Abstract
In myrmecophilous insects, interactions with ants are often a key factor determining persistence of their populations. Regional variation in host ant use is therefore an essential aspect to consider to provide adequate conservation practices for such species. In this study, we examined this important facet of species’ ecology in an endangered myrmecophilous butterfly Phengaris (=Maculinea) alcon (Lepidoptera, Lycaenidae). The investigations conducted in peripheral populations in Estonia allowed us to expand the knowledge of its host ant use to the northern distribution limit of the species. Our data indicate that in its northernmost populations, the xerophilous ecotype of Phengaris alcon is primarily parasitizing a single host ant species, Myrmica schencki. The data collected are in line with the emerging evidence suggesting that peripheral and core populations of P. alcon use different host ants, and peripheral populations tend to display higher host ant specificity. We also show that, at its northern range margin, P. alcon might be more limited by the availability of its sole larval food plant in the region, Gentiana cruciata, than the densities of its host ant. Finally, we found a strong negative correlation between Myrmica spp. and Lasius spp. colony densities, suggesting that interspecific competition between ants could have a substantial influence on host ant availability of Phengaris butterflies, and thus should be taken into account in conservation plans of these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Associations with ants (myrmecophily) have been documented in numerous arthropod taxa (Hölldobler and Wilson 1990; McIver and Stonedahl 1993). In butterflies, ant associations are almost exclusively restricted to the representatives of the family Lycaenidae (Fiedler 1991; Pierce et al. 2002). The degree of larval myrmecophily in this family ranges from loose facultative interactions in which larvae are only occasionally tended by ants to complex obligate associations in which ant attendance is crucial for the butterflies’ survival (Ballmer and Pratt 1992; Fiedler 1991, 2006; Pierce et al. 2002). Consistently, in the latter species, interactions with ants have often been considered a key factor for successful conservation of their populations (Elmes et al. 1998; Als et al. 2004; Thomas et al. 2009).
Among the myrmecophilous lycaenids, the Palearctic genus Phengaris Doherty, 1891 (senior synonym of Maculinea Van Eecke, 1915; Fric et al. 2007) particularly stands out by their intricate interactions with ants. The first three larval instars of these butterflies develop on flowers and seeds of their host plants, whereas they complete their development as obligate social parasites in the nests of Myrmica Latreille, 1804 ants (Thomas et al. 1989). Based on how they exploit their hosts, the species of this butterfly genus can be divided into two groups. In particular, the caterpillars of so called cuckoo-feeders (e.g. P. alcon) are mostly fed directly by the worker ants with their regurgitations, trophic eggs and dead prey, while predatory species (P. arion, Phengaris nausithous and Phengaris teleius) feed only on ant brood (Thomas and Wardlaw 1992; Thomas and Elmes 1998).
Although Phengaris larvae are adopted by workers of any Myrmica species they encounter (Elmes et al. 1991; Thomas 2002; Schönrogge et al. 2004), their survival inside the nests of different ant species differs to the extent that each Phengaris species is considered to have only one or a few suitable Myrmica hosts (Thomas et al. 1989; Elmes et al. 1991, 2004; Akino et al. 1999, but see; Pech et al. 2007). Nevertheless, recent studies have shown remarkable local and regional variation in intraspecific host ant use in European Phengaris butterflies (Als et al. 2001; Stankiewicz et al. 2004; Tartally et al. 2014). Regional variation in host ant use is therefore an important aspect to consider in order to effectively manage the populations of these endangered butterflies (e.g. Tartally et al. 2008; Sielezniew et al. 2010a, b).
The alcon blue (P. alcon Denis and Schiffermüller 1775), one of the most endangered butterfly species in Europe (e.g. Munguira and Martin 1999), shows high variability in host ant use throughout its range. In earlier studies on host specificity of P. alcon, its hygrophilous (P. alcon s. str.; P. alcon H hereafter; see Pech et al. 2004; Bereczki et al. 2005, 2006; Steiner et al. 2006) and xerophilous ecotype (P. rebeli auct. Nec Hirschke; P.alcon X hereafter; see Als et al. 2004; Pecsenye et al. 2007; Tartally et al. 2014) have been shown to specialize on Myrmica ruginodis and M. schencki, respectively (Thomas et al. 1989). More recent studies, however, have changed this view showing that both ecotypes can successfully exploit quite a number of different Myrmica species (Fig. 1a, b). Nevertheless, in different parts of the range the butterfly still displays much narrower host specialization, involving specific physiological adaptations to different hosts (Thomas et al. 2013). Populations close to range margins are of particular interest, as they are often genetically and ecologically divergent from central populations, and may therefore be valuable for sustaining the evolutionary potential of the species (Lesica and Allendorf 1995; Hill et al. 2011; Therry et al. 2014). Higher host specificity towards species distribution margins is one example of how peripheral populations are proposed to differ from core populations (e.g. Martin and Pullins 2004; Schmidt and Hughes 2006), but additional data, especially from peripheral populations are required before such conclusions can be generalized to P. alcon (Tartally et al. 2008).
