Abstract
Southern California’s sage scrub (SS) ecosystem is severely threatened by suburban development and invasion by non-native grasses, but how these threats impact the arthropod community is poorly understood. Native ants, which face the additional threat of being displaced by non-native Argentine ants, may be particularly at risk of local and regional extirpation. In this study, we surveyed the ant communities in the SS and non-native grassland habitats at the Robert J Bernard Biological Field Station (BFS) and surrounding suburban habitat, and compared patterns of species richness and composition among habitat types. We also compared ant richness and composition at the BFS to 40 coastal SS fragments previously surveyed in San Diego County to better understand how ant communities in interior and coastal SS fragments differ. Ant composition significantly differed among all three habitat types at and surrounding the BFS, but species richness did not. Comparisons between the BFS and coastal fragments indicate that interior SS fragments harbor unique ant species and more species relative to fragment area. Increased richness and unique ant assemblages are probably associated with the limited ability of invasive Argentine ants to colonize the non-native grassland and SS at the BFS. Because many southern California invertebrates are narrowly endemic to low elevation areas, patterns of habitat specificity seen with ants highlight that maintaining a mosaic of SS and non-native grassland habitat, particularly in interior areas where activity and diversity of non-native invertebrate species may be restricted, may be critical to preserving biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Covering <2 % of the Earth’s surface, Mediterranean climate ecosystems support extraordinary biodiversity, including an estimated 48,950 (~20 % of the world’s total) species of vascular plants (Cowling et al. 1996; Medail and Quezel 1997). However, this mild climate is also home to over 250 million human residents (Cox and Underwood 2011). The resulting development and habitat modification make Mediterranean ecosystems some of the most threatened worldwide (Sala et al. 2000). This is especially true in southern California and northern Mexico, which have more than double the human population density of any other Mediterranean region (Underwood et al. 2009; Riordan and Rundel 2014). Moreover, southern California’s human population is disproportionately concentrated in its lowlands, 20 % of which are now urban, with much of the rest suburban, agricultural, or otherwise disturbed (Underwood et al. 2009). These lowlands were once dominated by sage scrub (SS), a major scrubland ecosystem type that ranges from central California, USA to northern Baja, Mexico (Küchler 1977; Westman 1981; Rundel 2007). Estimates suggest that stands of SS have been reduced by more than 50 % in the last century (Minnich and Dezzani 1998; Talluto and Suding 2008) and by as much as 90 % from its prehistoric range (Westman 1981), with much of what remains found in small, isolated fragments (Soulé et al. 1988; Alberts et al. 1993; Suarez et al. 1998; Bolger et al. 2000).
In addition to habitat loss, biodiversity in the SS ecosystem is threatened by the establishment and spread of non-native species. For example, non-native annual grasslands, dominated by European grasses, are increasingly replacing SS habitat following disturbances (Eliason and Allen 1997; DeSimone and Zedler 2001; Cox et al. 2014). Increasing fire frequencies, high levels of nitrogen deposition and drought all contribute to the conversion of SS to non-native grasslands (Cox et al. 2014; Goldstein and Suding 2014; Kimball et al. 2014). Once non-native grasses are established, they promote more frequent fires perpetuating a cycle of grass dominance (D’Antonio and Vitousek 1992; Keeley et al. 2005). Invasion of non-native grasses into SS has been shown to alter ecosystem function and negatively influence ground-dwelling arthropod populations (Wolkovich et al. 2009a). Significant declines in arthropod diversity have also followed the establishment of the non-native Argentine ant, Linepithema humile, in SS (Suarez et al. 1998; Bolger et al. 2000; Tillberg et al. 2007). Argentine ants are less tolerant of heat and desiccation than native ants and the ability to invade SS has been shown to be regulated by climate and water availability (Suarez et al. 1998; Holway et al. 2002; Menke and Holway 2006).
Despite all the threats they face, the diversity and distributions of arthropods in southern California are not well understood. For example, there have been no direct comparisons among arthropod communities in SS and the other dominant habitat types (such as non-native grasslands and urban/suburban areas) that typically surround SS fragments. Studies that have investigated SS arthropods have primarily focused on the effects of: (1) SS fragmentation (Suarez et al. 1998; Bolger et al. 2000, 2008), (2) non-native grass invasion (Wolkovich et al. 2009a), (3) fire (Matsuda et al. 2011), or (4) the ecology and impacts of the invasive Argentine ant (Suarez et al. 1998; Holway et al. 2002; Menke and Holway 2006) on native arthropod diversity and community composition. While these studies have informed our understanding of how arthropod communities respond to a variety of disturbances that shape southern California ecosystems (e.g., native arthropod diversity, excluding spiders, correlates positively with fragment size and negatively with isolation; Suarez et al. 1998; Bolger et al. 2000, 2008), we still do not understand how arthropod communities are structured in areas that have type-converted from SS to non-native grasslands or suburban/urban areas. In addition, while SS fragments are found across much of southern California (e.g., Orange, Los Angeles, Riverside, Ventura, Santa Barbara, and western San Bernardino counties), some as far as 80 km from the coastline (Rundel 2007), most research on arthropods has been concentrated in coastal (within ~15 km the coast) San Diego County (Suarez et al. 1998; Bolger et al. 2000; Wolkovich et al. 2009a; Matsuda et al. 2011; but see Holway et al. 2002). Interior SS fragments are climatically different and can host distinct SS plant communities (Riordan and Rundel 2009). In addition, these interior SS fragments are typically more arid, which could make them more resistant to Argentine ant invasion (Holway et al. 2002). Determining which habitats support various native ant species and how the proximity to the coast influences arthropod community composition contribute to a broader understanding of how species are distributed throughout southern California and which may be at risk of local and regional expatriation. In addition, this approach provides novel information critical to addressing the role of native SS fragments in preserving regional biodiversity, and whether non-native habitat types offer any regional conservation significance.
