Abstract
A worldwide decline of many bee species has been reported, but conversely some species seems to be in expansion. Nonetheless species truly in expansion may be overestimated, especially when they are considered as a whole, and information about intraspecific lineages is lacking. The objective of this study was to test whether the bumblebee species Bombus morio and B. pauloensis will be safe under future climate changes. Specifically, test if these bees will suffer geographic decline or expansion; test whether each phylogeographic lineage within B. pauloensis will respond differently in modeling of future geographic distribution given climate change; find stable areas holding high genetic diversity based on predicted future climatic changes; and test whether these areas are covered by existing protected areas. To reach the objectives we performed analyses using phylogeographic data already available and climate change information to model the demography of the panmictic B. morio and the phylogeographic lineages in B. pauloensis. Our results suggest that both species will suffer a reduction in suitable area and that the reduction in distribution is masked for B. pauloensis. When each clade was separately analyzed, the ones in the edge of the species distribution are the most likely to decline. We found a large future refuge in eastern state of São Paulo and state of Rio de Janeiro for B. morio and for the clades of B. pauloensis. This refuge seems to show high levels of species richness and endemism for different taxa. Thus, by protecting this area we will be preserving not only the pattern of biodiversity but also the processes that generate and maintain them for many other species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently there are more than 20,000 species of bees accepted in the world (Ascher and Pickering 2014). The ecological services they provide through pollination are unquestionable and have high ecologic and economical impacts (Kremen et al. 2008; Fontaine et al. 2006; Vamosi et al. 2006; Olesen et al. 2007; Pauw 2007; Potts et al. 2016). Unfortunately recent reports have indicated a worldwide bee decline (Cane and Tepedino 2001; Biesmeijer et al. 2006; De la Rúa et al. 2009). Moreover, a regional Red List assessment indicates that 9% of bees are threatened (Potts et al. 2016). Although this assessment is available only for Europe, it should be considered as an alert in a global scenario. The cause of the decline in general may be associated to extrinsic factors as habitat loss and fragmentation, agriculture intensification, excessive use of pesticides, pathogens, industrialization, invasive alien species, and global climatic changes. Also intrinsic genetic and ecological factors as haplodiploidy and complementary sex determination (related to production of unviable or sterile diploid males), social behavior and specialist feeding habit may be involved (Zayed 2009, and references therein; Potts et al. 2016).
Specifically, bumblebees are important pollinators of natural flora and crops (Corbet et al. 1991; Kevan 1991; Memmott et al. 2004; Pywell et al. 2006; Velthuis and Doorn 2006; Goulson et al. 2008; Goulson 2010). They present important and sometimes advantageous features when compared to honeybees, e.g., a greater capacity for foraging in cold and rainy conditions, visiting crops with longer flowers due to their long tongue, and performing buzz pollination, important for plants with poricidal anthers, as Solanaceae (Goulson 2010, and references therein). Nevertheless, many studies have documented a recent decline of bumblebees species, mainly in areas that suffered high habit modification by anthropic actions related to agriculture intensification and urbanization (Williams 1982, 1986; Carvell 2002; Goulson 2006; Batra 1995; Ellis et al. 2006; McFrederick and LeBuhn 2006; Pywell et al. 2006; Fitzpatrick et al. 2007; Kosior et al. 2007; Colla and Packer 2008; Goulson et al. 2008; Grixti et al. 2009; Williams et al. 2009; Williams and Osborne 2009; Cameron et al. 2011; Morales et al. 2013).
Shifts in pollinator diversity and ranges are well documented in Europe and North America bumblebees (Potts et al. 2016), but there is little information for Latin America, Africa and Asia (Potts et al. 2016). While European species appear mainly affected by a change-over in agricultural practices impacting food and nest resources (Williams and Osborne 2009), in the United States, species decline has been associated with a high prevalence of the microsporidian pathogen Nosema bombi (Cameron et al. 2011). In Chile and southern Argentina, the introduced species Bombus ruderatus and Bombus terrestris have replaced Bombus dahlbomii, formerly the most abundant pollinator (Morales et al. 2013). Today, B. dahlbomii is absent in many areas, including those where the two introduced species were first recorded (Morales et al. 2013). In Brazil, a recent report indicated that B. bellicosus has suffered drastic decline and extinction in the northern portion of its distribution range, probably due to habitat loss, pollution, and climate change (Martins and Melo 2010; Martins et al. 2015).
