Abstract
Based on metapopulation theory, isolation, patch size and habitat quality within patches have recently been identified as the most critical parameters determining the persistence of species. In the special case of flightless and sedentary Orthoptera species, taking into account the low dispersal ability, species survival probably depends more on habitat quality than on isolation. The aim of this study was to document how landscape (patch size, isolation and climate) and microhabitat (vegetation structure, microclimate and land use) factors influence patch occupancy and population densities, respectively, of a flightless bush-cricket (Metrioptera brachyptera) in fragmented calcareous grasslands. In summer 2005 patch occupancy of M. brachyptera was assessed in 68 calcareous grassland patches of the Diemel Valley (central Germany). Among these, 26 patches with 80 plots were selected to characterise M. brachyptera habitats in detail. On each plot, bush-cricket density was sampled in an area of 20 m2 using a 0.5 m2 box quadrat. At the landscape level (patches) in 46 (68%) of 68 studied calcareous grassland patches M. brachyptera was present. Patch occupancy increased with annual precipitation and patch size but was independent of altitude, annual temperature and isolation. At the microhabitat level (plots), population density of M. brachyptera decreased with land-use intensity and increased with vegetation height. In addition, a high litter accumulation was adverse for M. brachyptera. Given the low explanatory power of isolation for patch occupancy, conservation of flightless and sedentary insects, such as M. brachyptera, should primarily focus on improving habitat quality. For M. brachyptera and other stenotopic calcareous grassland species we therefore recommend traditional rough grazing with sheep, which creates a heterogenous habitat structure and avoids the accumulation of too much litter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All around the world, land-use change is among the most severe drivers of terrestrial biodiversity loss (Sala et al. 2000). Thus, landscape structure and the consequences of habitat degradation and fragmentation on species survival has attracted considerable interest in ecology (Hanski and Ovaskainen 2000; Fahrig 2003). Based on metapopulation theory, patch isolation, patch size and habitat quality within patches have been identified as the most critical parameters determining persistence of species in cultivated landscapes in many invertebrates (Thomas et al. 2001; Anthes et al. 2003; Eichel and Fartmann 2008). Species that form metapopulations, like most butterflies (Hanski 1999), depend on regular exchange of individuals between subpopulations and, hence, are strongly affected by fragmentation (Casula 2006; Polus et al. 2007). But how can a flightless and sedentary species persist in a fragmented landscape? Among central European Orthoptera, only a few species reach the mobility of butterflies, and most of them have a low dispersal ability (Reinhardt et al. 2005). Moreover, in contrast to butterflies, many orthopterans are able to persist in fairly small (Köhler 1996; Maes et al. 2006; Theuerkauf and Rouys 2006) and often isolated habitat patches (Reinhardt and Köhler 2002). Taking this into account, one should expect that persistence of flightless and sedentary Orthoptera species depends more on habitat quality than on isolation.
However, several studies have addressed the influence of landscape structure on Orthoptera populations and have confirmed some of the findings of metapopulation theory; smaller patches are more often unoccupied and the respective local populations had a higher likelihood of extinction (Kindvall and Ahlén 1992; Hjermann and Ims 1996; Berggren et al. 2001; Carlsson and Kindvall 2001). Colonised patches were usually less isolated than unoccupied ones (Kindvall and Ahlén 1992; Hjermann and Ims 1996; Carlsson and Kindvall 2001), and connectivity in form of linear elements promoted movement between patches and colonisation success (Berggren et al. 2001; Diekötter et al. 2007).
Habitat quality in Orthoptera is mainly determined by land use (Kruess and Tscharntke 2002; Marini et al. 2009), vegetation structure (Poniatowski and Fartmann 2008) and microclimate (Willott and Hassall 1998; Gardiner and Dover 2008). Predation, competition and food availability are partly interrelated with the aforementioned parameters and usually play a minor role for the persistence of species in cultivated European landscapes (Ingrisch and Köhler 1998). Yet, whereas dispersal and mobility of many flightless orthopterans were studied in detail (e.g. Kindvall 1999; Berggren et al. 2002; Diekötter et al. 2005), knowledge of the key factors that determine habitat quality of the species is often still poor.
The aim of this study was to document which key factors determine the distribution of a flightless bush-cricket (Metrioptera brachyptera) in fragmented calcareous grasslands. Therefore, two different scales were used: At the landscape level we tested the impact of patch size, isolation and climate on patch occupancy and at the microhabitat level we examined the effect of vegetation structure, microclimate and land use on population density.