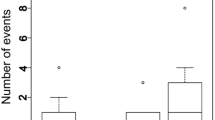
Host ants of Phengaris alcon. a Xerophilous ecotype, b hygrophilous ecotype in Europe [data sources: Austria (Steiner et al. 2003 and; Tartally et al. 2014); Belgium (van Dyck et al. 2000); Denmark (Als et al. 2001); Estonia (this study); France (Elmes et al. 1994; Stoeckel and Mercier 2001); Germany (Meyer-Hozak 2000; Küer and Fartmann 2005); Hungary (Tartally et al. 2008); Italy (Witek et al. 2013); Lithuania (Stankiewicz et al. 2004); Netherlands (Thomas et al. 1989; Radchuk et al. 2012); Poland (Steiner et al. 2003; Sielezniew and Stankiewicz 2004, 2007; Sielezniew et al. 2010b; Thomas et al. 2013); Portugal (Arnaldo et al. 2011); Romania (Tartally et al. 2008); Spain (Elmes et al. 1994; Thomas et al. 2013); Sweden (Elmes et al. 1994); Switzerland (Jutzeler 1989); Ukraine (Witek et al. 2008)]
In this study, we expand the knowledge of host ant use of the xerophilous ecotype of P. alcon to the northern distribution limit of the species. The investigated peripheral populations, recently discovered (in 2012) in the northern part of Estonia, Northern Europe, are by far the northernmost P. alcon X populations known. Moreover, Gentiana cruciata L., the sole larval host plant of P. alcon X in the region also reaches its northern range limit in Estonia (Kukk and Kull 2005; Backbone Taxonomy 2016). Given the poor dispersal ability of the food plant, a rapid northward range expansion, recently shown for numerous other European butterflies (Devictor et al. 2012), is unlikely in the case of P. alcon X. Hence, the study area can be considered as the true northern distribution limit of this ecotype. As a novel aspect, we also examine the potential effect of non-host ant species (Lasius spp. in particular) sharing the habitat with Myrmica on host ant availability in P. alcon X.
Materials and methods
Study species
The butterfly inhabits nutrient-poor xerothermic and calcareous grasslands where females lay eggs on flowerheads and leaves of G. cruciata (Munguira and Martin 1999). Young caterpillars develop quickly through three instars feeding on green seeds or flowers of the host plant. After the third (final) moult they descend to the ground and wait for worker ants of Myrmica spp. to adopt them into their underground nests (Elmes et al. 1991). In the nests, the caterpillars live as social parasites by tricking the nurse ant workers (using chemical and acoustic mimicry) to feed them with regurgitations and prey (cuckoo-type feeders; Thomas and Elmes 1998). Here the caterpillars acquire ca 98 % of their final biomass (Thomas and Elmes 1998) before emerging after 10 or 22 months (in the case of biennial development; Schönrogge et al. 2000). The pupal stage is also spent in the ant nest. In Estonia, P. alcon X adults are on the wing in July and August.
Fieldwork
Host ant use by P. alcon X was studied in two habitat patches, about 6.5 km apart, in Northern Estonia (Fig. 1a; exact localities are not presented because of the vulnerable status of the species in the study region), in one of the two locations in Estonia where the species is known to occur. The landscape of the area is dominated by arable land and forests in which calcareous grasslands, potentially suitable for P. alcon X, form a mosaic of small discontinuous patches. Neither of the grasslands (13.2 and 0.3 ha) in which fieldwork was conducted are actively managed and are therefore characterized by relatively high average turf height (ca 15 cm). As the data derived from the smaller grassland were insufficient for separate statistical analyses, the data from both grasslands were pooled.
