Argentinean Patagonia. Stud Neotrop Fauna Environ 46:49–58
Rahbek C (1997) The relationship among area, elevation and regional species richness in Neotropical birds. Am Nat 149:875–902
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org. Accessed 14 Apr 2018.
Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D, Ripley MB (2013). Package ‘mass’. Cran R, 538.
Rognes K (1997) The Calliphoridae (blowflies) (Diptera: Oestrioidea) are not a monophyletic group. Cladistics 13(1–2):27–66
Sánchez-Bayo F, Wyckhuys KAG (2019) World decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27
Santos, AJ (2009) Estimativas de riqueza em espécies. In: Cullen Jr L, Valladares-Padua C, Rudy R (Org.). Métodos de estudo em biologia da conservação e manejo da vida silvestre, 2ª ed. Ed. Universidade Federal do Paraná, Curitiba, pp 19–41.
Shewell GE (1987a) Calliphoridae. In: Mcalpine JF (ed) Manual of Nearctic Diptera, 2ndª edn. Research Branch Agriculture Canada, Ottawa, pp 1133–1145
Shewell GE (1987b) Sarcophagidae. In: McAlpine JF (ed) Manual of Neartic Diptera, 2ndª edn. Research Branch Agriculture Canada, Ottawa, pp 1159–1186
Svenning JC (1999) Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. J Ecol 87:55–65
Svenning JC (2001) On the role of microenvironmental heterogeneity in the ecology and diversification of Neotropical rain-forest palms (Arecaceae). Bot Rev 67:1–53
Sousa JRP, Esposito MC, Carvalho-Filho FS (2010) A fauna de califorídeos (Díptera) das matas e clareiras com diferentes coberturas vegetais da Base de Extração Petrolífera, bacia do Rio Urucu, Coari, Amazonas. Rev Bras Entomol 54:270–276
Sousa JRP, Esposito MC, Carvalho-Filho FS (2011a) Composition, abundance and richness of Sarcophagidae (Diptera: Oestroidea) in forests and forest gaps with different vegetation cover. Neotrop Entomol 40:20–27
Sousa JRP, Esposito MC, Carvalho-Filho FS (2011b) Diversity of Calliphoridae and Sarcophagidae (Diptera, Oestroidea) in continuous forest and gaps at different stages of regeneration in the Urucu oilfield in western Brazilian Amazonia. Rev Bras Entomol 55:578–582
Sousa JRP, Esposito MC, Carvalho-Filho FS, Juen L (2014) The Potential Uses Of Sarcosaprophagous Flesh Flies And Blowflies For The Evaluation Of The Regeneration And Conservation Of Forest Clearings: A Case Study in the Amazon forest. J Insect Sci 14:1–5
Sousa JRP, Carvalho-Filho FS, Esposito MC (2015) Distribution and abundance of necrophagous flies (Diptera: Calliphoridae and Sarcophagidae) in Maranhao Northeastern Brazil. J Insect Sci. https://doi.org/10.1093/jisesa/iev054
Sousa JRP, Carvalho-Filho FS, Juen L, Esposito MC (2016) Evaluating the effects of different vegetation types on necrophagous fly communities (Diptera: Calliphoridae; Sarcophagidae): implications for conservation. PLoS ONE. https://doi.org/10.1371/journal.pone.0164826
Tavares PD, Uzeda MC, Pires AS (2019) Biodiversity conservation in agricultural landscapes: the importance of the matrix. Floresta Ambient. https://doi.org/10.1590/2179-8087.066417
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann MC, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Tibana R (1976) Duas redescrições e uma descrição de espécie nova do gênero Helicobia (Diptera, Sarcophagidae). Rev Bras Biol 36:723–729
Tibana R (1981) Estudo sobre 7 espécies de Helicobia Coquillett, 1895. (Diptera, Sarcophagidae). Rev Bras Biol 41:625–634
Tibana R, Xerez R (1985) Uma nova espécie de Retrocitomyia Lopes, 1982, (Diptera, Sarcophagidae). Rev Bras Biol 45:485–488
Wallis-de-Vries MF, Parkinson AE, Dulphy JP, Sayer M, Diana E (2007) Effects of livestock breed and grazing intensity on biodiversity and production in grazing systems. 4. Effects on animal diversity. Grass Forage Sci 62:185–197
Willmer PG (1982) Thermoregulatory mechanisms in Sarcophaga. Oecologia 53:382–385
Whitworth TL, Yusseff-Vanegas S (2019) A revision of the genera and species of the Neotropical family Mesembrinellidae (Diptera: Oestroidea). Zootaxa. https://doi.org/10.11646/zootaxa.4659.1.1
Wolff M, Kosmann C (2016) Families Calliphoridae and Mesembrinellidae. Zootaxa 4122(1):856–875. https://doi.org/10.11646/zootaxa.4122.1.72
Valverde-Castro C, Buenaventura E, Sanchez-Rodriguez JD, Wolff M (2017) Flesh flies (Diptera: Sarcophagidae: Sarcophaginae) from the Colombian Guajira biogeographic province, an approach to their ecology and distribution. Zoologia. https://doi.org/10.3897/zoologia.34.e12277
Vasconcelos SD, Cruz TM, Salgado RL, Thyssen PJ (2013) Dipterans associated with a decomposing animal carcass in a rainforest fragment in Brazil: notes on the early arrival and colonization by necrophagous species. J Insect Sci. https://doi.org/10.1673/031.013.14501
Yepes-Gaurisas D, Sanchez-Rodriguez JD, Mello-Patiu CA, Wolff EM (2013) Synanthropy Of Sarcophagidae (Diptera) in La Pintada, Antioquia-Colombia. Rev Biol Trop 61:1275–1287
Acknowledgments
This study was part of the doctoral thesis of JRPS at the UFPA/MPEG Graduate Program in Zoology. We thank the Maranhão State Research Foundation for funding and providing the first author with a doctoral stipend. Leandro Juen (process: (304710/2019-9) and Maria Cristina Esposito (process: 309572/2013-4) thank CNPq (National Council for Scientific and Technological Development) for productivity scholarships. We are also grateful to the Brazilian Coordination for Higher Education Personnel Training (CAPES) for funding the senior internship scholarship of LJ at the University of Florida through PROCAD-AMAZONIA/CAPES (process 88881.474457/2020-01). We also thank the UFPA Dean’s Office for Graduate Studies UFPA (PROPESP) and Pará State Research Foundation (Decree 01/2019 PROPESP/FADESP-UFPA) for their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa, J.R.P., da Silva Carvalho-Filho, F., Juen, L. et al. The effects of cattle ranching on the communities of necrophagous flies (Diptera: Calliphoridae, Mesembrinellidae and Sarcophagidae) in Northeastern Brazil. J Insect Conserv 24, 705–717 (2020). https://doi.org/10.1007/s10841-020-00246-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00246-y