Methods
Study organism
The bog bush-cricket Metrioptera brachyptera (Linnaeus, 1761) (Orthoptera: Tettigoniidae) is a medium-sized bush-cricket, 11–21 mm in total length and usually flightless (Marshall and Haes 1988). Individuals capable of flight (macropterous form) are very rare (the proportion of macropters in a population is generally less than 1%, Poniatowski and Fartmann, unpublished data) and have as yet not been found outside the native habitat (Schouten et al. 2007). Moreover, M. brachyptera has a high habitat specificity (Schouten et al. 2007); i.e. M. brachyptera inhabits only a few habitat types such as wet heathlands or stands of purple moorgrass (Marshall and Haes 1988; Kleukers et al. 2004). However, the species can also be found in semi-dry calcareous grasslands (Bruckhaus 1994), occasionally even in high densities (Poniatowski and Fartmann 2008). The habitats of M. brachyptera are characterised as dense vegetation with long turf (e.g. Bruckhaus 1994). More detailed information on habitat requirements is rare (Poniatowski and Fartmann 2007).
Study area
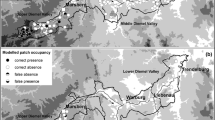
The study area (hereafter called Diemel Valley) of about 500 km2 is located in central Germany along the border between the federal states of North Rhine-Westphalia and Hesse (51°22′N/8°38′E and 51°38′N/9°25′E) at an elevation of 160–480 m a.s.l. (Fig. 1). The climate is suboceanic and varies greatly according to altitude (Müller-Wille 1981). The Upper Diemel Valley (300–500 m a.s.l.) is the coldest and wettest section with mean temperatures of 6.5–8°C and an annual precipitation of 700–1,000 mm. The Middle and Lower Diemel Valley (<300 m a.s.l.) in the eastern part of the study area have a relatively mild climate with less than 800 mm annual precipitation and an average annual temperature of up to 9°C (Müller-Temme 1986; MURL NRW 1989; Fartmann 2004). The area south of Warburg in the Middle Diemel Valley has an exposed position; with an annual precipitation of 600–650 mm it is the driest part of the whole study area (Müller-Temme 1986). Most of the study area consists of limestone with often extended semi-dry grasslands. The surrounding matrix of these habitat islands is dominated by a patchwork of woodland, improved grassland and arable fields. On the whole, it is the largest area of calcareous grasslands in the northern half of Germany (Fartmann 2004, 2006).
Patch occupancy
The distribution of insects is generally influenced by landscape factors, such as patch isolation, altitude and annual precipitation (e.g. Thomas et al. 1998; Willott and Hassall 1998; WallisDeVries 2004), as well as microhabitat factors such as vegetation structure, microclimate and land-use intensity (e.g. Gardiner and Dover 2008; Poniatowski and Fartmann 2008; Marini et al. 2009). For this reason, the habitat was evaluated at the landscape level and the microhabitat level:
Landscape level (patches)
Between mid-July and mid-August 2005 all known semi-dry calcareous grasslands (N patches = 68) having a mean size of 7.8 ha (range = 0.02–69.00 ha; SD = 12.90 ha) were searched at least once for M. brachyptera individuals (in the study area the species is restricted to this habitat type; Poniatowski and Fartmann 2008). Whenever the species was not detected during the first visit, a second survey was carried out a few days later. All surveys were conducted under favourable weather conditions (>15°C and sunny) between 1000 and 1600 hours (cf. Kindvall and Ahlén 1992; Berggren et al. 2002). Following Hermann (1999) the search was conducted in a success-oriented manner, predominantly on those parts of a patch that promised a high likelihood of finding the species. As the high-frequency song of M. brachyptera is only detectable within a couple of metres, the survey was supported by an ultrasound detector (Berggren et al. 2001). A patch was classified as occupied if at least one individual of M. brachyptera was detected. Patches were regarded as discrete when they were isolated from the nearest neighbouring patch by over 50 m of woodland, improved grassland or arable fields (Fartmann 2006). Isolation of the patches was measured as the geometric mean of the next three populated patches (Eichel and Fartmann 2008). Mean isolation of the patches was 2.4 km (range = 0.7–6.8 km; SD = 1.4). Also, for each of the patches, altitude (mean = 241 m a.s.l.; range = 160–480 m a.s.l.; SD = 65.9 m a.s.l.), mean annual precipitation (mean = 707 mm; range = 625–975 mm; SD = 76.8 mm) and mean annual temperature (mean = 8.1°C; range = 6.75–8.75°C; SD = 0.3°C) were ascertained using the climate atlas of North Rhine-Westphalia (resolution precipitation: 50 mm; resolution temperature: 0.5°C) (MURL NRW 1989; Table 1).