Fieldwork to obtain data on host ant specificity was conducted in the middle of May 2014, well before the flight period of the butterfly. For sampling, an a priori selected set of coordinates was determined, and host plants growing closest to these preselected coordinates were chosen. Myrmica colonies around these host plants were examined for butterfly larvae and pupae in square plots of 2 × 2 m. The size of the sample plots was chosen to match the approximate foraging range of Myrmica workers (Elmes et al. 1998). Ant colonies were localized by partial removal of the vegetation. All colonies were carefully opened and examined for the presence of P. alcon X juveniles. If the presence of the butterfly was established in upper chambers of nests, no further disturbance was undertaken. To facilitate survival of ant colonies, the ground and vegetation were restored as close as possible to pre-excavation conditions after inspection. Negative effects of this methodology on the ant colonies and populations are considered to be minor (Sielezniew et al. 2010a). As ground-dwelling ant communities are strongly structured by interspecific competition (e.g. Savolainen and Vepsäläinen 1988; Hölldobler and Wilson 1990), we also searched and counted Lasius spp. nests in all sample plots. For each Myrmica nest, we measured its distance to the nearest host plant and estimated the colony size using the methodology described by Skorka et al. (2006). Samples of 5–10 workers from each examined ant colony were preserved in 75 % alcohol for further identification. All sampled ants were identified to the species level using the key of Radchenko and Elmes (2010). Examining ant colonies in well-defined plots allowed us to evaluate their density in the study area.
Data analyses
To test for host ant specificity, a contingency table (with each Myrmica species treated separately) was used to calculate the Chi squared statistic, the significance of which was tested by Monte Carlo simulation procedure. In particular, each colony was randomly reassigned to one of the Myrmica species observed (with the constraint that the total number of colonies of each species was the same as observed), and each time the value of the Chi squared statistic was calculated. The simulation was repeated 100,000 times. The presence-absence data of P. alcon X larvae in host ant colonies were further analysed using logistic regression with Firth’s correction for separation (Firth 1993). Colony distance from the host plant and colony size entered the model as continuous independent variables. Due to the relatively small sample size and limited sampling scale, statistical testing of the presence of spatial autocorrelation in the model residuals was not attempted. Instead, best models were checked visually for spatial autocorrelation (both Pearson and deviance residuals). As there was no obvious spatial pattern in the residuals of any of the best models, we used non-spatial models. All the analyses were performed in R version 3.1.1 (R Development Core Team 2014), logistic regression was conducted using the logistf (Heinze et al. 2013) package.
Results
In 29 plots (116 m2) sampled, we found and examined altogether 56 Myrmica colonies belonging to five species (Table 1). M. schencki and M. sabuleti were the most abundant Myrmica species, constituting almost one-third and one-fourth, respectively, of all colonies inspected (Table 1). We documented a total of 17 P. alcon X individuals (11 larvae and 6 pupae), all from four colonies of M. schencki (Table 1; Fig. 2). All infested colonies were found in different plots. A significant p value (p = 0.044) derived from Monte Carlo 2 × 5 contingency table test suggests that host ant use of P. alcon was not random. Besides the host ant records determined in this study, two additional documentations of host ant use from the same population, recorded in an unsystematic manner, are also from M. schencki colonies. The caterpillars of P. alcon X were more likely to parasitize those M. schencki colonies that were closer to the butterfly’s host plants (logistic regression: X 2 = 7.05; p = 0.008; Table 2). In fact, all infested colonies were within a one meter radius from host plants (Table 2). The average number of worker ants in colonies infested with P. alcon X (all M. schencki) was more than two times higher than in uninfested M. schencki colonies (Table 2). However, due to a small sample size and relatively high variation in worker numbers, this difference between infested and uninfested colonies remained marginally non-significant (logistic regression; X 2 = 1.67; p = 0.096; Table 2). The overall density of M. schencki colonies across the study area was estimated to be about 1,550 colonies/ha.
Besides Myrmica colonies, the study sites harboured very high densities of Lasius spp. colonies, potential competitors of Myrmica ants. An average of four Lasius colonies was recorded in each 2 × 2 m sampling plot, corresponding to an estimate of 10,000 colonies per ha. There was a strong and highly significant negative correlation between colony densities of Myrmica spp. and Lasius spp. across sample plots (Spearman rank correlation: r s = −0.76, N = 29, p < 0.0001). Correlations remained consistently negative when Myrmica species were analysed separately; of these, statistical significance was attained in case of M. schencki (Spearman rank correlation: r s = −0.56, N = 29, p = 0.002), the local host ant of P. alcon X. No correlation (r s < 0.1) was found between colony densities of Lasius ants and colony sizes of Myrmica ants within sampling plots.
