Abstract
A mismatch of resource availability in certain periods can lead to spillover of insects between habitats, resulting in temporal differences in insect diversity. Urban gardens are important anthropogenic habitats but it is unknown whether, when and why spillover of beneficial insects occurs between gardens and agricultural habitats. We used trap nests for Hymenoptera to monthly monitor bee and wasp abundance and species richness in 12 gardens and 12 rapeseed fields. Half of the gardens and rapeseed fields were located in the urban–rural interface and bordered each other (a garden paired with a rapeseed field) and the other half were isolated in the rural landscape (isolated rapeseed fields) and in the urban city centre (isolated gardens). In general, gardens in the urban–rural interface comprised the highest richness of bees and wasps. The abundance of bees but not of wasps was highest in paired habitats and peaked at full rapeseed blooming, indicating that mass-flowering rapeseed offers foraging resources for bees nesting in adjacent gardens. Thus, bees nest and increase their populations in both areas, benefiting from the mass-flowering resource in the agricultural habitat as well as the nesting resources from gardens, suggesting spillover of bees but not of wasps between paired gardens and rapeseed fields. Our study highlights the value of gardens in the urban–rural interface for the biodiversity of functionally important insects. Implementing urban gardening and small-scale agriculture in cities and suburban habitats can promote local pollinator populations and benefit adjacent croplands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization and agricultural intensification lead to an increase of anthropogenic landscapes and a simultaneous loss of natural and semi-natural habitats (Grimm et al. 2008; Rand et al. 2006; Samnegård et al. 2011). Many studies investigated the effects of urbanization on population changes between urbanized and natural ecosystems of various animal taxa (e.g. Croci et al. 2008; Kadlec et al. 2008; Magura et al. 2009; Schüepp et al. 2011; Staniforth 2002; Zanette et al. 2005). These studies show that urbanization can either increase or decrease species richness depending on the taxonomic group, spatial scale or intensity of urbanization (McKinney 2008).
A negative effect of urbanization and destruction of natural habitat was observed for bees and wasps (Grimm et al. 2008; Samnegård et al. 2011; Saure 1996; Steffan-Dewenter 2002), which are important insects for pollination and predation services (biological control) (Steffan-Dewenter 2002). However, some studies in urban landscapes showed that even in a densely populated urban area, valuable resources for these functionally important insects are available (Fetridge et al. 2008; McIntyre and Hostetler 2001; Saure 1996; Zanette et al. 2005). This highlights the importance of urban green spaces for biodiversity conservation (Fetridge et al. 2008; Matteson and Langellotto 2011).
Anthropogenic landscapes are mosaics of habitats with different qualities, for example agricultural fields in rural areas bordering natural or semi-natural habitats. Shared boundaries between these habitats increase steadily through fragmentation caused by urban expansion and agricultural land-use intensification (Watling and Orrock 2010). The flow of organisms between different habitats across these boundaries can have important effects on food web dynamics (Blitzer et al. 2012; Tscharntke et al. 2005). The cross-edge flow of insects due to high productivity and availability of resources in one of the two adjacent habitats in certain time periods is termed spillover of insects (Rand et al. 2006). Spillover leads to temporally variable and distinct diversity and abundance of insects in each habitat, which may influence ecosystem functions mediated by insects such as herbivory, parasitism, pollination and predation (Blitzer et al. 2012; Rand and Louda 2006; Rand et al. 2006).
On the one hand, natural and semi-natural habitats enhance insect diversity and their ecological functions in adjacent agricultural fields by offering diverse forage resources and nest sites that are not available in crop monocultures (Bianchi et al. 2008; Garibaldi et al. 2011; Rand and Louda 2006; Samnegård et al. 2011; Steffan-Dewenter 2002). On the other hand, the availability of resources in crop fields at certain times of the year can also benefit insect diversity in the surrounding landscape (Tscharntke et al. 2005). Especially mass-flowering crops like rapeseed (Brassica napus L.) provide substantial forage resources for flower-visiting and pollinating insects during bloom (Hoyle et al. 2007), therefore benefiting for example population growth of bumblebees (Westphal et al. 2009). Goulson et al. (2010) showed that the positive influence of gardens on populations of three bumblebee species extended at least 1 km into the surrounding farmland. However, spillover of trap-nesting bees and wasps in the urban–rural interface (i.e. between agricultural landscapes and urban areas) has so far not been investigated.