Microhabitat level (plots)
In order to characterise details of M. brachyptera habitat choice, 26 occupied patches were selected. The patches cover the whole study area (Upper Diemel Valley: N patches = 9; Middle Diemel Valley: N patches = 7; Lower Diemel Valley: N patches = 10) and they were representative for the calcareous grasslands in the Diemel Valley (Poniatowski and Fartmann 2008). Depending on their structural heterogeneity, 1–7 plots per patch were defined (80 plots in total; 36 occupied; 44 unoccupied). Each plot had a homogenous vegetation structure according to Sänger (1977) and could be differentiated by its structural composition from the other plots per patch. To avoid edge effects (Schirmel et al. 2010) the size of the plot was at least 500 m2 and the bush-cricket densities were recorded in the centre of the plot (for bush-cricket sampling see next sub-section). The measurement of environmental parameters took place after the quantitative sampling of bush-crickets in an undisturbed section of the plot. For each plot, several environmental parameters, such as inclination, vegetation height and density, were measured (Table 1); for further details see Poniatowski and Fartmann (2008). Additionally, land-use intensity per plot was ascertained (Table 2), because management regimes frequently differed at the plot level. For this purpose landowners were interviewed.
Bush-cricket sampling
Quantitative sampling (counts of bush-cricket densities) took place once per plot between mid-July and mid-August 2005. Densities were recorded with a box quadrat (Ingrisch and Köhler 1998; Gardiner et al. 2005), which, according to Gardiner and Hill (2006), is the best sampling method to ascertain bush-cricket abundance. The box quadrat had an area of 0.5 m2 (0.71 × 0.71 m) with white gauze covered sides of 0.8 m height. It was randomly dropped over the vegetation at 40 different points per plot; i.e., in total an area of 20 m2 was studied on each plot (e.g. Fartmann et al. 2008; Poniatowski and Fartmann 2008).
Statistical analysis
For non-parametic comparisons of two independent and not normally distributed samples (Kolmogorov–Smirnov test), Mann–Whitney U test (MWU) was used. Differences between more than two variables were analysed using Kruskal–Wallis H (KW) test. Binomial generalized linear model (GLM) was applied to assess which environmental parameters possessed the highest explanatory power for the patch occupancy of the species on the landscape level (patches). For the analysis, only such environmental parameters were used that were constant within a patch (e.g. altitude, annual precipitation and patch isolation; Table 1). At the microhabitat level (plots), parameters that affect bush-cricket densities within the patches (e.g. inclination, litter layer and vegetation height; Table 1) were analysed by generalized linear mixed-effects model (GLMM: lmer, Bates et al. 2008). The variable patch was set up as a random factor (Table 1). A quasi-Poisson error structure was used to counteract over-dispersion. Non-significant predictors were excluded from the final model by stepwise backward-selection (P > 0.05). The significance of the predictor variables were assessed with likelihood ratio tests (Type III test). To assess inter-correlation between predictor variables Principal Component Analysis (PCA) was used (Table 1). Statistical analyses were performed using R-2.9.0 (R Development Core Team 2009), Canoco 4.5 and SPSS 11.5 statistical packages. For graphical analysis, we used SigmaPlot 11.0.
Results
Patch occupancy (landscape level)
M. brachyptera is widespread in the study area. In 46 (68%) of 68 studied calcareous grassland patches the species was present (Fig. 1). The Lower Diemel Valley is the stronghold of M. brachyptera in the study area (18 of 20 patches [90%] occupied). In contrast, M. brachyptera was absent in many semi-dry grasslands in the Middle Diemel Valley (18 of 36 patches [50%] occupied). Most of the unoccupied patches were in an area with low precipitation in the south of Warburg. The occupied patches were predominantly found at the edge of the Middle Diemel Valley with higher precipitation. Although the number of remaining semi-dry grasslands is markedly lower in the Upper Diemel Valley than in the other two subareas, M. brachyptera is nearly always present (10 of 12 patches [83%] occupied).