In Brazil there are just eight species of bumblebees recognized: B. applanatus, B. bahiensis, B. bellicosus, B. brasiliensis, B. brevivillus, B. morio, B. pauloensis, and B. transversalis, being the first two species recently described (Santos et al. 2015; Françoso et al. 2016b). They are important pollinators of several crops: blueberry (da Silveira et al. 2011); Brazil nut (Maués 2002; Giannini et al. 2015); eggplant (D`Avila and Marchini 2005; Montemor and Souza 2009; Giannini et al. 2015); gabiroba, guava, jurubeba, sweet passion fruit, squash, urucum (Giannini et al. 2015), tomato (Aldana et al. 2007; Giannini et al. 2015); yellow passion fruit (Yamamoto et al. 2012; Giannini et al. 2015); beans (D’Avila and Marchini 2005); and sunflowers (D’Avila and Marchini 2005; Giannini et al. 2015). Nonetheless, little is known about their conservation status, and consequently there is no policy for the protection and management of these bees.
Future changes in climate are likely to affect species range, distribution of the biological diversity, and taxa evolutionary history (Thuiller et al. 2011; Kerr et al. 2015). The geographic distribution modeling based on future projections has been used to predict how species will respond to climatic changes (Hannah et al. 2002; Kramer et al. 2010; Loiselle et al. 2010; Pearman et al. 2010; Maiorano et al. 2011; Saupe et al. 2011; Taubmann et al. 2011; Watt et al. 2011; Giannini et al. 2012; D`Amen et al. 2013). Giannini et al. (2012), using two different scenarios for future projections, an optimistic and a pessimistic, to the years 2050 and 2080, concluded that 9 of 10 species of Brazilian bees would have their suitable areas decreased. While a number of causal factors may be involved, the response of different species to habitat loss is likely a key component of this phenomenon (Lozier and Cameron 2009). Lozier et al. (2011) observed expansion in some bumblebee species in North America, despite recent habitat fragmentation. The authors discuss the role of marginal habitats, such as patches of weedy flowers adjacent to highways and agricultural fields, or gardens in heavily urbanized areas, as potential food resources for bumblebees. Furthermore, the high capacity for dispersal (Macfarlane 1995; Buttermore 1997; Kraus et al. 2009; Lepais et al. 2010) may maintain population structure via immigration (Francisco et al. 2016; Françoso et al. 2016a). Although the decline of bees is global and currently discussed, many species of Bombus are not affected and remain constant or even in expansion (Goulson et al. 2008). Nevertheless, species truly in expansion may be overestimated. Normally the species are analyzed as a unit, lacking important information about lineages. That is, one lineage may be expanding but an overall loss of genetic diversity may be accounting in other lineages. The infra-specific genetic data are crucial for the preservation of the historical components of diversity and to maintain the potential for species to mount adaptive responses to environmental changes (D’Amen et al. 2013).
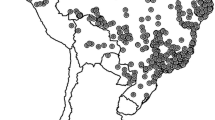
Françoso et al. (2016a), encompassing the two most widely distributed bumblebee species in Brazil, B. morio and B. pauloensis, provided valuable contributions on the demography, distribution, and genetic structure. Their results suggest no genetic structure for B. morio by mitochondrial and microsatellite data, except for very distinct samples from Teodoro Sampaio city that likely reflect a new species or subspecies corroborating previous data obtained by Francisco et al. (2016). On the other hand, B. pauloensis has three conspicuous lineages with deep phylogeographic division according to mitochondrial data and morphology: central (C), north (N), and south (S) clades. These lineages differ in the climates they experience and have substantial geographic separation (Françoso et al. 2016a; Fig. 1). As the phylogeographic data provide an important set, and once the genetic structure of B. morio and B. pauloensis is known, our main goal is to test whether both species and the lineages of B. pauloensis will be safe under future climate changes, considering the existing protected areas. Specifically the objectives were to test: (i) whether these bee species will suffer geographic decline or expansion; (ii) whether each phylogeographic lineage within B. pauloensis will respond differently in modeling of future geographic distribution given climate change; (iii) whether there are stable areas with high genetic diversity based on predicted future climatic changes; and (iv) whether these areas are covered by existing protected areas. These objectives were addressed by performing geographic distribution modeling and projected future climate change.

Adapted from Françoso et al. (2016a)
Phylogeographic patterns found in Bombus morio (a) and Bombus pauloensis (b) by using molecular data concerning 1570 bp of mitochondrial DNA (Cytochrome C Oxidase I, Cytochrome B, the large ribosomal RNA subunit, and cluster 4 of tRNA, covering a region of Cytochrome C Oxidase II and ATPase 8 genes, and tRNAlys and tRNAasp). Grey areas in the map correspond to altitudes above 750 m. Main main clade, TS Teodoro Sampaio clade, C central clade, N north clade, S south clade.
