Abstract
Many ant assemblages are organised in competitive hierarchies. Myrmica rugulosa, a submissive species at the bottom of the ant competition hierarchy, temporarily took over groups of the myrmecophilic aphid Stomaphis quercus, which is strictly associated with the territorial ant species Lasius fuliginosus. Such previously unknown intervention happened repeatedly on the nest tree of L. fuliginosus. Masses of M. rugulosa intruded into bark crevices harbouring the aphids, and blocked with their own bodies access of L. fuliginosus to the aphids. Lasius fuliginosus showed no aggression towards the intruders, but walked around and on M. rugulosa clusters, palpating foreign ants with their antennae. Their only countermeasure was to briefly drag or carry individual M. rugulosa workers that pressed themselves down and froze for the moment, and then resumed their activities. We interpret the behaviour of M. rugulosa, in the context of its already known interspecific relations, as a specific competitive means of existence with higher-ranked species. The behaviour—by strict definition neither interference nor exploitation competition—effectively combines submissiveness, appeasement and tenacity. We discuss similarities between such behaviour and related behaviour reported earlier in a few other Myrmica species and Manica rubida while confronting physically stronger ants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In studies on the structuring of ant communities, interspecific competition has obtained much attention, with special emphasis on various dominance-related exclusions of subordinate species by aggressive top-dominants (for a recent review, see (Cerdá et al., 2013). In evaluating the role of competition in community assembly of ants Vepsäläinen and Pisarski (1982), however, made the point that if competition and replacement are seen on one side of the coin, competition and coexistence reside on the other side. Coexistence by exploitative competition of submissive species is possible by shifts in daily activity or food quality (Savolainen and Vepsäläinen, 1988), or microhabitat (Savolainen and Vepsäläinen, 1989), or when single foragers locate scattered food items before aggressive, often territorial species (Reznikova, 1983), and minimise damaging physical contact by dodging away (Dlusskij, 1967; Savolainen, 1991) or by intimidating behaviour (Czechowski, 1979; Vepsäläinen and Pisarski, 1982).
In efforts to generalise competitive relations among species and the role of competition (as such a term too diffuse to have any practical use) in structuring species assemblages, context-dependent variation in interspecific relations may easily escape notion. In the following, we will describe our unique observations on Myrmica rugulosa, one of the lowest-ranking submissive ant species of Europe (Czechowski, 2004; Seifert, 2007), which occasionally took over aphid resources tended by Lasius fuliginosus, a competitively top-dominant species (Dlusskij, 1981). The aphid in question, the arboreal Stomaphis quercus, is an obligatory myrmecophilic species, trophobiotically associated with and throughout the year dependent on L. fuliginosus (Goidanich, 1957; Depa et al., 2012). We will use available information on similar behaviour of ants when confronted with physically or chemically dominant species, to set our observations in a more general context, and to point out still open questions for future study.
The observations
The events (recorded by KV) took place in 2013, in southernmost Finland (Sipoo, Nikkilä), in a grassy yard with a few trees, including two big birches (Betula pendula) growing 1.2 m apart from each other. The birches were occupied by L. fuliginosus, which nested between the tree roots. On the trunk of the main nest birch (ETRS-TM35FIN coordinates: N 6693934, E 404977, accuracy a couple of metres), from the ground up to over 2 m, we located in bark crevices groups of S. quercus aphids tended by L. fuliginosus (this is the first documentation of S. quercus in Finland). Most of the aphids were deep in the crevices, their presence only deducible from gatherings of L. fuliginosus workers, though part of the aphids could be located by closer examination. The birches were surrounded by a polydomous colony of M. rugulosa, with its numerous nest entrances in the ground dispersed within a radius of several metres.
We started observations on July 23rd after catching sight of a spectacular intrusion of M. rugulosa to the core of L. fuliginosus workers tending their aphids, and continued the observations irregularly to August 7th (later checks of the site, from August 26th onward, displayed no sign of further conflict). The intrusions by M. rugulosa came in waves, with varying intensity. Within the period of conflict (16 days), we visited the site at least once during 11 days, and recorded invasion by M. rugulosa in 7 days. Daily activity of the intrusions showed no pattern; we saw the earliest conflict before noon (then in full fling) and the last, again a strong one at 20:35. Short daily point checks may have missed earlier or later invasions, but 1 day with frequent checks from 10:30 to 19:40 revealed no M. rugulosa on the aphid tree (Table 1).