In this study, we examined differences in ant species richness and composition in three major southern California habitat types: SS, non-native grassland, and suburban areas. We focused on the ant fauna because ants are the most extensively studied arthropod taxon in the region, the fauna is well described and understanding how ant species utilize the various habitat types may help to inform a broader understanding of the distributions of other arthropod assemblages in southern California. We exploited the proximity of the three habitat types at and adjacent to the Robert J. Bernard Field Station (BFS) located in eastern Los Angeles County, CA to mitigate the influence of confounding variables such as dispersal or climate variation. Our objectives were to survey and describe the ant communities in each habitat type, and determine which species depend on a specific habitat type. In addition, we compared the ant communities at the BFS to the 40 fragments surveyed by Suarez et al. (1998) in San Diego County in an initial effort to understand how ant communities in more northern, inland, and xeric SS fragments may differ.
Methods
Study site
Sampling was conducted at the Robert J. Bernard Biological Field Station (BFS) and in the surrounding suburban area. The BFS comprises approximately 35 ha in the town of Claremont, CA, near the eastern edge of Los Angeles County (Online Resource 1; map of study area). It sits at the foot of the San Gabriel Mountains and receives an average annual rainfall of 45.5 cm. It is important to note that rainfall is extremely variable among years. The average summer maximum and winter minimum temperatures are 32 and 4 °C, respectively, and over 90 % of the precipitation occurs in the winter months. As such, plant growth is restricted by low light intensity and cooler temperatures in the winter and drought in the summer.
The BFS harbors two distinct habitat types: native SS and non-native grassland (Online Resource 2; pictures of these two habitat types). The SS covers ~25 ha and is relatively intact with interstitial areas between plants consisting of bare ground and soil crusts typical of intact SS (Mooney 1977). The drought-deciduous shrubs coastal sagebrush (Artemisia californica), yerba santa (Eriodictyon trichocalyx), and flat-top buckwheat brush (Eriogonum fasciculatum) are the three most abundant plant species. The California SS ecosystem is broken into different plant associations: coastal sage scrub, interior sage scrub and island sage scrub (Rundel 2007). While the BFS has plant species (e.g., Rhus integrifolia and Malosma laurina) that are typical of the coastal sage scrub association and low abundances of other species (e.g., Saliva apiana) that typify interior sage scrub communities, broad alluvial fans that flank the San Gabriel Mountains support a plant community that is typically considered within the broader interior sage scrub association (Rundel 2007). As such, the BFS represents an interesting study site because it is more arid, but is relatively similar in shrub composition to SS sites closer to the coast. The non-native grassland is small (~3.5 ha) but persistent (>40 years in age). It is dominated by non-native grasses (Bromus spp.), which are active between autumn and spring. European grasses became dominant in this area following removal of a citrus orchard sometime before 1976. The two remaining portions of the BFS include: (1) an area with a few buildings surrounded by non-native landscaping (~2 ha) and (2) an area where non-native grasslands were being recolonized by native shrubs (~5.5 ha). A fire in September 2013 completely burned the area being recolonized by shrubs, but did not affect any intact SS or non-native grassland sites in this study.
The BFS is surrounded on all sides by human modified landscapes that receive regular water subsidies through irrigation. To the north and east of the BFS are suburban neighborhoods where yards and gardens are the only unpaved habitat. South of the BFS are the Claremont Colleges and residential areas, which are similarly interspersed with gardens and lawns typical of suburban Los Angeles County. On its west side, the BFS borders Rancho Santa Ana Botanic Garden (RSABG), a nationally recognized institution dedicated to the study and conservation of California native plants (www.rsabg.org). RSABG cultivates native California plant species, but these species come from across the state and collections do not represent the local native vegetation. In addition, RSABG is subject to regular irrigation and maintenance. As such, RSABG and the area around the buildings within the BFS that have non-native landscaping and receive water subsidies more closely reflect garden conditions than native habitat. Though many habitat characteristics, such as plant community composition and management history (e.g., soil and nutrient amendment schedules and planting regimes), were highly variable among these landscapes, they were all grouped together in the suburban category due to the strong effect of human modification and irrigation in promoting Argentine ant invasion (Human et al. 1998; Menke and Holway 2006).