Urban landscapes are characterized by densely built-up areas with high levels of paved surfaces, buildings and few green spaces with generally low plant diversity. Rural landscapes are dominated by large, monoculture crop fields interspersed with semi-natural habitat such as forests and hedges. Urban and rural landscapes connect in the urban–rural interface, creating a mixture of both landscape types with different habitats such as residential gardens, urban green spaces, buildings, paved surfaces, crop fields and semi-natural and natural habitats.
Gardens at city margins are part of the urban–rural interface and their diversity of ornamental, vegetable but also native plants may play a positive role for the diversity of insects (Goulson et al. 2002). Pawelek et al. (2009) could show that bee species richness increased when gardens were specifically designed to harbour plant species that benefitted wild bees. Gardens can therefore enhance ecosystem services such as pollination by bees and predation by wasps (Fetridge et al. 2008; Goddard et al. 2010; Goulson et al. 2010; Pawelek et al. 2009; Samnegård et al. 2011).
To investigate the role of different habitats for the diversity of solitary bees and wasps in an urban–rural interface, we focused on the spillover of trap-nesting bees and wasps between paired gardens and rapeseed fields, addressing the following hypotheses:
-
(1)
Gardens and rapeseed fields in the urban–rural interface comprise higher diversity of bees and wasps than isolated gardens (in the city centre) and isolated rapeseed fields (isolated from urban areas);
-
(2)
Bees and wasps spill over from paired gardens to rapeseed fields in the urban–rural interface, due to the mass-flowering of rapeseed fields in late spring;
-
(3)
Landscape and site variables influence bee and wasp abundance and species richness differently, during and after the peak of rapeseed blooming.
We chose trap-nesting bees and wasps as target organisms since they play a functional role as pollinators or predators in many ecosystems and because they are bioindicators, limited by the availability of nesting and foraging resources, making them sensitive to habitat changes (Gathmann and Tscharntke 1999; Klein et al. 2006).
Materials and methods
Study area and site selection
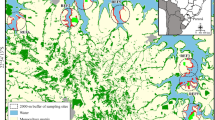
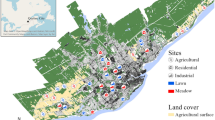
The study was conducted in the city of Lüneburg (Lower-Saxony, northern Germany) and its surrounding landscape (53°15′9″N, 10°24′52″E) (Fig. 1). The city of Lüneburg comprises around 73,000 citizens with a density of 1,045 inhabitants per km2 (Landesbetrieb für Statistik und Kommunikationstechnologie Niedersachsen–Bevölkerungsfortschreibung). Lüneburg encompasses approximately 70 km2 and includes a variety of small (50 m2) to medium sized (600 m2) private gardens with grassy areas, ornamental and vegetable plants. Gardens at the edge of the city are similarly managed to gardens in the urban city centre but are generally larger in size (600–2,000 m2) and are often bordering the rural landscape directly. The rural landscape can be described as a mosaic of pine and mixed beech forests, arable fields dominated by oilseed rape and grasslands including heathland with several villages interspersed.
Map of the study area and locations of sites along the urban–rural gradient in Lüneburg, Germany. Isolated gardens are located in the city centre; paired gardens and fields are gardens and rapeseed fields as part of the urban–rural interface; isolated fields are rapeseed fields located in rural area, isolated from the city
Gardens and rapeseed fields were selected as habitat types (Fig. 1). They were either isolated from each other or paired (bordering each other directly). Isolated gardens (N = 6) were located in the urban city centre and isolated rapeseed fields (N = 6) were located in the rural landscape 2–3 km away from the urban area and surrounded by other crop fields. The paired gardens (N = 6) and rapeseed fields (N = 6) were part of the urban–rural interface. These paired habitats were chosen to investigate spillover between these habitats. For now on we will refer to paired gardens as the component gardens, and paired rapeseed fields, as the component rapeseed fields, of the paired habitats. All pairs and isolated sites were separated from each other with a minimum distance of 1 km (Fig. 1). Brassica napus L. (rapeseed) was selected as our target crop field because it provides mass resources like pollen and nectar for many insects during its peak flowering period.