Methods
Modeling future geographic distribution
For input locality data, we used the same points described in Françoso et al. (2016a): 102 different georeferenced occurrence records for B. morio and 71 for B. pauloensis. Distribution models were based on the top contributing bioclimatic variables (Table 1) from the WorldClim data set (Hijmans et al. 2005) using Maximum Entropy algorithm in Maxent, v.3.3.3k (Phillips et al. 2006; Phillips and Dudik 2008), a method that performed well in comparison to alternative approaches (Elith et al. 2006). The top contribution bioclimatic variables were chosen according to the estimates of relative contributions of the environmental variables to the Maxent model and the jackknife test of variable importance. The environmental variables were analyzed separately and excluded if verified: (1) low contribution and lower gain when in isolation, and (2) increase or no change in the gain when omitted. If the area under the curve (AUC) value was decreased, the environment variable was kept; conversely, if the AUC value increased, the environment variable was definitely excluded. Maxent generates models using only presence records. We developed present-day models (5 km resolution) and then projected them into the future conditions (2050 and 2070). Global Climate Model (GCM) data at 2.5 min spatial resolution from CMIP5 (IPCC Fifth Assessment) for four representative scenarios based on CO2 concentration pathways (RCP2.6, RCP4.5, RCP6.0 and RCP8.5) data was downscaled and calibrated (bias corrected) using WorldClim 1.4 as baseline “current” climate according to WorldClim (http://www.worldclim.org/cmip5_2.5m). Each RCP differs greatly in the rate of radiative forcing and emissions, being RCP2.6 the scenario with lower emissions; and RCP8.5 the scenario with higher emissions. Four GCM models were used: BCC-CSM1-1, CCSM4, HadGEM2-AO, and MIROC5. We modeled averages of ten replicates using the “crossvalidate” option for each model and scenario. Model performance was evaluated using the AUC calculated by Maxent. AUCs > 0.75 are typically considered adequate for species distribution modeling applications (Pearce and Ferrier 2000). The average of the four GCM models with each one of the four scenarios of carbon emission were used for final considerations.
We classified the suitability for each species and for the clades in B. pauloensis into three thresholds: maximum suitability, corresponding to a predicted suitability higher than 75%; medium suitability, with values above 50%; and minimum suitability, representing suitability above 25%. Based on this information, we evaluated the percent reduction of suitable area for each species and infra-specific clade based on the predicted future climate change.
All maps and the data summarization were made in R (R Core Team 2013), using Geosphere (Hijmans 2014a, b), Maptools (Bivand and Lewin-Koh 2013), Raster (Hijmans 2014a, b), rgdal (Bivand et al. 2013), and rgeos (Bivand and Rundel 2014).
Conservation
The cell values obtained in the distribution modeling were extracted under the different simulations (current, 2050 and 2070) to measure the predicted reduction in suitable area. The intersection of 2070 distribution models from both species (including the C, N, and S clades of B. pauloensis) was used to find future stable areas (i.e., refuges) with high genetic diversity. The simulations were performed firstly considering B. morio and B. pauloensis, and secondly considering B. morio and the three clades of B. pauloensis. These areas were compared to current protected areas, including Full Protection Conservation Units (FPCU) and Sustainable Use Conservation Units (SUCU) (Supplementary Material 1; available from http://siscom.ibama.gov.br).
Results
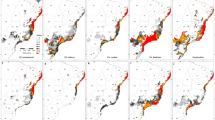
We verified high AUC values for all projections for both species (> 0.97). The current modeling and future projections of distribution using the average of four GCM models, each one with four scenarios, as well the reduction in distribution area for each GCM model and scenario for B. morio, B. pauloensis and its three clades are represented in Fig. 2. The simulations performed separately for each GCM model with each scenario presented a high variation, which means they are very distinct from each other (graphics in Fig. 2 and Supplementary Material 2). In 2070, according to the four GCM models together (average), the prediction is a strong reduction in the distribution area, nearly 45% for B. morio, 49% for B. pauloensis (all clades together), and 37, 71 and 60% for the C, N, and S B. pauloensis clades, respectively, with suitability above 50% (Fig. 3).
Current and future distribution modeling for Bombus morio (main clade) and Bombus pauloensis (all clades together, and Central, North, and South clades separately, according to Françoso et al. 2016a), obtained using the average of four GCM models (BCC-CSM1-1, CCSM4, HadGEM2-AO, and MIROC5), each under four representative CO2 concentration pathways (RCP26, RCP45, RCP60 and RCP85). Suitability range is represented by the grey scale on the projections, in which lighter and darker gray means minimum and maximum probabilities, respectively. Graphs represent the distributional area reduction from modern day to 2050 and 2070 for each model and scenario combination of predicted presence. The axes of ordinates show the occupied area in a normalized ratio
Bombus morio and B. pauloensis (analyzed as a whole) would face a reduction in their distribution on the northern portions of their ranges, mainly in the state of Minas Gerais. The B. pauloensis clades would be affected differentially according to future predictions. Clade C should be the most stable, while clade N will suffer the stronger reduction from the south of state of Minas Gerais until the north of state of São Paulo, and clade S will be reduced mainly from west to east of its original distribution. In general, north areas will face a reduction in the suitability, while the opposite will happen in south areas.