Sampling protocol
Pitfall trapping was conducted at 40 plots over five 2-week periods at approximately 3-month intervals in order to observe seasonal and inter-annual variation in the ant community (spring 2013: 29–30 March to 11–12 April; summer 2013: 1–3 to 15–17 July; autumn 2013: 28 September to 12 October; winter 2014: 14–16 to 28–30 January; and spring 2014: 12–14 to 26–28 March). Sixteen plots were distributed systematically across the SS habitat, each at least 75 m from its nearest neighbor. Eight plots were distributed systematically throughout the non-native grassland, but were closer together (~40 m from its nearest neighbor) due to the smaller size of the patch. The remaining sixteen plots were in the suburban habitat and ranged from ~30 to 1000 m in distance from the BFS. These sites were chosen because of access and because they were representative of the variety of suburban environments.
Each plot contained three pitfall traps and most sites (excluding many in the suburban habitat where sidewalks and other obstacles prevented it) were configured so the three pitfall traps formed a north-pointing equilateral triangle with each trap 10 m from the others. Using the trap design employed by Higgins (2010), pitfall traps were constructed so they could remain in the field for 2 weeks between charging of a trap and collection of specimens. Each pitfall trap consisted of a heavy Pyrex glass “test tube” (3.2 cm diameter, 25 cm deep) inserted in a PVC sleeve (3.8 cm in diameter, 28 cm long) buried in the ground. The opening of each trap was flush with the soil surface. When charged, test tubes were half filled with ~75 ml of propylene glycol, which is non-toxic and is an excellent short-term preservative for arthropods. Each pitfall trap was covered with a PVC rain shield with holes ~3–4 cm above the ground to allow arthropods access while restricting access to most vertebrates. Between sampling periods, PVC sleeves were capped to prevent them from filling with dirt or inadvertently capturing vertebrates.
Following collection, pitfall trap contents were transferred into 80 % ethanol. Ants were separated from each pitfall trap using a dissecting microscope. All individuals were identified to the lowest taxonomic level possible, which was most often to species. Ants collected at the BFS were compared to those collected by Suarez et al. (1998) (kept in a reference collection maintained by Phil Ward, University of California at Davis) to confirm consistency of species identification. All specimens collected in this study are stored in the Bernard Field Station Invertebrate Collection.
Analyses
Difference among habitats and seasons
To determine how well we inventoried ant species richness in each habitat type, percent inventory completeness was determined by calculating the ratio of total collected species to predicted species present using the non-parametric estimator Chao 2 function, executed in PRIMER v.6 (Clarke and Gorley 2006). The Chao2 function is less sensitive to problems related to sample coverage, species distribution, or variation in species capture probability than other estimators (Colwell et al. 2004).
To investigate whether there were differences in ant communities among habitats and among seasons, we performed multiple analysis of similarity (ANOSIM) tests (a multivariate permutation-based hypothesis test) using PRIMER v.6. Prior to running each ANOSIM test, a resemblance matrix was generated using the Bray-Curtis coefficient, which is preferred to Euclidean distance because it does not treat the absence of a species at two sites as an indication of similarity (Legendre and Legendre 1983). Each resemblance matrix was calculated using the proportion of traps occupied by each species at each plot (number of traps occupied/number of operational traps at each plot). We did this because: (1) the number of ants caught in a pitfall trap is dependent on the proximity of the trap to the colony entrance, (2) some pitfall traps were destroyed during each season (range 2.5–9.2 %) and removed from the analysis, and (3) differences in ant activity may confound estimates of abundance based on numbers of individuals collected in a trap. Thus, using the proportion of traps occupied at each plot allows for estimation of which species are present and widespread at a given site.
To test for differences among (1) habitats while controlling for differences among seasons and (2) seasons while controlling for differences among habitats, we ran a Two-Way Crossed ANOSIM test (9999 permutations) using habitat and season as factors. This analysis is a permutation analogue to a complete randomized two-factor ANOVA that does not require the assumption of equal variances. Pair-wise differences among treatments (differences among habitats within seasons and differences among seasons within habitats) were examined only following a significant ANOSIM test. We adjusted α-values for multiple testing using the conservative Bonferroni correction procedure (three pairwise habitat comparisons: α = 0.016; ten pairwise season comparisons: α = 0.005). We also generated a multi-dimensional scaling (MDS) plot to visualize differences among all plots in each habitat (i.e. using the data from all five seasonal collections).
To further investigate differences in ant communities among habitats and seasons, we ran multiple One-Way ANOSIM tests (9999 permutations). We ran five One-Way ANOSIM tests using habitat as a factor to examine differences among habitats in each season (spring, summer, autumn 2013; winter, spring 2014). We also ran three One-Way ANOSIM tests using season as a factor to examine differences among seasons in each habitat type (SS, non-native grassland, suburban). The α-values were adjusted for multiple testing for the ANOSIM tests examining differences among habitats, ANOSIM tests examining differences among seasons, and for each set of pairwise comparisons (ten pairwise season comparisons within each habitat: α = 0.005; three pairwise habitat comparisons with each season: α = 0.016). To visualize differences among habitat types in each season we generated five MDS plots, one for each season.
Following significant One-Way ANOSIM tests we ran One-Way Similarity Percentage (SIMPER) analyses. These analyses quantified the relative contributions of each individual species in driving the observed differences between habitats and seasons.