Discussion
Our data indicate that, at its northern distribution margin, the xerophilous ecotype of P. alcon is primarily parasitizing a single host ant species. In particular, all P. alcon X caterpillars and pupae (altogether 17 individuals recorded in this study plus two additional documentations) were found exclusively in the colonies of Myrmica schencki (Table 1). Four other Myrmica species, three of which (M. sabuleti, M. scabrinodis and M. rugulosa) have been previously documented as host ants of P. alcon X elsewhere (see Fig. 1, for a summary of P. alcon host ants in Europe), were also relatively abundant in the study area, but none of their nests were infested.
Recent studies have shown that P. alcon X occurs in two ecologically and physiologically different forms in Europe, one of them exploiting M. schencki and the other M. sabuleti as the primary host ant (Thomas et al. 2013). While the latter form occurs mainly in Central Europe, the form parazitising M. schencki tends to dominate in more peripheral areas of the butterfly’s European range (see Fig. 1 in Thomas et al. 2013). Although based on a relatively small number of host records, our results of host ant use at the species’ northern range limit are in line with this pattern. Moreover, we did not find any secondary host ants in our P. alcon X population, which is consistent with the idea of higher host specificity near species’ range margins (Martin and Pullins 2004; Schmidt and Hughes 2006). Interestingly, M. schencki has been considered one of the most xerothermophilous Myrmica species in Europe (Elmes et al. 1998) and has been associated with bare ground patches (Sielezniew et al. 2010b). However, our findings of relatively high densities of M. schencki colonies in rather dense vegetation highlight the need to review habitat requirements of this species in a broader geographical context.
Given the restricted foraging range of Myrmica ants, a spatial overlap of the two principal larval resources—host plant and host ant—is a necessary precondition for viability of Phengaris populations. Depending on the relative abundance, either of the two resources can be more limiting for population size and growth. Our data suggest that, Estonian populations of P. alcon X are primarily limited by the availability of host plants rather than host ants. Similarly to P. alcon, G. cruciata reaches its northern limit in Estonia (GBIF Backbone Taxonomy 2016), and its populations in suitable habitats are relatively sparse. The density of G. cruciata in the study area did not exceed 50 plants/ha which is far lower than the suggested optimum host plant density for P. alcon X populations (1,500 plants/ha, Clarke et al. 1998). A relatively high average egg load on individual plants (9.2 eggs per plant shoot: Vilbas et al., submitted manuscript) is also consistent with the limiting role that host plant density is likely to play.
Nevertheless, the high density of host ant colonies may to a certain extent compensate the scarcity of food plants by ensuring that a high proportion of host plants is within the foraging range of potential host ants. In particular, population viability models for predatory Phengaris have shown that densities of about 500 host ant colonies per ha are necessary for long-term population persistence of such species (e.g. Griebeler and Seitz 2002). The respective values for cuckoo-type feeders like P. alcon are evidently lower as the carrying capacity of Myrmica colonies for such species is usually higher (Thomas and Wardlaw 1992; Thomas and Elmes 1998). Consistently, similar studies conducted in different locations in Europe have reported host ant densities in Phengaris habitats to be often under 1,000 colonies/ha (Stankiewicz et al. 2004; Sielezniew et al. 2010a; Vilbas et al. 2015) and sometimes even as low as 300 colonies/ha (Sielezniew et al. 2010a). In our study area, the mean density of host ant colonies was considerably higher (1,550 colonies/ha, overall Myrmica density: 4,900 colonies/ha; Table 1). In this light, host ant densities per se are unlikely to threaten the persistence of P. alcon X populations at the northern range margin of the species.
A typical foraging zone of Myrmica workers has been shown to be approximately two metres (Elmes et al. 1998), which means that caterpillars feeding on food plants within this range are potential canditates for adoption. In the plots examined, however, all the 17 P. alcon X caterpillars and pupae were found within a much narrower range: indeed, all infested colonies were located within one metre distance from the butterfly’s food plants (Table 2). The confinement of infestations to the immediate proximity of the food plants is likely to be explained by the high host ant density in the study area. Workers of colonies closer to the butterfly’s food plant had a higher chance of encountering Phengaris larvae, and their colonies were thus more likely to become parasitized. As a methodological point, restricting the search area to one metre around the host plant could therefore notably increase the chance of finding Phengaris caterpillars and pupae in habitats with high densities of Myrmica colonies.