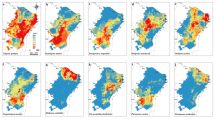
To investigate whether the current protect areas are proper to protect both species of bumblebees in current days and in the future, the suitability intersection under current and 2070 projections using both species together, and both species but considering the internal clades of B. pauloensis were stacked with the protected areas (Fig. 4). The intersection area using B. morio and the clades of B. pauloensis is deeply smaller in comparison to the whole species. According to the simulations using both species together, from current days to 2070 there will be a reduction in area of 48% and 74% under medium and maximum suitability, respectively. The intersection of B. morio and the clades of B. pauloensis distribution modeling in 2017 showed three common areas that can be named as future refuges. Two of these areas are close in distance and are located in the coast of southeast of Brazil, mainly in the east of states of São Paulo and Rio de Janeiro, and will be referred here as “large future refuge”. The third area is very small and located in the south of state of Espírito Santo, and will be name here as “small future refuge”.
Intersection of current and future (2070) distribution modeling of Bombus morio and Bombus pauloensis (whole species and the threes clades separately: Central, North, and South clades) in different levels of suitability, overlapped with conservation unit layers. Suitability ranges in maximum, medium, and minimum probabilities (greater than 75%, 50–75%, and 25–50%, respectively) of predicted presence. The intersection of 2070 distribution models from both species (including the C, N, and S clades of B. pauloensis) was used to find future stable areas (red dashes). The two figures below represent the diversity of clades (B. morio, C, N and S clades of B. pauloensis) in the distribution for 2070 projections
The number of clades (B. morio and B. pauloensis clades C, N and S) in the distribution was performed for current and 2070 projections (Fig. 4). In 2070, there will be two areas with medium suitability proper for the four clades together, located inside the large future refuge in the states of São Paulo and Rio de Janeiro. Compared with current protected areas, these future refuges apparently seem to be protected, especially the large one, since the Serra do Mar region is covered by a mosaic of conservation units (Fig. 4).
Discussion
According to future projections, until 2070 both B. morio and B. pauloensis will have a decrease of nearly 50% in the distribution in areas of medium suitability. This reduction will may not affect so drastically B. morio, since it has high dispersal capacity (Moure and Sakagami 1962); and does not present genetic differentiation through its geographic distribution indicating panmixia (Francisco et al. 2016; Françoso et al. 2016a). Therefore, the panmixia could be ensured and the genetic diversity would be easily recovered. However, the reduction in the distribution of B. pauloensis considered as a unit was masked in comparison to the predictions performed for each clade separately. While the clade C will likely be less affected by future climatic changes (reduction of 37%), clades N and S will suffer a reduction of ca. 71 and 60%, respectively.
Models that do not distinguish infra-specific units often fail to identify potential risks of climate changes to lineages (Pearman et al. 2010; D`Amen et al. 2013). In fact, the clades of B. pauloensis will respond very differently to future changes. The clade C showed to be the most stable in future projections analysis. Probably its current geographic distribution region may represent the species center of origin and diversity. Yet, the two other clades within B. pauloensis seem to have been originated in eastern São Paulo state (Françoso et al. 2016a). According to Françoso et al. (2016a) the clade C is in Hardy–Weinberg equilibrium, i.e., microsatellite data indicated random mating and no selective pressure among the genotypes from generation to generation (Ridley 2004). These data reinforce the hypothesis of population stability and no expansion. In contrast, the clades N and S will suffer a remarkable decline, especially clade N. These clades have been originated recently (Last Glacial Maximum and Last Inter Glacial in the Pleistocene, respectively), are in expansion, and present homozygote excess (Françoso et al. 2016a). Furthermore, they encompass the edges of the species distribution, which are areas most likely to change. Populations at the edge of the species distribution, even if not necessarily related to low genetic variability (Lira-Noriega and Manthey 2013), probably have a high chance of extinction (Doherty et al. 2003). Reduced levels of genetic diversity, which is expected in declining species, is not necessarily observed (Lozier and Cameron 2009; Lozier et al. 2011) since diversity loss through genetic drift in small effective populations can be replenished by migrants (Cameron et al. 2011), unless a severe bottleneck there has occurred. Williams (1986, 1988), to explain the pattern of species loss in the United Kingdom, at least in part, argued that each bumblebee species occupies a particular climatic range. In the center of this range the species should be able to forage most profitably, and persist in a range of habitats including those that are not ideal. If the quality of a habitat declines, the populations near the edge of their climatic range are the first to become extinct. Bombus bellicosus is a good example. The species distribution ranges from Argentina to state of Paraná, Brazil, and was abundant until the early 1980s. In recent surveys conducted from 2002 to 2005, no specimens were recovered in state of Paraná, at sites they used to occur (Martins and Melo 2010).