Comparisons between the BFS and San Diego SS fragments
To compare the ant community at the BFS to the communities in San Diego SS fragments, we created a species presence/absence matrix based on our collections and the species Suarez et al. (1998) recorded at 40 SS fragments and the larger “core area” at Elliot Chaparral Reserve. Our data included ants from both SS and grassland habitats within the BFS, but excluded ants only collected in the suburban areas. Using this matrix, we generated a resemblance matrix in PRIMER v.6 using the Bray-Curtis coefficient. To test for differences in ant community composition between San Diego and the BFS, we ran a One-Way ANOSIM test (only 42 possible permutations, since we had only one inland site: BFS) with location (BFS or San Diego) as a factor. We also generated an MDS plot to visualize differences between the ant community at the BFS and those in each San Diego fragment.
To further facilitate comparisons of diversity between the BFS and the San Diego fragments, we examined the species richness per unit area at the BFS and compared it with what was reported in coastal San Diego fragments by Suarez et al. (1998). Suarez et al. (1998) did not distinguish between SS and grassland habitat, but instead reported the total area and the percent of native vegetation, which ranged from 5 to 90 %. Since the SS and non-native grassland habitats are clearly delineated at the BFS, we combined the areas of the SS and grassland habitats to obtain the total area.
Results
In total, we collected 9810 ants and recorded 27 species and 17 genera (Table 1). Only two known non-native species were collected: L. humile (the Argentine ant) and Cardiocondyla maruitanica, native to South America and subtropical regions of the old world (Africa and Asia), respectively. However, at least three species (Camponotus sp1, Nylanderia vividula, and Stenamma punctatoventre) are typically collected in woodlands or human-modified landscapes (AntWeb 2015) and are probably not native to dry southern California SS. In addition, Brachymyrmex sp1 has not previously been reported in California (Ward 2005; AntWeb 2015) and is suspected to be non-native.
Difference in ant communities among habitats and seasons in and adjacent to the Bernard Field Station
Comparison of observed species richness to the number of species predicted to be in each habitat by the Chao2 estimator indicates >90 % inventory completeness for the non-native grassland and suburban habitat types, but only 65.2 % completeness in the SS habitat (Table 2).
The Two-Way ANOSIM test revealed that ant communities differed among habitats (R = 0.474, P = 0.0001) and seasons (R = 0.066, P = 0.0002). Pairwise comparisons revealed that all three habitat types harbored distinct ant communities (Table 3). One-Way ANOSIM tests examining differences among habitats in each season showed differences were consistent across seasons: spring 2013 (R = 0.352, P = 0.0001), summer 2013 (R = 0.517, P = 0.0001), autumn 2013 (R = 0.562, P = 0.0001), winter 2014 (R = 0.441, P = 0.0001), spring 2014 (R = 0.498, P = 0.0001) (Table 4). MDS plots for each season and the five seasons combined showed clear clustering according to habitat, indicating that each habitat type harbors a unique ant community (Fig. 1; Tables 1, 2).
MDS ordination of sites showing differences in ant composition and abundance among habitat types in each season, a spring 2013, b summer 2013, c autumn 2013, d winter 2014, e spring 2014, and f all seasons combined. Proximate sites are more similar in ant composition and abundance. Ovals encompass all suburban sites. Three sites (two grassland and one SS) were removed from the MDS plots to allow better visibility of the main clusters
One-Way ANOSIM tests examining differences among seasons in each habitat found that ant communities significantly differed among seasons in SS (R = 0.122, P = 0.007) and non-native grassland (R = 0.107, P = 0.033) habitat types, but not the suburban habitat (R = −0.001, P = 0.471) (Table 5).
SIMPER analyses revealed that differences in the abundances of Argentine ants (L. humile) and parasitic thief ants (Solenopsis molesta (sensulato)) were the primary drivers of dissimilarity among habitat types (Online Resources 3–7, SIMPER tables examining the contribution of the various ant species to the dissimilarity among the three habitat types for each of the five seasons). Argentine ants appeared in 94–100 % of suburban sites, compared to 21–53 % of SS sites and just 0–9 % of grassland sites (Fig. 2a). The proportion of pitfall traps occupied by Argentine ants and the number of Argentine ants per occupied trap followed similar patterns (Fig. 2b, c). The presence or absence of native ant species was also an important driver of differences among habitats (Online Resources 3–7) and seasons (Online Resources 8, 9, SIMPER tables examining the contribution of the various ant species to the dissimilarity among seasons within the sage scrub and non-native habitat types). Seventeen species (59 %) were found in just one of the three habitat types. Six (Brachymyrmex depilis, Veromessor chamberlini, Myrmecocystus testaceus, Myrmecocystus wheeleri, Pheidole pilifera, and Temnothorax nevadensis) were only collected in the SS, three (Veromessor andrei, Myrmecocystus mimicus, and Pheidole cerebrosior) in the non-native grassland, and eight (Brachymyrmex sp1, Camponotus sp1, Cardiocondyla mauritanica, Hypoponera opacior, Monomorium ergatogyna, N. vividula, Prenolepis imparis, and S. punctatoventre) in the suburban habitat. Four species were rare, appearing at only a single plot in a single season: B. depilis, M. wheeleri, and P. pilifera in the SS and Camponotus sp1 in the suburban habitat.