At the landscape level (patches) patch occupancy was positively correlated with patch size and mean annual precipitation (Table 3). M. brachyptera prefers areas with more than 650 mm annual precipitation. The smallest occupied habitat patch was 0.05 ha (mean = 9.9 ha; SD = 14.9 ha). In contrast, altitude, patch isolation and mean annual temperature did not contribute to the model.
Habitat quality (microhabitat level)
The habitats of M. brachyptera in the Diemel Valley are characterised by an almost closed vegetation coverage (median = 95%; Fig. 2), a medium high field layer (median = 15 cm; Fig. 3) and a dense vegetation up to 15 cm height (Fig. 4). The percentage of bare ground and stony surface is low (Fig. 2) and reaches 15% at most. Litter and cryptogams, in contrast, cover up to 95% (median = 50 and 25%, respectively; Fig. 2).
Horizontal structure (coverage [%]) of calcareous grassland plots occupied (N = 36) and unoccupied (N = 44) by Metrioptera brachyptera. Box-plots show 10th and 90th percentile (whiskers), 25th and 75th percentile (boundary of the box), and median (thick line); Mann–Whitney U test for significance (α = 0.05): * P < 0.05, ** P < 0.01, *** P < 0.001
Height of field layer in calcareous grassland plots occupied (N = 36) and unoccupied (N = 44) by Metrioptera brachyptera. Open dots (outliers) (see also Fig. 2)
Horizontal vegetation density in calcareous grassland plots occupied (N = 36) and unoccupied (N = 44) by Metrioptera brachyptera (see also Fig. 2)
Bush-cricket densities were highest on plots with moderate land-use intensity (median = 3.0 adults/10 m2), but on abandoned plots and plots that were sporadically grazed (absent/low land-use intensity) maximum values were much higher (maximum = 14.0 adults/10 m2; Fig. 5). Calcareous grasslands with high land-use intensity were mostly avoided (Table 2); single individuals of M. brachyptera were detected only very rarely (Fig. 5).
Adult densities of Metrioptera brachyptera and land-use intensity of all surveyed plots (N = 80). For classification of land-use intensities see Table 2. Kruskal–Wallis H test: χ² = 40.144, df = 2, P < 0.001. Different symbols indicate significant differences at the P < 0.05 level (Mann–Whitney U test for significance, α = 0.05) (see also Fig. 2)
At the microhabitat level (plots) the distribution pattern of M. brachyptera is best explained by a combination of land-use intensity as well as vegetation height and litter coverage (Table 4); population density decreased with land-use intensity and a high litter accumulation and increased with vegetation height. All other environmental parameters had no significant influence.
Discussion
The persistence of M. brachyptera in the fragmented landscape of our study area is determined by mean annual precipitation and patch size, but not by patch isolation. This is in line with the assumption of Reinhardt and Köhler (2002) according to which the majority of Orthoptera populations are isolated and metapopulation aspects play a minor role.
However, there are several studies that found a correlation between isolation and patch occupancy in Orthoptera species (Appelt and Poethke 1997; Carlsson and Kindvall 2001); even in flightless/less mobile species (Kindvall and Ahlén 1992; Hjermann and Ims 1996). Why then did we not find a correlation? Most of these studies examined metapopulation parameters without considering habitat quality, although habitat quality is one of the three most important metapopulation parameters (e.g. Thomas et al. 2001; Anthes et al. 2003; Eichel and Fartmann 2008). Nevertheless, we believe that the observed patterns are still valid for Orthoptera, but—in comparison to butterflies (cf. Maes et al. 2006)—on a much smaller scale, particularly for flightless species (cf. Kindvall and Ahlén 1992; Hjermann and Ims 1996).
It is undisputable that immigrants are beneficial for the genetic diversity, and therewith the long-term survival of isolated populations (Frankham et al. 2009). However, we believe that M. brachyptera and probably many other orthopterans (cf. Köhler 1996) are able to persist in highly isolated populations over a very long time without individual exchanges; given that the habitat requirements of the species are continuously fulfilled. One example are the degenerated bogs with M. brachyptera populations in the Münsterland (North-West Germany), which lie in an intensively used agricultural landscape and have been isolated for more than 100 years (Bömer 1893). M. brachyptera populations in these habitat islands have been known for more than 60 years (Röber 1951) and they still exist (Poniatowski, unpublished data). Indeed, there is a long-winged (macropterous) form of M. brachyptera, which is probably able to fly, but these individuals are quite rare. In the Netherlands for instance, only three long-winged individuals of M. brachyptera have been found yet (Kleukers et al. 2004).