Future refuge
The large future refuge, located in the east of the states of São Paulo and Rio de Janeiro, has been already suggested for 9 of 14 species analyzed, including spiders, harvestmen, scorpions, amphibians, birds, and mammals (Porto et al. 2013). Furthermore this area is also very important by the high level of species richness and endemism of different taxa including mammals (Costa et al. 2000), birds (da Silva et al. 2004), and harvestmen (Pinto-da-Rocha et al. 2005). This stable area was firstly identified through the intersection of three distribution models for each species under different climatic scenarios: current, 6000 and 21,000 years ago (Porto et al. 2013). In addition, we found two common areas composed by Atlantic Forest inside the large future refuge in the states of São Paulo and Rio de Janeiro, suitable for B. morio and the three clades of B. pauloensis together.
Although this large future refuge apparently seems to be protected, the Atlantic Forest is extremely degraded. Its natural reserves protect only 9% of the remaining forest and 1% of the original forest (Ribeiro et al. 2009). Unfortunately, most of these areas are small, the distance between fragments is large, and are near to large cities, as São Paulo. Therefore, there is no guarantee that these reserves will be sufficient to protect this important refuge, even knowing that bumblebees can survive in marginal and very disturbed habitats as agricultural fields (Lozier et al. 2011) or urbanized areas. This way, the guidelines for conservation suggested by Ribeiro et al. (2009) must be considered, which includes prioritizing conservation of large mature forest fragments; management of smaller fragments to maintain functionally linked mosaics; managing the matrix surrounding fragments to minimize edge effects and improve connectivity; and take restoration actions, particularly in certain key areas.
The importance of phylogeographic lineages in conservation
The uniqueness of a population (in terms of its allelic composition) and its diversity levels are criteria for the selection of priority populations to be conserved (Petit et al. 1998). Considering the criteria for recognition of management units (MU) as populations with a significant genetic diversity (Moritz 1994), we suggest that the three phylogeographic groups of B. pauloensis should be treated as different MUs. Therefore, significant well-preserved natural areas containing representative genetic diversity should be identified in each of the three groups to evaluate the current system of conservation units.
Preserving central populations in a species’ niche may protect populations with higher genetic diversity rather than those populations that are environmentally peripheral (Lira-Noriega and Manthey 2013). The protection of this large future refuge will be preserving not only the pattern of biodiversity but also the processes that generated and maintained it (Smith et al. 2001), and not only for B. morio and B. pauloensis, but also for many other species. Furthermore, we strongly recommend that population genetics and conservation studies should consider the center and edge of distribution and the diversity of species, since a population in decline or expansion can just be in the edge or center of the species distribution. Studies focusing on phylogeography, geographic distribution modeling, and the suitability values of distribution are useful tools to improve our understanding on process and population species dynamics.
References
Aldana J, Cure JR, Almanza MT, Vecil D, Rodríguez D (2007) Efecto de Bombus atratus (Hymenoptera: Apidae) sobre la productividad de tomate (Lycopersicon esculentum Mill.) bajo invernadero en la Sabana de Bogotá, Colombia. Agron Colomb 25:62–72
Ascher JS, Pickering J (2014) Discover life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). http://www.discoverlife.org/mp/20qguide=Apoidea_species. Accessed 21 Feb 2017
Batra WT (1995) Bees and pollination in our changing environment. Apidologie 26:361–370
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Bivand R, Lewin-Koh N (2013) Maptools: tools for reading and handling spatial objects. R package version 0, pp 8–27. http://CRAN.Rproject.org/package=maptools
Bivand R, Rundel C (2014) rgeos: interface to geometry engine—open source (GEOS). R package version 0.3-5. http://CRAN.R-project.org/package=rgeos
Bivand R, Keitt T, Rowlingson B (2013) rgdal: bindings for the geospatial data. R package version 0.8-13. http://CRAN.R-project.org/package=rgdaland