Comparisons between the BFS and San Diego SS fragments
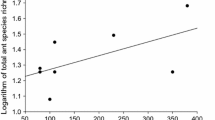
The ant community at the BFS (which includes both the intact SS and non-native grassland, but excludes the suburban habitat) significantly differed from the communities in SS fragments (which were similarly characterized by a mixture of native and non-native vegetation, ranging from 5 to 90 % native SS cover) in San Diego (R = 0.631; P = 0.048) surveyed by Suarez et al. (1998; Fig. 3). Of the 19 species we collected at SS and non-native grassland sites at the BFS, four (19 %) were not reported by Suarez et al. (1998) in San Diego fragments or the Elliot Reserve (Table 1). Also, using the data from San Diego County as a baseline, the BFS had higher species richness than projected for a fragment of its size (Fig. 4).
MDS ordination showing differences in ant compositions between SS fragments in San Diego surveyed by Suarez et al. (1998) (empty circles) and the BFS (filled circle)
Discussion
Our study demonstrates that different low elevation habitat types in Los Angeles County harbor unique ant communities. Of the 19 species at the BFS (SS and non-native grassland habitats combined), ten (53 %) were never collected in the suburban habitat, and six (32 %) and three species (16 %) were collected exclusively in the SS and non-native grassland, respectively. While the developed Los Angeles basin is classified as SS (Küchler 1977), reconstruction of the early Spanish explorations in the late 1700’s suggest that much of this area was covered with extensive fields of flowers (native forbs and native perennial grasses; Minnich 2008). As such, it seems likely that some native ant species may be better adapted to persist in habitats with structure similar to non-native grasslands. Our study supports this assertion, with two species restricted to the non-native grassland (M. mimicus and V. andrei) being closely related congeners of species that were only collected in SS (M. testaceus, M. wheeleri and V. chamberlini). In addition, V. andrei, which was restricted to the non-native grassland in our study, has been shown to increase in abundance in recently burned SS sites, which often have more non-native grasses and less shrub cover due to differences in rates of recolonization (Matsuda et al. 2011).
Comparisons between SS and non-native grasslands have been limited, in part because researchers frequently classify mixed fragments as SS even when a significant percentage (75 % cover or more) of the vegetation is non-native (Suarez et al. 1998; Matsuda et al. 2011). Understanding how ant communities will be influenced following type conversion from SS to non-native grassland is valuable for informing restoration and management efforts. For example, Suarez et al. (1998) found a negative correlation between the amount of non-native vegetation and the number of native ant species, and Wolkovich et al. (2009a) found that the abundance of several common arthropods, including native ant species, declined in SS after invasion by non-native grasses. Together, these results suggest that non-native grasses may reduce ant richness. However, species richness at the BFS increases when the SS and non-native grassland habitats are combined, since the grassland contributes novel species. In most other studied sites, the space between SS shrubs is filled with non-native grasses (e.g., Suarez et al. 1998; Wolkovich et al. 2009a, b; Matsuda et al. 2011). In contrast, the BFS has two distinct habitat types, with the SS differing from the non-native grassland in that much of the area between plants is open. As such, our study is unique in its ability to tease out which ants utilize intact SS habitat and type-converted non-native grasslands, and suggests that both habitat types are of conservation significance and may be required to preserve maximum native ant diversity in the region.
In addition to modifications of habitat structure that follow non-native grass incursion, Wolkovich et al. (2009a) suggested that ant species specialized to arid systems may be more affected by non-native grass invasions than more widespread ant species that may be able tolerate the cooler and moister microclimate. This hypothesis was largely driven by two observations by Wolkovich et al. (2009a, b, 2010): (1) increased litter associated with non-native grass invasions increases soil moisture and reduces soil temperatures, and (2) that abundances of Forelius mccooki and Pheidole vistana, both arid specialists, decreased in areas of high grass invasion. In contrast to this pattern, we collected F. mccooki in a higher proportion of traps in the non-native grassland than SS. This suggests that the effects of grass invasion on native ants may differ among SS sites. While it has not been quantified, incident solar radiation and, consequently, temperature are presumably higher at the soil surface in the non-native grassland than the SS at the BFS due to lack of shrub cover. As a result, the non-native grassland may be more arid than the SS, particularly in the summer and autumn months. In the rainier winter and spring months, soil moisture may also be depleted more quickly in the non-native grassland than the SS due to the higher rate of transpiration by grasses compared to shrubs (Clary et al. 2004).