M. brachyptera is a sedentary species (Kleinert 1992) and movement of (flightless) bush-crickets is generally very limited. Most of them move only a few metres per day; especially when they are moving in their preferred habitat (Kindvall and Ahlén 1992; Hein et al. 2003; Diekötter et al. 2005, 2007). For example, most of the 440 studied Metrioptera bicolor individuals moved less than 1 m per day and the maximum daily movement distance was a mere 40 m (Kindvall 1999). It might be argued that the movement ratio is higher in an unsuitable matrix, but one must keep in mind, that many animals—such as M. bicolor (Kindvall and Ahlén 1992)—are unwilling to leave their native habitat patches (Stamps et al. 1987). This is probably the case for the habitat specialist M. brachyptera. The species is restricted to semi-dry grasslands in the Diemel Valley (Poniatowski and Fartmann 2008), and we never observed any individual outside the native habitat.
Taking this into account, an exchange of individuals between (sub) populations seems to be impossible for flightless and sedentary Orthoptera species in highly fragmented landscapes like the Diemel Valley (mean of the next suitable habitat patch: 2.4 km). This is in line with the findings of Wettstein and Schmid (1999), who found no effect of habitat fragmentation on Orthoptera persistence. According to their study, Orthoptera species’ richness was, beside altitude, only influenced by habitat quality.
In the case of M. brachyptera habitat quality is determined by a medium high field layer. Additionally, a moderate litter and cryptogam (i.e. moss) coverage seems to be important. In combination with sufficient precipitation, these structures lead to a mesic microclimate near the soil surface (Fartmann 2004, 2006), which is necessary for successful embryonic development (cf. Ingrisch 1979).
Conservation
Harrison and Bruna (1999) stressed that many scientists and conservationists deal with fashionable terms like metapopulation and corridor but ignore the loss of habitat quality within habitat fragments. Following the findings of our study for the conservation of flightless and sedentary Orthoptera species as well as many other immobile insects, it would be more appropriate to improve habitat quality within patches than to establish a better connectivity between patches (cf. Reinhardt and Köhler 2002). Of course, a better connectivity between patches would be desirable, too. However, for sedentary habitat specialists this is often not practicable, because they need well connected stepping stones or even real corridors with a suitable vegetation structure. Hence, conservation of flightless and sedentary species should primary focus on improving habitat quality.
In general, larger patches are of higher interest, as they provide a connection to metapopulation theory. The bigger the patch, the higher the habitat heterogeneity should be, and thus the likelihood of having parts with high habitat quality increases (cf. Thomas et al. 2001; Schouten et al. 2007). Especially due to climate change, spatial heterogeneity of patches becomes important elsewhere: habitat heterogeneity can act as a climatic buffer and can reduce the risk of population extinction (Kindvall 1996; Berggren et al. 2001; Fartmann 2006).
The semi-dry calcareous grasslands of the Diemel Valley are protected by the EU Habitats Directive and are of special relevance for the persistence of M. brachyptera. In almost 70% of the studied calcareous grassland patches the habitat specialist occurred. As a chortobiont species the densities of M. brachyptera are highest on abandoned patches (Fig. 5). However, this is only the case if the coverage of litter is moderate. As with other Orthoptera species a consecutive litter accumulation is adverse for M. brachyptera because sooner or later it leads to a cooler microclimate and, consequently, lower densities (Fartmann and Mattes 1997). Therefore, the range of densities in abandoned and sporadically used patches is very wide. As an optimal land use we recommend the traditional rough grazing with sheep, which creates a heterogeneous habitat structure and avoids the accumulation of too much litter (Fartmann and Mattes 1997). This type of land use is not only beneficial for M. brachyptera but also for a large number of other stenotopic species breeding in calcareous grassland such as Hamearis lucina (Fartmann 2006; Anthes et al. 2008) and Melitaea aurelia (Eichel and Fartmann 2008).