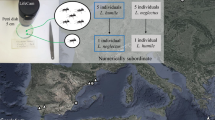
During the most successful intrusions, we estimated the amount of M. rugulosa workers on the trunk of the main nest birch of L. fuliginosus in one or more thousands. The intruders were concentrated in one to several clusters in and around deep bark crevices, with much lower densities on larger trunk areas. The clusters formed a dense and thick carpet of M. rugulosa (typically about 4 × 10 cm2 by area), with workers ranging from the surface of the bark deep into the crevices, and consequently blocking access of L. fuliginosus to their ‘own’ aphids (see Online Resource 1 Fig. S1). Only with lower densities or no M. rugulosa could some aphids be discerned in the bark crevices (see Online Resource 1 Figs. S2, S3) or occasionally to ascend the trunk (Fig. 1).
We were able to observe only once petering out of the intrusion. That day (7.8.; Table 1), thousands of M. rugulosa crowded on the trunk (documented for 40 min), but when checked after 80 min, their number had dropped to hundreds, and after still 1.5 h only 10–20 workers were left. Another half an hour later six M. rugulosa were seen on the trunk, low at the base, being carried off in the mandibles of L. fuliginosus workers. Our scarce behavioural data do not allow speculations on the reasons for withdrawal of M. rugulosa from the aphids they had temporarily reached.
Successful temporary takeovers by M. rugulosa of aphid gatherings in one or a few bark crevices took place at variable heights, sometimes from the ground up to 10 cm, sometimes twice that high (Table 1). As we were able to locate Stomaphis aphids in bark crevices at the height of 1.5 m, once above 2 m (the maximum reached by us standing on the ground), only a lesser part of the aphids on the trunk were ever reached by the intruders.
The L. fuliginosus workers, much less numerous than M. rugulosa at these aggregations, lingered close by or walked deliberately on the carpets of the foreign ants, palpating them with the antennae. Individual L. fuliginosus workers took with their mandibles an individual M. rugulosa worker, usually by hind leg, antenna or petiolus, dragged or carried it a few centimetres back, and then just released it. The M. rugulosa worker, when studied by L. fuliginosus, pressed itself down and froze, then underwent dragging (sometimes visibly resisting by clasping to rough surface with its feet)—after getting loose, it soon resumed attempts to move back towards the aphids. During the most hectic dragging and carrying by L. fuliginosus, occasionally a cluster of five to ten M. rugulosa workers, clasping together, tumbled down the trunk. Palpation and dragging was common behaviour by L. fuliginosus during less intense conflicts (and on the ground, around the nest tree), but when mass intrusions reached the aphid crevices, carrying took over (see Online Resource 2 Video S1; video legend in Online Resource 3). We noticed no sign of direct mutual or unilateral aggressiveness (either physical or chemical, e.g. use of dendrolasin by L. fuliginosus).
During the intrusions, part of the M. rugulosa workers carried a piece of grass, moss or lichen, or a birch seed—both at the feet of the birches and on the trunk. On the ground, they thus built small heaps of plant material, and on the trunk took some pieces up to the bark crevices with aphids. Whatever the intended function of the behaviour (e.g. building temporary shelters for looted aphids), searching through the heaps did not reveal anything. Not even attempts by M. rugulosa to drag out aphids from the crevices were noticed. Without doubt, however, M. rugulosa were successful in obtaining honeydew from Stomaphis. This could be deduced from swollen abdomens of many workers that looked similar to those of M. rugulosa on maple syrup baits (placed at the site) and some odd workers coming down the trunk of a big pine close by, evidently after visiting aphids high up in the tree (uncommon behaviour by M. rugulosa at least in the study site).
We located nests of M. rugulosa around the study birch by serving pieces of oat flakes close to the birch and tracking foragers to their nest entrances. The closest nest was located 60 cm from the study birch, and six nests were found within <2 m. One forager travelled to a distance of 4.6 m before it was lost. Because we did not observe any traffic of M. rugulosa from or to a nest during the invasions to the study birch, we do not know the source nests of the intruders. Anyhow, the high peak numbers of workers on the aphid tree imply that workers came from more than one nest.