Buttermore RE (1997) Observations of successful Bombus terrestris (L.) (Hymenoptera: Apidae) colonies in southern Tasmania. Aust J Entomol 36:251–254
Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL (2011) Patterns of widespread decline in North American bumble bees. Proc Natl Acad Sci USA 108:662–667
Cane JH, Tepedino VJ (2001) Causes and extent of declines among native North American invertebrate pollinators: detection, evidence, and consequences. Conserv Ecol 5:1
Carvell C (2002) Habitat use and conservation of bumblebees (Bombus spp.) under different grassland management regimes. Biol Conserv 103:33–49
Colla SR, Packer L (2008) Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidea), with special focus on Bombus affinis Cresson. Biodivers Conserv 17:1379–1391
Corbet SA, Williams IH, Osborne JL (1991) Bees and the pollination of crops and wildflowers in the European community. Bee World 72:47–49
Costa LP, Leite YLR, da Fonseca GAB, da Fonseca MT (2000) Biogeography of South American forest mammals: endemism and diversity in the Atlantic forest. Biotropica 32:872–881
D’Amen M, Zimmermann NE, Pearman PB (2013) Conservation of phylogeographic lineages under climate change. Global Ecol Biogeogr 22:93–104
D’Avila M, Marchini LC (2005) Polinização realizada por abelhas em culturas de importância econômica do Brasil. Bol Ind Anim 62:79–90
da Silva JMC, de Sousa MC, Castelletti CHM (2004) Areas of endemism for passerine birds in the Atlantic forest, South America. Glob Ecol Biogeogr 13:85–92
da Silveira TMT, Raseira MCB, Nava DE, Couto M (2011) Blueberry pollination in southern Brazil and their influence on fruit quality. Rev Bras Frutic 33:81–88
De la Rúa P, Jaffé R, Dall’Olio R, Muñoz I, Serrano J (2009) Biodiversity, conservation and current threats to European honeybees. Apidologie 40:263–284
Doherty PF, Boulinier T, Nichols JD (2003) Local extinction and turnover rates at the edge and interior of species’ ranges. Ann Bot Fenn 40:145–153
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, McC. Overton J, Peterson AT, Phillips SJ, Richardson K, Scachetti-Pereira R, Schapire RE, Soberón J, William S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Ellis JS, Knight ME, Darvill B, Goulson D (2006) Extremely low effective population sizes, genetic structuring and reduced genetic diversity in a threatened bumblebee species, Bombus sylvarum (Hymenoptera: Apidae). Mol Ecol 15:4375–4386
Fitzpatrick U, Murray MG, Paxton RJ, Breen J, Cotton D, Santorum V, Brown MJF (2007) Rarity and decline in bumblebees—a test of causes and correlates in the Irish fauna. Biol Conserv 136:185–194
Fontaine C, Dajoz I, Meriguet J, Loreau M (2006) Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol 4:e1
Francisco FO, Santiago LR, Mizusawa YM, Oldroyd BP, Arias MC (2016) Genetic structure of island and mainland population of a neotropical bumble bee species. J Insect Conserv 20:383–394
Françoso E, Zuntini AR, Carnaval AC, Arias MC (2016a) Comparative phyleogeography in the Atlantic forest and Brazilian savannas: pleistocene fluctuations and dispersal shape the spatial patterns in two bumblebees. BMC Evol Biol 6:267
Françoso E, Oliveira FF, Arias MC (2016b) An integrative approach identifies a new species of bumblebee (Hymenoptera: Apidae: Bombini) from northeastern Brazil. Apidologie 47:171–185
Giannini TC, Acosta AL, Garófalo CA, Saraiva AM, Alves-dos-Santos I, Imperatriz-Fonseca VL (2012) Pollination services at risk: bee habitats will decrease owing to climate change in Brazil. Ecol Model 244:127–131
Giannini TC, Boff S, Cordeiro GD, Cartolano EA Jr, Veiga AK, Imperatriz-Fonseca VL, Saraiva AM (2015) Crop pollinators in Brazil: a review of reported interactions. Apidologie 46:209–223
Goulson D (2006) The demise of the bumblebee in Britain. Biologist 53:294–299
Goulson D (2010) Bumblebees: behavior, ecology, and conservation, 2nd edn. Oxford University Press, Oxford
Goulson D, Lye GC, Darvil B (2008) Decline and conservation of bumble bees. Annu Rev Entomol 53:191–208
Grixti JC, Wong LT, Cameron SA, Favret C (2009) Decline of bumble bees (Bombus) in the North American Midwest. Biol Conserv 142:75–84
Hannah L, Midgley GF, Lovejoy T, Bonds WJ, Bush M, Lovett JC, Scott D, Woodward FI (2002) Conservation of biodiversity in a changing climate. Conserv Biol 16:264–268
Hijmans RJ (2014a) Raster: geographic data analysis and modeling. R package version 2.2-12. http://CRAN.R-project.org/package=raster
Hijmans RJ (2014b) Geosphere: spherical trigonometry. R package version 1.3-11. http://CRAN.R-project.org/package=geosphere