M. schencki colonies infested with P. alcon X were, on average, more than two times larger than uninfested colonies (the difference remained marginally non-significant though, probably because of a small sample size; Table 2). Given the detrimental effects of Phengaris larvae on ant colonies, the colony size difference at the time of infestation may have been even larger. Most likely, larger colonies become more frequently infested simply because they have higher numbers of foraging workers in the field (e.g. Herbers and Choiniere 1996; Palmer 2004). Moreover, larger colonies are less likely to abandon the nest site (Elmes et al. 1998) or collapse, either because of the infestation by Phengaris larvae or any other reasons (Thomas and Wardlaw 1992). Alternatively, larger colonies tend to be more polygynous (Elmes and Keller 1993; Sundström 1995), which may both reduce the aggressiveness of workers towards intruders (Fürst et al. 2012) and facilitate mimicking the cuticular chemistry of these colonies (Nash and Boomsma 2008).
Last but not least, we found a strong negative correlation between colony densities of Myrmica spp. and Lasius spp. The association remained significant when M. schencki, the local host ant of P. alcon X, was analysed separately. While other causal factors (e.g. differences in microhabitat preferences) behind this negative correlation cannot be excluded, interspecific competition which is known to be a major factor shaping spatial distribution patterns and species composition in ant communities (e.g. Savolainen and Vepsäläinen 1988; Hölldobler and Wilson 1990; Andersen and Patel 1994; Slipinski et al. 2014) probably plays a role here. In fact, it is somewhat surprising that studies addressing host ant availability in Phengaris butterflies have largely overlooked the possible interfering effect of competition between Myrmica ants and ants from other genera. In dry grasslands, like those inhabited by P. alcon X or P. arion, where butterflies’ host ants share the habitat with a number of abundant non-host ant species, there is a high potential for competitive interactions between ants to affect host ant availability for myrmecophilous butterflies. Myrmica species are some of the most subordinate in the ant competition hierarchy (e.g. Seifert 2007; Slipinski et al. 2014; Vepsäläinen and Czechowski 2014), and their abundance and distribution patterns are therefore, to a considerable extent, driven by other ant genera. Moreover, parasitism by Phengaris butterflies by itself dramatically reduces both the number and size of host ant colonies, giving other ant species an additional competitive advantage (Hochberg et al. 1994). Interspecific competition between ants could thus substantially influence the persistence of Phengaris populations. From the conservation point of view, habitat management practices adversely affecting Lasius spp. and other non-host ants may thus be beneficial for Phengaris butterflies.
References
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc B 266:1419–1426
Als TD, Nash DR, Boomsma JJ (2001) Adoption of parasitic Maculinea alcon caterpillars (Lepidoptera: Lycaenidae) by three Myrmica ant species. Anim Behav 62:99–106
Als TD, Vila R, Kandul NP, Nash DR, Yen SH, Hsu YF, Mignault AA, Boomsma JJ, Pierce NE (2004) The evolution of alternative parasitic life histories in large blue butterflies. Nature 432:386–390
Andersen AN, Patel AD (1994) Meat ants as dominant members of Australian ant communities: an experimental test of their influence on the foraging success and forager abundance of other species. Oecologia 98:15–24
Arnaldo PS, Wynhoff I, Soares P, da Conceição Rodrigues M, Aranha J, Csosz S, Maravalhas E, Tartally A (2011) Maculinea alcon exploits Myrmica aloba in Portugal: unusual host ant species of a myrmecophilous butterfly in a peripheral region. J Insect Conserv 15:465–467
Ballmer GR, Pratt GF (1992) Quantification of ant attendance (myrmecophily) of lycaenid larvae. J Res Lepid 30:95–112
Bereczki J, Pecsenye K, Peregovits L, Varga Z (2005) Pattern of genetic differentiation in the Maculinea alcon species group (Lepidoptera, Lycaenidae) in Central Europe. J Zool Syst Evol Res 43:157–165