Understanding the physical environment is also critical in predicting the influence of non-native Argentine ants on the native ant fauna (Holway et al. 2002). The non-native grassland and, to a lesser extent, the SS at the BFS seem to be effective at limiting the distribution and abundance of Argentine ants. On average, Argentine ants were found in just 2.6 % of pitfall traps in non-native grassland sites and 32.8 % of traps in the SS, while they were captured in 87 % of traps in the suburban habitat. In addition, average numbers of Argentine ants in pitfall traps (averages were calculated using only pitfall traps that captured Argentine ants) were much lower in both SS and non-native grassland habitat types, indicating that Argentine ant colonies are smaller and/or are less active in these habitats. These results are consistent with those of Holway et al. (2002) who found that in more xeric habitats Argentine ants are at low enough densities that native ants remain largely unaffected. Bolger (2007) suggested that Argentine ants may increase in abundance in habitat fragments when conditions are temporarily favorable, such as following seasonal rains. While we observed this pattern in the non-native grassland, where Argentine ants were only collected in the spring, in the SS the percentage of sites and traps in which Argentine ants were present seemed relatively consistent among seasons. In both non-suburban habitats, abundances remained well below two Argentine ants per trap day, a threshold that seems to have a significant impact on native ant richness (Suarez et al. 1998). The extremely low Argentine ant abundance in the non-native grassland at the BFS suggests that this habitat type may be especially good at providing a refuge for some native ant species in xeric areas.
The suburban habitat, which is typified by the presence of widespread and abundant Argentine ants, is surprisingly rich. For instance, we collected more ant species (17) at suburban sites than in any other habitat type. While eight species were unique to the suburban habitat type, many are most likely non-native. Several are commonly found in mesic woodland/riparian areas (e.g. those in the genera Camponotus, Hypoponera, and Stenamma) or disturbed habitats (e.g. N. vividula) across much of North America (Ward 2005; AntWeb 2015). These species are: (1) probably not adapted to the aridity of SS in eastern Los Angeles County and (2) dependent on suburban arboriculture and water subsidies for survival. This suggests that at least five (including the known non-native C. maruitanica) of the eight ant species that were only collected in the suburban environment are probably non-native. In addition to having more non-native species than the other habitat types, the suburban habitats also had more litter dwelling (hypogeic) species, such as those in the genera Brachymyrmex, Hypoponera, and Stenamma, which have been found to be more tolerant of Argentine ants than epigeic species (Ward 1987; Suarez et al. 1998). Of the native epigeic species found in the suburban habitat, several (e.g. Dorymymex insanus, Pheidole hyatti, Pogonomyrmex californicus and Solenopsis xyloni) were sparsely distributed, often only within a few hundred meters of the field station. Further studies are needed to ascertain if these species would be present in suburban areas if they were they not immediately adjacent to source populations such as the BFS.
Significant seasonal differences in the ant community were observed in both the SS and non-native grassland habitats. Limited seasonal turnover in the ant community in the suburban habitat is probably due in part to the presence of water subsidies, which can mitigate climatic extremes, such as increases in temperature and drought. The ant community in SS during the summer 2013 collection differed from most other seasons because most ant species were collected in a higher proportion of the pitfall traps. However, ant communities in the summer 2013 and winter 2014 exhibited the strongest differences, highlighting that a small subset of ants are more active in cooler months. Because various ant species in SS are active during different seasons and many ant species are patchily distributed, our sampling approach, once each season for five seasons, was insufficient to obtain a complete inventory of the SS ant community. Increased sampling effort within years and over multiple years to account for inter-annual variation in SS habitats is required to obtain a complete species inventory.
Even without a complete species inventory, it is evident that interior SS fragments, like the BFS, probably harbor more diverse ant communities with distinct ant assemblages. We collected 19 species of ants at the BFS, which is more than would be expected in a coastal SS fragment of similar size. This is consistent with the finding that xeric SS fragments have similar species richness as similar sized plots within unfragmented areas, while species richness is often reduced in more mesic SS fragments (Holway et al. 2002). Furthermore, we found four species that were not reported by Suarez et al. (1998) in the 40 SS fragments and Elliot Chaparral Reserve in San Diego. Of these, one, P. cerebrosior, was collected by A.V. Suarez in the San Diego area as part of a broader sampling effort (P.S. Ward personal communication), and another, M. mimicus, was collected in the San Diego National Wildlife Refuge, Sweetwater Unit by Wolkovich et al. (2009a). While further surveys are certainly required to determine whether or not the other two species, V. chamberlini and M. wheeleri, are actually absent from coastal San Diego SS fragments, finding increased species richness and any novel ant species in a survey of just one inland fragment is suggestive of marked differences in ant composition, diversity, abundance, and/or activity between coastal and inland fragments, and that further surveys of interior SS fragments will likely result in new species occurrences.
To what extent differences in ant community composition and diversity between coastal and inland fragments may be attributable to abiotic conditions, susceptibility to Argentine ant incursion, or interactions between these two factors requires further investigation. For example, surveys in inland and more northern SS patches are necessary to establish an accurate account of ant diversity across the entire range of the SS ecosystem. Additionally, surveys that categorize sites as SS or non-native grassland are needed throughout the region to better elucidate how different ant species utilize these habitat types. The extent to which increases in species richness are influenced by the ability of the Argentine ant to invade a habitat patch also warrants further investigation. Suarez et al. (1998) reported that Argentine ants typically excluded all but a few tolerant native species within 200 m of fragment edges in San Diego. Because of the odd “L” shape of the BFS, there is no habitat >200 m from an edge. Higher maximum temperatures and aridity in inland, xeric sites have been shown to increase diversity by reducing the foraging success of Argentine ants relative to native species (Holway et al. 2002). Increased resistance to Argentine ant invasion, higher native ant diversity and the presence of unique ant species indicate that inland SS and non-native grassland patches are of high conservation importance.