Discussion
Similar intimidating behaviour of M. rugulosa as described above has been reported to take place with L. niger on sugar baits and at nests (Czechowski, 1979, 1994); de Vroey 1980), and likewise inhibit physical damage by the stronger ants. The behaviour is also known in other Myrmica species of the scabrinodis group, to which M. rugulosa belongs (Radchenko and Elmes, 2010)—in M. scabrinodis with L. niger on baits (K. Vepsäläinen, unpubl.) and in confrontation with Manica rubida (Le Masne, 1967), and in M. constricta (originally reported as M. rugulosa) with Formica sanguinea attacking their nest (Czechowski, 2004). Generalising, the behaviour of the stronger ants includes dragging or carrying the submissive ant a few centimetres and then letting it free; the submissives freeze when touched, dragged or carried, and after let free resume their activity (return to the bait, aphids or their nest). Also Leptothorax species (L. acervorum, L. muscorum) are known to effectively cut attacks by wood ants (the Formica rufa group) short by pressing themselves down and freezing (Vepsäläinen and Pisarski, 1982), which allows them coexistence with and indirect protection of their nests against potential competing ants (e.g. Lasius s. str., Myrmica spp., Tetramorium caespitum) by the wood ants (Savolainen and Vepsäläinen, 1989).
Our observations on M. rugulosa invading some of the aphid resources of the top-dominant L. fuliginosus, a species able to resist colonies of territorial wood ants (Czechowski et al., 2013), and to destroy colonies of subordinate ants, including those of Myrmica species (Markó et al., 2013), raise still open questions: What do our observations tell about the competition between one of the top-dominant species and the very lowest Myrmica species in the competitive hierarchy of ants? Why did the L. fuliginosus workers not use dendrolasin, their most effective means to repel even strongest alien ants, against M. rugulosa? What specific circumstances drove M. rugulosa into the seemingly exceptional intrusions to the aphids of this top-dominant species?
First, we suggest that competitive gains and losses are relative measures, and in the present case probably highly asymmetric. Even though we did not estimate methodologically adequately the sizes of the polydomous colony of M. rugulosa or the colony of L. fuliginosus, they certainly were of different orders of magnitude; probably with a workforce of several to many thousands and at least tens of thousands (perhaps some hundred of thousands), respectively. It thus seems that M. rugulosa successfully gained part of the alien aphid honeydew, but also that the losses to L. fuliginosus may have been relatively small owing to only a smaller part of the aphids exploited for only a short period of the season by the intruders—probably not a big loss to L. fuliginosus, but a substantial booty to M. rugulosa. Anyhow, it would be easier to understand the lack of physical and chemical measures by L. fuliginosus during the invasions that took the time and energy of quite a few of their workforce, if the intruders had used some appeasement pheromones—a topic for future research?
Second, the intrusions by M. rugulosa may have been caused by the long, hot and dry spells of weather, during which the vegetation at the site wilted, and perhaps killed its assumed root aphids. True, practically nothing is known about its root aphids, though some Myrmica species are known to associate with root aphids (Newton et al., 2011), including M. scabrinodis of the same species group (Depa and Wojciechowski, 2008). It is also possible that similar invasions by M. rugulosa are exceptional only in a statistical sense, i.e. rarely seen owing to the rarity of co-occurrence of M. rugulosa and L. fuliginosus colonies that tend Stomaphis quercus. It should be evident, however, that we were observing only exploitation of S. quercus by M. rugulosa. Without attendance by its unique host ant, L. fuliginosus, the aphid colony would die out, as the colony’s life cycle is dependent on host protection from egg to adult (Goidanich, 1957).
The few other above-ground exploitations of aphids by M. rugulosa known to us, have interesting similarities with our observations described in this note: once the tended aphid was Stomaphis graffii on Norway maple (Acer platanoides), whose eggs probably do not survive the winter if not taken care by its usual host ant, Lasius brunneus (Depa, 2012), and once Aphis sp. on Rumex nr. crispus (see Online Resource 1 Figs. S4, S5). On Rumex, the numerous M. rugulosa, when found, coexisted with a few L. niger, and it took several days before L. niger had successfully expelled M. rugulosa from the aphids (Czechowski, 1994). These two ant species also co-exploited Aphis sp. on Tropaeolum majus (Czechowski, 1979).
Finally, when generalising from our present observations over all known cases where the submissive M. rugulosa and its close relatives, and some other ants, have taken advantage of concentrated food resources (aphids, baits) by appeasement, our observations turn to an extreme variant of a more general behaviour. Usually, Myrmica species are known to shift to less preferred food and foraging times, and reduce aboveground foraging in presence of territorial ant species (Savolainen and Vepsäläinen, 1988, 1989), and references therein). It seems, however, that the effective appeasement behaviour of some Myrmica may open them access to relatively long-lasting concentrated food resources even in the presence of stronger, dominant and subdominant ant species. It is not known what triggers such intrusion of the submissive Myrmica to food usually monopolised by stronger ants, but extended shortage of their conventional food—or merely serendipitous opportunity—might be a sufficient trigger.