Bereczki J, Pecsenye K, Varga Z (2006) Geographical versus food plant differentiation in populations of Maculinea alcon (Lepidoptera: Lycaenidae) in Northern Hungary. Eur J Entomol 103:725–732
Clarke RT, Thomas JA, Elmes GW, Wardlaw JC, Munguira ML, Hochberg ME (1998) Population modelling of the spatial interactions between Maculinea rebeli, their initial foodplant Gentiana cruciata and Myrmica ants within a site. J Insect Conserv 2:29–37
Devictor V, van Swaay C, Brereton T, Brotons L, Chamberlain D, Heliölä J, Herrando S, Julliard R, Kuussaari M, Lindström Å, Reif J, Roy DB, Schweiger O, Settele J, Stefanescu C, Van Strien A, Van Turnhout C, Vermouzek Z, WallisDeVries M, Wynhoff I, Jiguet F (2012) Differences in the climatic debts of birds and butterflies at a continental scale. Nat Clim Change 2:121–124
Elmes GW, Keller L (1993) Ecological determinants of queen number in ants. In: Keller L (ed) Queen number and sociality in insects. Oxford University Press, Oxford, pp 294–307
Elmes GW, Clarke RT, Wardlaw JC (1991) Larvae of Maculinea rebeli, a large blue butterfly, and their Myrmica host ants: patterns of caterpillar growth and survival. J Zool 224:447–460
Elmes GW, Thomas JA, Hammarstedt O, Munguira ML, Martin J, van der Made JG (1994) Differences in host-ant specificity between Spanish, Dutch and Swedish populations of the endangered butterfly Maculinea alcon (Dennis et Schiff.) (Lepidoptera). Memorabilia Zool 48:55–68
Elmes GW, Thomas JA, Wardlaw JC, Hochberg ME, Clarke RT, Simcox DJ (1998) The ecology of Myrmica ants in relation to the conservation of Maculinea butterflies. J Insect Conserv 2:67–78
Elmes GW, Wardlaw JC, Schönrogge K, Thomas JA, Clarke RT (2004) Food stress causes differential survival of socially parasitic caterpillars of Maculinea rebeli integrated in colonies of host and non-host Myrmica ant species. Entomol Exp Appl 110:53–63
Fiedler K (1991) European and North West African Lycaenidae (Lepidoptera) and their associations with ants. J Res Lepid 28:239–257
Fiedler K (2006) Ant-associates of Palearctic lycaenid butterfly larvae (Hymenoptera: Formicidae; Lepidoptera: Lycaenidae): a review. Myrmecol Nachr 9:77–87
Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80:27–38
Fric Z, Wahlberg N, Pech P, Zrzavỳ J (2007) Phylogeny and classification of the Phengaris–Maculinea clade (Lepidoptera: Lycaenidae): total evidence and phylogenetic species concepts. Syst Entomol 32:558–567
Fürst MA, Durey M, Nash DR (2012) Testing the adjustable threshold model for intruder recognition on Myrmica ants in the context of social parasite. Proc R Soc B 279:516–522
GBIF Backbone Taxonomy (2016). doi:10.15468/39omei. Accessed 20 Sept 2016
Griebeler EM, Seitz A (2002) An individual based model for the conservation of the endangered large blue butterfly, Maculinea arion (Lepidoptera: Lycaenidae). Ecol Model 156:43–60
Heinze G, Ploner M, Dunkler D, Southworth H (2013) Package ‘logistf’: Firth’s bias reduced logistic regression. R package version 1.21. http://CRAN.R-project.org/package=logistf. Accessed 18 Nov 2015
Herbers JM, Choiniere E (1996) Foraging behaviour and structure in ants. Anim Behav 51:141–153
Hill JK, Griffiths HM, Thomas CD (2011) Climate change and evolutionary adaptations at species’ range margins. Annu Rev Entomol 56:143–159
Hochberg ME, Clarke RT, Elmes GW, Thomas JA (1994) Population dynamic consequences of direct and indirect interactions involving a large blue butterfly and its plant and red ant hosts. J Anim Ecol 63:375–391
Hölldobler B, Wilson EO (1990) The ants. Harvard University press, Cambridge, MA, pp 732
Jutzeler D (1989) Maculinea rebeli (Hirschke): Ein Raupenfund im Glarnerland (Lepidoptera: Lycaenidae). Mitt Entom Gesellschaft Basel 39:92–93
Küer A, Fartmann T (2005) Prominent shoots are preferred: microhabitat preferences of Maculinea alcon ([Denis Schiffermüller], 1775) in Northern Germany (Lycaenidae). Nota Lepid 27:309–319
Kukk T, Kull T (2005) Eesti taimede levikuatlas. Atlas of the Estonian flora. EMÜ põllumajandus-ja keskkonnainstituut, Tartu
Lesica P, Allendorf FW (1995) When are peripheral populations valuable for conservation?. Conserv Biol 9:753–760