References
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure—the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185. doi:10.1023/A:1027330422958
Anthes N, Fartmann T, Hermann G (2008) The Duke of Burgundy butterfly and its dukedom: larval niche variation in Hamearis lucina across Central Europe 12:3–14. doi: 10.1007/s10841-007-9084-7
Appelt M, Poethke H-J (1997) Metapopulation dynamics in a regional population of the blue-winged grasshopper (Oedipoda caerulescens; Linnaeus 1758). J Insect Conserv 1:205–214. doi:10.1023/A:1018468017604
Berggren Å, Carlson A, Kindvall O (2001) The effect of landscape composition on colonization success, growth rate and dispersal in introduced bush-crickets Metrioptera roeseli. J Anim Ecol 70:663–670. doi:10.1046/j.1365-2656.2001.00525.x
Berggren Å, Birath B, Kindvall O (2002) Effect of corridors and habitat edges on dispersal behavior, movement rates, and movement angles in Roesel’s bush-cricket (Metrioptera roeseli). Conserv Biol 16:1562–1569. doi:10.1046/j.1523-1739.2002.01203.x
Bömer A (1893) Die Moore Westfalens. Der Kreis Ahaus (Band 1). Buchdruckerei „Die Post”, Berlin
Bruckhaus A (1994) Das Springschreckenvorkommen von bewirtschafteten und unbewirtschafteten Kalkmagerrasen der Nordeifel. Articulata 9:1–14
Carlsson A, Kindvall O (2001) Spatial dynamics in a metapopulation network: recovery of a rare grasshopper Stauroderus scalaris from population refuges. Ecography 24:452–460. doi:10.1111/j.1600-0587.2001.tb00480.x
Casula P (2006) Evaluating hypotheses about dispersal in a vulnerable butterfly. Ecol Res 21:263–270. doi:10.1007/s11284-005-0130-1
Diekötter T, Csencsics D, Rothenbühler C, Billeter R, Edwards PJ (2005) Movement and dispersal patterns in the bush cricket Pholidoptera griseoaptera: the role of developmental stage and sex. Ecol Entomol 30:419–427. doi:10.1111/j.0307-6946.2005.00714.x
Diekötter T, Speelmans M, Dusoulier F, van Wingerden WKRE, Malfait JP, Crist TO, Edwards PJ, Dietz H (2007) Effects of landscape structure on movement patterns of the flightless bush cricket Pholidoptera griseoaptera. Environ Entomol 36:90–98
Eichel S, Fartmann T (2008) Management of calcareous grasslands for Nickerl’s fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation, and patch area. J Insect Conserv 12:677–688. doi:10.1007/s10841-007-9110-9
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fartmann T (2004) Die Schmetterlingsgemeinschaften der Halbtrockenrasen-Komplexe des Diemeltales. Biozönologie von Tagfaltern und Widderchen in einer alten Hudelandschaft. Abh Westf Mus Naturkunde 66:1–256
Fartmann T (2006) Oviposition preferences, adjacency of old woodland and isolation explain the distribution of the Duke of Burgundy butterfly (Hamearis lucina) in calcareous grasslands in central Germany. Ann Zool Fenn 43:335–347
Fartmann T, Mattes H (1997) Heuschreckenfauna und Grünland—Bewirtschaftungsmaßnahmen und Biotopmanagement. Arb Inst Landschaftsökol 3:179–188
Fartmann T, Behrens M, Loritz H (2008) Orthopteran communities in the conifer-broadleaved woodland zone of the Russian Far East. Eur J Entomol 105:673–680
Frankham R, Ballou JD, Briscoe DA (2009) Introduction to conservation genetics, 2nd edn. Cambridge University Press, Cambridge
Gardiner T, Dover J (2008) Is microclimate important for Orthoptera in open landscapes? J Insect Conserv 12:705–709. doi:10.1007/s10841-007-9104-7
Gardiner T, Hill J (2006) A comparison of three sampling techniques used to estimate population density and assemblage diversity of Orthoptera. J Orthoptera Res 15:45–51
Gardiner T, Hill J, Chesmore D (2005) Review of the methods frequently used to estimate the abundance of Orthoptera in grassland ecosystems. J Insect Conserv 9:151–173. doi:10.1007/s10841-005-2854-1