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Kerr JT, Pindar A, Galpern P, Packer L, Potts SG, Roberts SM, Rasmont P, Schweiger O, Colla SR, Richardson LL, Wagner DL, Gall LF, Sikes DS, Pantoja A (2015) Climate change impacts on bumblebees converge across continents. Science 349:177–180
Kevan PG (1991) Pollination: keystone process in sustainable global productivity. Acta Hort 288:103–110
Kosior A, Celary W, Olejniczak P, Fijat J, Król W, Solarz W, Plonka P (2007) The decline of the bumble bees and cockoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 41:79–88
Kramer K, Degen B, Buschbom J, Hickler T, Thuiller W, Sykes M, Winter W (2010) Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change-range, abundance, genetic diversity and adaptive response. Forest Ecol Manag 259:2213–2222
Kraus FB, Wolf S, Moritz RF (2009) Male flight distance and population substructure in the bumblebee Bombus terrestris. J Anim Ecol 78:247–252
Kremen C, Cameron A, Moilanen A, Phillips SJ, Thomas CD, Beentje H, Dransfield J, Fisher BL, Glaw F, Good TC, Harper GJ, Hijmans RJ, Lees DC, Louis E Jr, Nussbaum RA, Raxworthy CJ, Razafimpahanana A, Schatz GE, Vences M, Vieites DR, Wright PC, Zjhra ML (2008) Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 320:222–226
Lepais O, Darvill B, O`Connor S, Osborne JL, Sanderson RA, Cussans J, Goffe L, Goulson D (2010) Estimation of bumblebee queen dispersal distances using sibship reconstruction method. Mol Ecol 19:819–831
Lira-Noriega A, Manthey JD (2013) Relationship of genetic diversity and niche centrality: a survey and analysis. Evolution 68:1082–1093
Loiselle BA, Graham CH, Goerck JM, Ribeiro MC (2010) Assessing the impact of deforestation and climate change on the range size and environmental niche of bird species in the Atlantic forests, Brazil. J Biogeogr 37:1288–1301
Lozier JD, Cameron SA (2009) Comparative genetic analyses of historical and contemporary collections highlight contrasting demographic histories for the bumble bees Bombus pensylvanicus and B. impatiens in Illinois. Mol Ecol 18:1875–1886
Lozier JD, Strange JP, Stewart IJ, Cameron SA (2011) Patterns of range-wide genetic variation in six North American bumble bee (Apidae: Bombus) species. Mol Ecol 20:4870–4888
Macfarlane RP (1995) Distribution of bumble bees in New Zealand. N Z Entomol 8:29–36
Maiorano L, Falcucci A, Zimmermann NE, Psomas A, Pottier J, Baisero D, Rondinini C, Guisan A, Boitani L (2011) The future of terrestrial mammals in the Mediterranean basin under climate change. Philos Trans R Soc B 366:2681–2692
Martins AC, Melo GAR (2010) Has the bumblebee Bombus bellicosus gone extinct in the northern portion of its distribution range in Brazil? J Insect Conserv 14:207–210
Martins AC, Silva DP, De Marco Jr P, Melo GAR (2015) Species conservation under future climate change: the case of Bombus bellicosus, a potentially threatened South American bumble bee species. J Insect Conserv 19:33–43
Maués MM (2002) Reproductive phenology and pollination of the Brazil nut tree (Bertholletia excelsa Humb. and Bonpl. Lecythidaceae) in estern Amazonia. In: Kevan P, Imperatriz Fonseca VL (eds) Pollinating bees—the conservation link between agriculture and nature. Ministry of Environment, Brasília, pp 245–254
McFrederick Q, LeBuhn G (2006) Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol Conserv 129:372–382
Memmott J, Waser NM, Price MV (2004) Tolerance of pollination networks to species extinctions. Proc R Soc Lond B 271:2605–2611
Montemor KA, Souza DTM (2009) Biodiversidade de polinizadores e biologia floral em cultura de berinjela (Solanum melongena). Zootec Trop 27:97–103
Morales CL, Arbetman MP, Cameron SA, Aizen MA (2013) Rapid ecological replacement of a native bumble bee by invasive species. Front Ecol Environ 11:529–534
Moritz C (1994) Defining ‘evolutionary significant units’ for conservation. Trends Ecol Evol 9:373–375
Moure JS, Sakagami SF (1962) As mamangabas sociais do Brasil (Bombus Latreille) (Hymenoptera, Apoidea). Stud Entomol 5:65–194
Olesen JM, Bascompte J, Dupont YL, Jordano P (2007) The modularity of pollination networks. Proc Natl Acad Sci USA 104:19891–19896
Pauw A (2007) Collapse of a pollination web in small conservation areas. Ecology 88:1759–1769
Pearce JL, Ferrier S (2000) An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol Model 128:127–147
Pearman PB, D’Amen M, Graham CH, Thuiller W, Zimmermann N (2010) Within-taxon niche structure: niche conservatism, divergence and predicted effects of climate change. Ecography 33:990–1003
Petit RJ, Mousadik AE, Pons O (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol 12:844–855