Because many of the remaining SS fragments are threatened by type-conversion to non-native grasslands and/or conversion to suburban/urban habitats by future development (Cox et al. 2014; Goldstein and Suding 2014; Kimball et al. 2014; Riordan and Rundel 2014), understanding how such landscape transformations influence arthropod biodiversity is critical to conservation efforts at multiple scales. On a local scale, our research demonstrates that conversion of SS to non-native grassland will favor a unique subset of native ant species, while conversion to suburban habitat favors a surprisingly diverse but overwhelmingly non-native ant fauna. On a regional scale, our research highlights that non-native grasslands do provide conservation value and a mosaic of both intact SS and non-native grasslands, particularly in the absence of native grasslands, are needed to preserve maximum native ant diversity. Preservation of inland sites appears to be particularly important, as these sites offer increased resistance to Argentine ant invasion, higher native ant diversity, and unique native ant species. Because many of the native ants in this and Suarez’s et al. (1998) study have broad distributions throughout the southwest, widespread loss of SS habitat may not necessarily lead to their extinction. However, further examination of the distribution of the California endemic, V. chamberlini, is warranted since it was not found in the surveys of the San Diego area and was only collected in SS in our study. Because many southern California SS invertebrates are narrowly endemic to low elevation areas in southern California, including millipedes (Keaton 1960), pulmonate land snails (Pilsbry 1939; Roth and Sadeghian 2003), and insects (Hogue 1993), patterns of habitat specificity seen with ants may indicate that maintaining a network of inland SS and non-native grassland habitat is critical to preserving biodiversity.
References
Alberts AC, Richman AD, Tran D, Sauvajot R, McCalvin C, Bolger DT (1993) Effects of habitat fragmentation on populations of native and exotic plants in southern California coastal scrub. In: Keely JE (ed) Interface between ecology and land development in California. Southern California Academy of Science, Los Angeles, pp 103–110
AntWeb (2015). Available from http://www.antweb.org. Accessed 19 June 2015
Bolger DT (2007) Spatial and temporal variation in the Argentine ant edge effect: implications for the mechanism of edge limitation. Biol Conserv 136:295–305
Bolger DT, Suarez AV, Crooks KR, Morrison SA, Case TJ (2000) Arthropods in urban habitat fragments in Southern California: area, age, and edge effects. Ecol Appl 10:1230–1248
Bolger DT, Beard KH, Suarez AV, Case TJ (2008) Increased abundance of native and non-native spiders with habitat fragmentation. Divers Distrib 14:655–665
Branstetter MG (2012) Origin and diversification of the cryptic ant genus Stenamma Westwood (Hymenoptera: Formicidae), inferred from multilocus molecular data, biogeography, and natural history. Syst Entomol 37:478–496
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. Plymouth, PRIMER-E
Clary J, Savé R, Biel C, De Herralde F (2004) Water relations in competitive interactions of Mediterranean grasses and shrubs. Ann Appl Biol 144:149–155
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianoutsou M (1996) Plant diversity in Mediterranean-climate regions. Trends Ecol Evolut 11:362–366
Cox RL, Underwood EC (2011) The importance of conserving biodiversity outside of protected areas in Mediterranean ecosystems. PLoS One 6:e14508
Cox RD, Preston KL, Johnson RF, Minnich RA, Allen EB (2014) Influence of landscape-scale variables on vegetation conversion to exotic annual grassland in southern California, USA. Glob Ecol Conserv 2:190–203
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
DeSimone SA, Zedler PH (2001) Do shrub colonizers of southern California grassland fit generalities for other woodly colonizers? Ecol Appl 11:1101–1111
Eliason SA, Allen EB (1997) Exotic grass competition in suppressing native shrubland re-establishment. Restor Ecol 5:245–255
Goldstein LJ, Suding KN (2014) Intra-annual rainfall regime shifts competitive interactions between coastal sage scrub and invasive grasses. Ecology 95:425–435
Higgins JW (2010) Ground dwelling arthropod responses to successional endpoints: burned versus old growth pinyon-juniper woodlands. MA thesis, Northern Arizona University, USA
Hogue CL (1993) Insects of the Los Angeles basin, 2nd edn. Natural History Museum of Los Angeles, Los Angeles
Holway DA, Suarez AV, Case TJ (2002) Role of abiotic factors in governing susceptibility to invasion: a test with Argentine ants. Ecology 83:1610–1619
Human KG, Weiss S, Weiss A, Sandler B, Gordon DM (1998) Effects of abiotic factors on the distribution and activity of the invasive Argentine ant (Hymenoptera: Formicidae). Environ Entomol 27:822–833
Keaton WT (1960) A taxonomic study of the millipede family Spirobolidae (Diplopoda: Spirobolida). Mem Am Entomol Soc 17:1–146
Keeley JE, Baer-Keeley M, Fotheringham CJ (2005) Alien plant dynamics following fire in Mediterranean-climate California shrublands. Ecol Appl 15:2109–2125