Martin LA, Pullins AS (2004) Host-plant specialisation and habitat restriction in an endangered insect, Lycaena dispar batavus (Lepidoptera: Lycaenidae) I. Larval feeding and oviposition preferences. Eur J Entomol 101:51–56
McIver JD, Stonedahl G (1993) Myrmecomorphy: morphological and behavioural mimicry of ants. Annu Rev Entomol 38:351–379
Meyer-Hozak C (2000) Population biology of Maculinea rebeli (Lepidoptera: Lycaenidae) on the chalk grasslands of Eastern Westphalia (Germany) and implications for conservation. J Insect Conserv 4:63–72
Munguira ML, Martin J (1999) Action plan for the Maculinea butterflies in Europe. Nature and Environment 97. Council of Europe Publishing, Strasbourg, pp 64
Nash DR, Boomsma JJ (2008) Communication between hosts and social parasites. In: d’Ettorre P, Hughes DP (eds) Sociobiology of communication: an interdisciplinary approach. Oxford University Press, Oxford, pp 55–79
Palmer TM (2004) Wars of attrition: colony size determines competitive outcomes in a guild of African acacia ants. Anim Behav 68:993–1004
Pech P, Fric Z, Konvička M, Zrzavỳ J (2004) Phylogeny of Maculinea blues (Lepidoptera: Lycaenidae) based on morphological and ecological characters: evolution of parasitic myrmecophily. Cladistics 20:362–375
Pech P, Fric Z, Konvička M (2007) Species-specificity of the Phengaris (Maculinea)–Myrmica host system: fact or myth? (Lepidoptera: Lycaenidae; Hymenoptera: Formicidae). Sociobiology 50:983–1003
Pecsenye K, Bereczki J, Tihanyi B, Tóth A, Pergovits L, Varga Z (2007) Genetic differentiation among the Maculinea species (Lepidoptera: Lycaenidae) in Eastern Central Europe. Biol J Linn Soc 91:11–21
Pierce NE, Braby MF, Heath A, Lohman DJ, Mathew J, Rand DB, Travassos MA (2002) The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu Rev Entomol 47:733–771
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Radchenko AG, Elmes GW (2010) Myrmica ants (Hymenoptera: Formicidae) of the old world. Natura Optima Dux, Warsaw, Poland
Radchuk V, WallisDeVries MF, Schtickzelle N (2012) Spatially and financially explicit population viability analysis of Maculinea alcon in the Netherlands. PLoS One 7:e38684
Savolainen R, Vepsäläinen K (1988) A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51:135–155
Schmidt DJ, Hughes JM (2006) Genetic affinities among subspecies of a widespread Australian lycaenid butterfly, Ogyris amaryllis (Hewitson). Aust J Zool 54:429–446
Schönrogge K, Wardlaw JC, Thomas JA, Elmes GW (2000) Polymorphic growth rates in myrmecophilous insects. Proc R Soc B 267:771–777
Schönrogge K, Wardlaw JC, Peters AJ, Everett S, Thomas JA, Elmes GW (2004) Changes in chemical signature and host specificity from larval retrieval to full social intregration in the myrmecophilous butterfly Maculinea rebeli. J Chem Ecol 30:91–107
Seifert B (2007) Die Ameisen Mittel- und Nordeuropas. Lutra Verlagsund Vertriebsgesellschaft, Tauer, Görlitz
Sielezniew M, Stankiewicz AM (2004) Simultaneous exploitation of Myrmica vandeli and M. scabrinodis (Hymenoptera: Formicidae) colonies by the endangered myrmecophilous butterfly Maculinea alcon (Lepidoptera: Lycaenidae). Eur J Entomol 101:693–696
Sielezniew M, Stankiewicz AM (2007) Differences in the development of the closely related myrmecophilous butterflies Maculinea alcon and M. rebeli (Lepidoptera: Lycaenidae). Eur J Entomol 104:433–444
Sielezniew M, Dziekańska I, Stankiewicz-Fiedurek AM (2010a) Multiple host-ant use by the predatory social parasite Phengaris (=Maculinea) arion (Lepidoptera, Lycaenidae). J Insect Conserv 14:141–149
Sielezniew M, Włostowski M, Dziekańska I (2010b) Myrmica schencki (Hymenoptera: Formicidae) as the primary host of Phengaris (Maculinea) arion (Lepidoptera: Lycaenidae) at heathlands in Eastern Poland. Sociobiology 55:95–106
Skorka P, Witek, M, Woyciechowski M (2006) A simple and nondestructive method for estimation of worker population size in Myrmica ant nests. Insectes Soc 53:97–100
Slipinski P, Marko B, Rzeszowski K, Babik H, Czechowski W (2014) Lasius fuliginosus (Hymenoptera: Formicidae) shapes local ant assemblages. N W J Zool 10:404–412
Stankiewicz AM, Sielezniew M, Švitra G (2004) Myrmica schencki (Hymenoptera: Formicidae) rears Maculinea rebeli (Lepidoptera: Lycaenidae) in Lithuania: new evidence for geographical variation of host-ant specificity of an endangered butterfly. Myrmecol Nachr 7:51–54