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Pinto-da-Rocha R, da Silva MB, Bragagnolo C (2005) Faunistic similarity and historic biogeography of the harvestmen of southern and southeastern Atlantic Rain Forest of Brazil. J Arachnol 33:290–299
Porto TJ, Carnaval AC, da Rocha PLB (2013) Evaluating refugial models using species distribution models, model filling and inclusion: a case study with 14 Brazilian species. Divers Distrib 19:330–340
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ (2016) Safeguarding pollinators and their values to human well-being. Nature 40:220–229
Pywell RF, Warman EA, Hulmes L, Hulmes S, Nuttall P, Sparks TH, Critchley CNR, Sherwood A (2006) Effectiveness of new agri-environment schemes in providing foraging resources for bumble bees in intensively farmed landscapes. Biol Conserv 129:192–206
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. http://www.R-project.org/
Ribeiro MC, Metzger JP, Martensen AC, Ponzono FG, Hirota MM (2009) The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ridley M (2004) Evolution, 3rd edn. Blackwell Publishing, Turin
Santos JE, Santos FR, Silveira FA (2015) Hitting an unintended target: Phylogeography of Bombus brasiliensis Lepeletier, 1836 and the first new Braziliam bumblebee species in a century (Hymenoptera: Apidae). PLoS ONE 10:e0125847
Saupe EE, Papes M, Selden PA, Vetter RS (2011) Tracking a medically important spider: climate change, ecological niche modeling, and the brown recluse (Loxosceles reclusa). PLoS ONE 6:e17731
Smith TB, Kark S, Schneider CJ, Wayne RK, Moritz C (2001) Biodiversity hotspots and beyond: the need for preserving environmental transitions. Trends Ecol Evol 16:431
Taubmann J, Theissinger K, Feldheim KA, Laube I, Graf W, Haase P, Johannesen J, Pauls SU (2011) Modelling range shifts and assessing genetic diversity distribution of the montane aquatic mayfly Ameletus inopinatus in Europe under climate change scenarios. Conserv Genet 12:503–515
Thuiller W, Lavergne S, Roquet C, Boulangeat I, Lafourcade B, Araujo MB (2011) Consequences of climate change on the tree of life in Europe. Nature 470:531–534
Vamosi JC, Knight TM, Steets JA, Mazer SJ, Burd M, Ashman TL (2006) Pollination decays in biodiversity hotspots. Proc Natl Acad Sci USA 103:956–961
Velthuis HHW, van Doorn A (2006) A century of advances in bumblebee domestication and the economic and environmental aspects of commercialization for pollination. Apidologie 37:421–451
Watt MS, Stone JK, Hood IA, Manning LK (2011) Using a climatic niche model to predict the direct and indirect impacts of climate change on the distribution of Douglas-fir in New Zealand. Glob Change Biol 17:3608–3619
Williams PH (1982) The distribution and decline of British bumble bees (Bombus Latr.). J Apic Res 21:236–245
Williams PH (1986) Environmental change and the distribution of British bumble bees (Bombus Latr.). Bee World 67:50–61
Williams PH (1988) Habitat use by bumble bees (Bombus spp.). Ecol Entomol 13:223–237
Williams PH, Osborne JL (2009) Bumblebee vulnerability and conservation world-wide. Apidologie 40:367–387
Williams PH, Colla S, Xie Z-H (2009) Bumblebee vulnerability: common correlates of winners and losers across three continents. Conserv Biol 23:931–940
Yamamoto M, da Silva CI, Augusto SC, Barbosa AAA, Oliveira PE (2012) The role of bee diversity in pollination and fruit set of yellow passion fruit (Passiflora edulis forma flavicarpa, Passifloraceae) crop in Central Brazil. Apidologie 43:515–526
Zayed A (2009) Bee genetics and conservation. Apidologie 40:237–262
Acknowledgements
We would like to thank J. Richard Abbott for English review of the earlier version of the manuscript, the Missouri Botanical Garden (Saint Louis, MO, USA) where this manuscript was written, Flavio de Oliveira Francisco for comments, and Susy Coelho for the laboratory maintenance. Financial support was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (Proc. 10/50597-5 and 13/12530-4; Ph.D. and scholarship to EF 2009/07124-1, 2010/20548-2 and 2013/03961-1) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (research fellowship to MCA).
Author information
Authors and Affiliations
Contributions
EF, did the research and wrote the manuscript; ARZ, assistance in data analysis, R scripts and advice; and MCA, advice, guidance and support in preparing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Françoso, E., Zuntini, A.R. & Arias, M.C. Combining phylogeography and future climate change for conservation of Bombus morio and B. pauloensis (Hymenoptera: Apidae). J Insect Conserv 23, 63–73 (2019). https://doi.org/10.1007/s10841-018-0114-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-018-0114-4