References
Cerdá X., Arnan X. and Retana J. 2013. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology? Myrmecol. News 18: 131-147
Czechowski W. 1979. Competition between Lasius niger (L.) and Myrmica rugulosa Nyl. (Hymenoptera, Formicidae). Ann. Zool. 34: 437-451
Czechowski W. 1994. Aphids, honeydew, ants. Przyroda Polska 10(453): 5 [In Polish]
Czechowski W. 2004. Submissive posture of Myrmica rugulosa in its interspecific relations (Hymenoptera, Formicidae). Przegląd Zoologiczny 48: 19-28 [In Polish with English summary]
Czechowski W., Markó B., Radchenko A. and Ślipiński P. 2013. Long-term partitioning of space between two territorial species of ants (Hymenoptera: Formicidae) and their effect on subordinate species. Eur. J. Entomol. 110: 327-337
Depa Ł. 2012. Abundance of Stomaphis graffii Cholod. (Hemiptera) on maple trees in Poland. Cent. Eur. J. Biol. 7: 284-287
Depa Ł., Mróz E. and Szawaryn K. 2012. Molecular identity of Stomaphis quercus (Hemiptera: Aphidoidea: Lachnidae) and description of a new species. Eur. J. Entomol. 109: 435-444
Depa Ł. and Wojciechowski W. 2008. Ant-root aphid relations in different plant associations. Pol. J. Entomol. 77: 151-163
Dlusskij G.M. 1967. Ants of the Genus Formica (Hymenoptera, Formicidae, g. Formica). Nauka, Moscow [In Russian]
Dlusskij G.M. 1981. Desert Ants. Nauka, Moscow [in Russian]
Goidanich A. 1957. Le migrazioni coatte mirmecogene dello Stomaphis quercus Linnaeus, Afide olociclico monoico omotopo (Hemiptera Aphidoidea Lachnidae). Boll. Ist. Entomol. Univ. Bologna 23: 93-131
Le Masne G. 1967. Les transports mutuels autour des nids de Neomyrma rubida Latr.: un nouveau type de relations interspecifiques chez les fourmis. C.R. 5th Congr. IUSSI, Toulouse, 5-10 July 1965, pp 303-322
Markó B., Czechowski W. and Radchenko A. 2013. Combining competition with predation: drastic effect of Lasius fuliginosus (Latr.) on subordinate ant species at the northern limit of its distribution. Ann. Zool. 63: 107-111
Newton J.S., Glasier J., Maw H.E.L., Proctor H.C. and Foottit R.G. 2011. Ants and subterranean Sternorrhyncha in a native grassland in east-central Alberta, Canada. Can. Entomol. 143: 518-523
Reznikova Zh.I. 1983. Interspecific Relations in Ants. Nauka, Novosibirsk [in Russian]
Radchenko A.G. and Elmes G.W. 2010. Myrmica Ants (Hymenoptera, Formicidae) of the Old World. Fauna Mundi, vol. 4, Natura optima dux Foundation, Warsaw
Savolainen R. 1991. Interference by wood ants influences size selection and retrieval rate of prey by Formica fusca. Behav. Ecol. Sociobiol. 28: 1-7
Savolainen R. and Vepsäläinen K. 1988. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51: 135-155
Savolainen R. and Vepsäläinen K. 1989. Niche differentiation of ant species within territories of the wood ant Formica polyctena. Oikos 56: 3-16
Seifert B. 2007. Die Ameisen Mittel- und Nordeuropas. Lutra Verlags- und Vertriebsgesellschaft, Görlitz
Vepsäläinen K. and Pisarski B. 1982. Assembly of island ant communities. Ann. Zool. Fennici 19: 327-335
Vroey C. de 1980. Relations interspécifiques chez les fourmis. In: Ecologie des Insectes Sociaux (Cherix D., Ed). C.R. Coll. Ann. UIEIS, Lausanne, 7-8 Sept. 1979, Lausanne, pp 107-113
Acknowledgments
We thank Łukasz Depa (University of Silesia, Katowice), who most generously answered our many questions on Stomaphis quercus and other aphid species, and let us use his photo on Myrmica rugulosa tending Stomaphis graffii. Two reviewers provided constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vepsäläinen, K., Czechowski, W. Against the odds of the ant competition hierarchy: submissive Myrmica rugulosa block access of the dominant Lasius fuliginosus to its aphids. Insect. Soc. 61, 89–93 (2014). https://doi.org/10.1007/s00040-013-0332-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-013-0332-4