Steiner FM, Sielezniew M, Schlick-Steiner BC, Höttinger A, Stankiewicz H, Górnicki A (2003) Host specificity revisited: new data on Myrmica host ants of the lycaenid butterfly Maculinea rebeli. J Insect Conserv 7:1–6
Steiner FM, Schlick-Steiner BC, Höttinger H, Nikiforov A, Moder A, Christian E (2006) Maculinea alcon and M. rebeli (Insecta: Lepidoptera: Lycaenidae): one or two alcon blues? Larval cuticular compounds and egg morphology of East Austrian populations. Ann Nathist Mus Wien 107B:165–180
Stoeckel S, Mercier JL (2001) Maculinea alcon (Lepidoptera, Lycaenidae) en Brenne: analyse des relations entre la plante hôte Gentiana pneumonanthe et la fourmi hôte Myrmica scabrinodis (Hymenoptera, Formicidae). Symbioses 4:11–17
Sundström L (1995) Sex allocation and colony maintenance in monogyne and polygyne colonies of Formica truncorum (Hymenoptera: Formicidae): the impact of kinship and mating structure. Am Nat 146:182–201
Tartally A, Nash DR, Lengyel S, Varga Z (2008) Patterns of host ant use by sympatric populations of Maculinea alcon and M.‘rebeli’ in the Carpathian basin. Insectes Soc 55:370–381
Tartally A, Koschuh A, Varga Z (2014) The re-discovered Maculinea rebeli (Hirschke, 1904): host ant usage, parasitoid and initial food plant around the type locality with taxonomical aspects (Lepidoptera, Lycaenidae). ZooKeys 406:25–40
Therry L, Nilsson-Örtman V, Bonte D, Stoks R (2014) Rapid evolution of larval life history, adult immune function and flight muscles in a poleward-moving damselfly. J Evol Biol 27:141–152
Thomas JA (2002) Larval niche selection and evening exposure enhance adoption of a predacious social parasite, Maculinea arion (large blue butterfly), by Myrmica ants. Oecologia 132:531–537
Thomas JA, Elmes GW (1998) Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies trough trophallaxis rather than by predation. Ecol Entomol 23:457–464
Thomas JA, Wardlaw JC (1992) The capacity of a Myrmica ant nest to support a predacious species of Maculinea butterfly. Oecologia 91:101–109
Thomas JA, Elmes GW, Wardlaw JC, Woyciechowski M (1989) Host specificity among Maculinea butterflies in Myrmica ant nests. Oecologia 79:452–457
Thomas JA, Simcox DJ, Clarke RT (2009) Successful conservation of a threatened Maculinea butterfly. Science 325:80–83
Thomas JA, Elmes GW, Sielzeniew M, Stankiewicz-Fiedurek A, Simcox DJ, Settele J, Schönrogge K (2013) Mimetic host shifts in an endangered social parasite of ants. Proc R Soc B 280:20122336
Van Dyck H, Oostermeijer JG, Talloen W, Feenstra V, van der Hidde A, Wynhoff I (2000) Does the presence of ant nests matter for oviposition to a specialized myrmecophilous Maculinea butterfly?. Proc R Soc B 267:861–866
Vepsäläinen K, Czechowski W (2014) Against the odds of the ant competition hierarchy: submissive Myrmica rugulosa block access of the dominant Lasius fuliginosus to its aphids. Insectes Soc 61:89–93
Vilbas M, Teder T, Tiitsaar A, Kaasik A, Esperk T (2015) Habitat use of the endangered parasitic butterfly Phengaris arion close to its northern distribution limit. Insect Conserv Divers 8:252–260
Witek M, Śliwińska EB, Skórka P, Nowicki P, Wantuch M, Vrabec V, Settele J, Woyciechowski M (2008) Host ant specificity of large blue butterflies Phengaris (Maculinea) (Lepidoptera: Lycaenidae) inhabiting humid grasslands in East-Central Europe. Eur J Entomol 105:871–877
Witek M, Casacci LP, Barbero F, Patricelli D, Sala M, Bossi S, Maffei M, Woyciechowski M, Balletto E, Bonelli S (2013) Interspecific relationships in co-occurring populations of social parasites and their host ants. Biol J Linn Soc 109:699–709
Acknowledgments
We are grateful to Kertu Jaik for assistance in the field, and Ants Kaasik for statistical advice. This study was supported by the institutional research funding IUT20-33 of the Estonian Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilbas, M., Esperk, T. & Teder, T. Host ant use of the Alcon blue butterfly at the northern range margin. J Insect Conserv 20, 879–886 (2016). https://doi.org/10.1007/s10841-016-9921-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-016-9921-7