Kimball S, Goulden ML, Suding KN, Parker S (2014) Altered water and nitrogen input shifts succession in a southern California coastal sage community. Ecol Appl 24:1390–1404
Küchler AW (1977) The map of the natural vegetation of California. In: Barbour MJ, Major J (eds) Terrestrial vegetation of California. Wiley, New York, pp 909–938
Legendre L, Legendre P (1983) Numerical ecology. Elsevier Scientific, New York, New York
Matsuda T, Turschak G, Brehme C, Rochester C, Mitrovich M, Fisher R (2011) Effects of large-scale wildfires on ground foraging ants (Hymenoptera: Formicidae) in Southern California. Environ Entomol 40:204–216
Medail F, Quezel P (1997) Hot-spots analysis for conservation of plant biodiversity in the Mediterranean basin. Ann Mo Bot Gard 84:112–127
Menke SB, Holway DA (2006) Abiotic factors control invasion by Argentine ants at the community scale. J Anim Ecol 75:368–376
Minnich RA (2008) California’s fading wildflowers: lost legacy and biological invasions. University of California Press, Berkeley
Minnich RA, Dezzani RJ (1998) Historical decline of coastal sage scrub in the Riverside-Perris Plain, California. Western Birds 29:366–391
Mooney HA (1977) Southern coastal scrub. In: Barbour MG, Major J (eds) Terrestrial vegetation of California. Wiley, New York, pp 471–489
Pilsbry HA (1939) Land mollusca of North America north of Mexico. Volume 1, Part 1. Academy of Natural Sciences of Philadelphia, Philadelphia, USA
Riordan EC, Rundel PW (2009) Modelling the distribution of a threatened habitat: the California sage scrub. J Biogeogr 36:2176–2188
Riordan EC, Rundel PW (2014) Land use compounds habitat losses under projected climate change in a threatened California ecosystem. PLoS One 9:e86487
Roth BA, Sadeghian P (2003) Check list of the land snails and slugs of California. Santa Barbara Museum of Natural History Contributions in Sciences, no. 3., Santa Barbara, CA. USA
Rundel PW (2007) Sage scrub. In: Barbour MG, Keeler Wolf T, Schoenherr AA (eds) Terrestrial vegetation of California. University of California Press, Berkeley, pp 208–228
Sala OS, Chapin FS III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sannwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Soulé ME, Bolger DT, Alberts AC, Sauvajot R, Wright J, Sorice M, Hill S (1988) Reconstructed dynamics of rapid extinction of chaparral requiring birds in urban habitat islands. Conserv Biol 2:75–92
Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal Southern California. Ecology 79:2041–2056
Talluto MV, Suding KN (2008) Historical change in coastal sage scrub in Southern Californica, USA in relation to fire frequency and air pollution. Landsc Ecol 23:803–815
Tillberg CV, Holway DA, LeBrun EG, Suarez AV (2007) Trophic ecology of Argentine ants in their native and introduced ranges. PNAS 104:20856–20861
Underwood EC, Viers JH, Klausmeyer KR, Cox RL, Shaw MR (2009) Threats and biodiversity in the Mediterranean biome. Divers Distrib 15:188–197
Ward PS (1987) Distribution of the introduced Argentine ant (Iridomyrmex humilis) in natural habitats of the lower Sacramento Valley and its effects on the indigenous ant fauna. Hilgardia 55:1–16
Ward PS (2005) A synoptic review of the ants of California (Hymenoptera: Formicidae). Zootaxa 936:1–68
Westman WE (1981) Diversity relations and succession in Californian coastal sage scrub. Ecology 62:170–184
Wolkovich EM, Bolger DT, Holway DA (2009a) Complex responses to invasive grass litter by ground arthropods in a Mediterranean scrub ecosystem. Oecologia 161:697–708
Wolkovich EM, Bolger DT, Cottingham KL (2009b) Invasive grass litter facilitates native shrubs through abiotic effects. J Veg Sci 20:1121–1131
Wolkovich EM, Lipson DA, Virginia RA, Cottingham KA, Bolger DT (2010) Grass invasion causes rapid increases in ecosystem carbon and nitrogen storage in a semiarid shrubland. Glob Change Biol 16:1351–1365
Acknowledgments
This study was made possible by funding from the Howard Hughes Medical Institute’s 2012 Colleges Initiative (HHMI Grant #52007555) and the Department of Biology at Pomona College and was conducted with permission by the Claremont Colleges’ Robert J. Bernard Field Station. We would like to thank Nina Karnovsky for reviewing earlier versions of this manuscript and Kim Franklin who helped with species identification. Phil Ward and Matthew Prebus provided access to the voucher specimens deposited by Andrew Suarez at the Bohart Museum of Entomology at the University of California, Davis. The authors are grateful to Ashish Streatfield, Kristen Park, Taylor Montgomery, Jeanette Rios, Brenna Gormally and Madeline Bossi for help setting up pitfall traps and processing trap contents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Staubus, W.J., Boyd, E.S., Adams, T.A. et al. Ant communities in native sage scrub, non-native grassland, and suburban habitats in Los Angeles County, USA: conservation implications. J Insect Conserv 19, 669–680 (2015). https://doi.org/10.1007/s10841-015-9790-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-015-9790-5