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Ovaskainen O (2000) The metapopulation capacity of a fragmented landscape. Nature 404:755–758. doi:10.1038/35008063
Harrison S, Bruna E (1999) Habitat fragmentation and large-scale conservation: what do we know for sure? Ecography 22:225–232. doi:10.1111/j.1600-0587.1999.tb00496.x
Hein S, Gombert J, Hovestadt T, Poethke H-J (2003) Movement patterns of the bush cricket Platycleis albopunctata in different types of habitat: matrix is not always matrix. Ecol Entomol 28:432–438. doi:10.1046/j.1365-2311.2003.00531.x
Hermann G (1999) Methoden zur qualitativen Erfassung von Tagfaltern. In: Settele J, Feldmann R, Reinhardt R (eds) Die Tagfalter Deutschlands. Ulmer, Stuttgart, pp 124–143
Hjermann DO, Ims RA (1996) Landscape ecology of the wart-biter Decticus verrucivorus in a patchy landscape. J Anim Ecol 65:768–780
Ingrisch S (1979) Experimentell-ökologische Freilanduntersuchungen zur Monotopbindung der Laubheuschrecken (Orthoptera: Tettigoniidae) im Vogelsberg. Beitr Naturkunde Osthessen 15:33–95
Ingrisch S, Köhler G (1998) Die Heuschrecken Mitteleuropas. Westarp Wissenschaften, Magdeburg
Kindvall O (1996) Habitat heterogeneity and survival in a bush cricket metapopulation. Ecology 77:207–214
Kindvall O (1999) Dispersal in a metapopulation of the bush cricket, Metrioptera bicolor (Orthoptera: Tettigoniidae). J Anim Ecol 68:172–185. doi:10.1046/j.1365-2656.1999.00273.x
Kindvall O, Ahlén I (1992) Geometrical factors and metapopulation dynamics of the bush cricket, Metrioptera bicolor Philippi (Orthoptera: Tettigoniidae). Conserv Biol 6:520–529. doi:10.1046/j.1523-1739.1992.06040520.x
Kleinert H (1992) Entwicklung eines Biotopbewertungskonzeptes am Beispiel der Saltatoria (Orthoptera). Articulata Beiheft 1:1–117
Kleukers RMJC, van Nieukerken EJ, Odé B, Willemse LPM, van Wingerden WKRE (2004) De sprinkhanen en krekels van Nederland (Orthoptera), 2nd edn. Nederlandse Fauna 1. Nationaal Natuurhistorisch Museum, KNNV Uitgeverij & EIS-Nederland, Leiden
Köhler G (1996) The ecological background of population vulnerability in Central European grasshoppers and bush-crickets: a brief review. In: Settle J, Margules CR, Poschold P, Henle K (eds) Species survival in fragmented landscapes. Kluwer, Dordrecht, pp 290–298
Kruess A, Tscharntke T (2002) Grazing intensity and the diversity of grasshoppers, butterflies, and trap-nesting bees and wasps. Conserv Biol 16:1570–1580
Maes D, Ghesquiere A, Logie M, Bonte D (2006) Habitat use and mobility of two threatened coastal dune insects: implications for conservation. J Insect Conserv 10:105–115. doi:10.1007/s10841-006-6287-2
Marini L, Fontana P, Battisti A, Gaston KJ (2009) Response of orthopteran diversity to abandonment of semi-natural meadows. Agric Ecosyst Environ 132:232–236. doi:10.1016/j.agee.2009.04.003
Marshall JA, Haes ECM (1988) Grasshoppers and allied insects of Great Britain and Ireland. Harley, Colchester
Müller-Temme E (1986) Niederschläge in raum-zeitlicher Verteilung. Geographisch-landeskundlicher Atlas II, Lieferung 6. Geographische Kommission für Westfalen, Münster
Müller-Wille W (1981) Westfalen. Landschaftliche Ordnung und Bindung eines Landes, 2nd edn. Aschendorffsche Verlagsbuchhandlung, Münster
MURL NRW (Minister für Umwelt, Raumordnung und Landwirtschaft des Landes Nordrhein-Westfalen) (ed) (1989) Klima-Atlas von Nordrhein-Westfalen. Selbstverlag, Düsseldorf
Polus E, Vandewoestijne S, Choutt J, Baguette M (2007) Tracking the effects of one century of habitat loss and fragmentation on calcareous grassland butterfly communities. Biodivers Conserv 16:3423–3436. doi:10.1007/s10531-006-9008-y
Poniatowski D, Fartmann T (2007) Kleinräumig heterogen strukturierte Hochheiden in mikroklimatisch günstiger Lage—Lebensräume der Kurzflügeligen Beißschrecke (Metrioptera brachyptera) im Quellgebiet der Diemel (Südwestfalen/Nordhessen). Articulata 22:153–171
Poniatowski D, Fartmann T (2008) The classification of insect communities: Lessons from Orthoptera assemblages of semi-dry calcareous grasslands in central Germany. Eur J Entomol 105:659–671
Reinhardt K, Köhler G (2002) Bedeutung aktueller Befunde der Verhaltensökologie für den Artenschutz. Dargestellt am Beispiel der Heuschrecken. Natursch Landschaftspl 34:171–180
Reinhardt K, Köhler G, Maas S, Detzel P (2005) Low dispersal ability and habitat specificity promote ectinctions in rare but not in widespread species: the Orthoptera of Germany. Ecography 28:593–602. doi:10.1111/j.2005.0906-7590.04285.x
Röber H (1951) Die Dermapteren und Orthopteren Westfalens in ökologischer Betrachtung. Abh Landesmus Naturkunde Münster 14:1–60
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Biodiversity—global biodiversity scenarios for the year 2100. Science 287:1770–1774. doi:10.1126/science.287.5459.1770
Sänger K (1977) Über die Beziehungen zwischen Heuschrecken (Orthoptera: Saltatoria) und der Raumstruktur ihrer Habitate. Zool Jb Syst 104:433–488
Schirmel J, Blindow I, Fartmann T (2010) The importance of habitat mosaics for Orthoptera (Caelifera and Ensifera) in dry heathlands. Eur J Entomol 107:129–132
Schouten MA, Verweij PA, Barendregt A, Kleukers RJM, de Ruiter PC (2007) Nested assemblages of Orthoptera species in the Netherlands: the importance of habitat features and life-history traits. J Biogeogr 34:1938–1946. doi:10.1111/j.1365-2699.2007.01742.x
Stamps JA, Buechner M, Krishnan VV (1987) The effect of edge permeability and habitat geometry on emigration from patches of habitat. Am Nat 129:533–552
Theuerkauf J, Rouys S (2006) Do Orthoptera need human land use in Central Europe? The role of habitat patch size and linear corridors in the Bialowieza Forest, Poland. Biodiv Conserv 15:1497–1508. doi:10.1007/s10531-005-2356-1
Thomas JA, Simcox DJ, Wardlaw JC, Elmes GW, Hochberg ME, Clarke RT (1998) Effects of latitude, altitude and climate on the habitat and conservation of the endangered butterfly Maculinea arion and its Myrmica ant hosts. J Insect Conserv 2:39–46. doi:10.1023/A:1009640706218
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc R Soc London Ser B 268:1791–1796. doi:10.1098/rspb.2001.1693
Tonne F (1954) Besser bauen mit Besonnungs- und Tageslicht-Planung. Hofmann, Schorndorf
WallisDeVries MF (2004) A quantitative conservation approach for the endangered butterfly Maculinea alcon. Conserv Biol 18:489–499. doi:10.1111/j.1523-1739.2004.00336.x
Wettstein W, Schmid B (1999) Conservation of arthropod diversity in montane wetlands: effect of altitude, habitat quality and habitat fragmentation on butterflies and grasshoppers. J Appl Ecol 36:363–373. doi:10.1046/j.1365-2664.1999.00404.x
Willott SJ, Hassall M (1998) Life-history responses of British grasshoppers (Orthoptera: Acrididae) to temperature change. Funct Ecol 12:232–241. doi:10.1046/j.1365-2435.1998.00180.x
Acknowledgments
We are very grateful to A. M. Schulte (Meschede) for obtaining information on the distribution of M. brachyptera in the Diemel Valley. Many thanks go to Nils Anthes (University of Tübingen) and two anonymous reviewers for helpful comments on an earlier version of the manuscript. Moreover, we would like to thank Jan Thiele (University of Münster) for help with R. The Biologische Station Hochsauerlandkreis e.V. and the Akademie für ökologische Landeserforschung e.V. partly funded the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poniatowski, D., Fartmann, T. What determines the distribution of a flightless bush-cricket (Metrioptera brachyptera) in a fragmented landscape?. J Insect Conserv 14, 637–645 (2010). https://doi.org/10.1007/s10841-010-9293-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-010-9293-3