Landscape and site characteristics
Landscape variables that characterize the surrounding landscape for each of the 24 study sites were calculated for a radius of 1 km using ArcGIS (ESRI 2011). This distance was selected because the average foraging distance of trap-nesting species is less than 1 km (Gathmann and Tscharntke 2002). Landscape variables were expressed as the percentage quantity within the 1 km radius. The following three landscape variables were considered: (1) percentage of arable fields, (2) percentage of sealed areas (paved surfaces) and (3) percentage of forests. The degree of urbanization was defined using the percentage of sealed areas, where high percentages equalled a high degree of urbanization.
The following three site variables were measured to characterize each study site: (1) age of the site in years, (2) size of the site (size of the connected garden area and size of each rapeseed field) in m2 and (3) number of flowering plant species per month. The size of each site was measured using ArcGIS. Size of the gardens was determined as the size of the entire joint area of gardens in the neighbourhood, limited by roads, buildings or agricultural fields. The age of each garden was determined by interviewing the garden owners to establish the time when the area was converted into a garden. Because rapeseed is an annual crop, the age of rapeseed fields was set to be 1 year.
The number of flowering plant species in gardens per month and study site was determined by identifying all plant species within a 5 m radius around each of the two trap-nest poles in June 2011. We chose the 5 m radius because the vegetative areas of some gardens, especially in the city center, were small and this radius around each trap nest already comprised the entire vegetative garden size. In order to estimate their flowering times, the LEDA trait database was used (Kleyer et al. 2008).
Trap nests for bees and wasps
Eight standardized trap nests for bees and wasps were exposed in each of the 24 study sites (total of 192 trap nests) from 15 April to 30 October 2011. Trap nests were made of a 10.5 cm diameter plastic tube filled with approximately 150 internodes (21 cm long) of common reed Phragmites australis (Cav.) Trin. The diameters of the internodes varied between 2 and 20 mm to cover nesting requirements for a wide range of species (Gathmann and Tscharntke 1999; Holzschuh et al. 2010). Four traps were fixed on one wooden post, 1.0–1.2 m above ground level. In each study site, two posts with four trap nests were exposed in an east–west orientation, with a distance of approximately 10 m between the posts.
Occupied nests were collected every 4 weeks from the end of April until the end of October in 2011. Nests were identified as occupied internodes (internodes closed with material such as sand, clay or resin containing bee and wasp nests). After each monthly collection in 2011, nests were kept at room temperature (around 22 °C) in the laboratory to rear the specimens until the early autumn 2011. For overwintering species, nests were placed at 4 °C cold storage (Steffan-Dewenter and Leschke 2003; Steffan-Dewenter and Schiele 2008). In early spring 2012 (March), nests were warmed up to room temperature for hatching and species identification. The monthly collections also avoided risking the loss of individuals of bivoltine species. The collected filled internodes (nests) were replaced with empty reed internodes directly in the field. In October, the nests were collected in the beginning of the second week (as part of the monthly collection cycle) and the traps were completely removed from the sites between 25 and 30 October. All remaining reed internodes were opened to detect additional nests and found nests were treated as described above.
For each internode containing a nest, the number of brood cells was determined, reflecting the abundance of species. After rearing, bees and wasps were determined to species level. When no adults emerged, specimen was identified to genus level using nest characteristics (Gathmann and Tscharntke 1999). The species were determined using Gathmann and Tscharntke (1999) and the reference collection for trap-nesting bees and wasps of the Agroecology group of the University of Göttingen, Germany. Further literature used for species identifications were Ascher and Pickering (2014), Bellmann (2005), Müller et al. (1997), Schmid-Egger (2004) and Westrich (2011). The abundance and richness analyses for October included all nests collected in the beginning and at the end of the month.
Statistical analyses
Statistical analyses were performed using the software “R” (R Development Core Team 2012). To test whether abundance and species richness of bees and wasps differed between habitat types and location, and to identify specific differences between the landscape characteristics (Liow et al. 2001) non-linear Kruskal–Wallis comparisons and subsequent multiple comparison post hoc tests were performed separately for each month and with the selected landscape and site variables (pgirmess package) (Giraudoux 2012).
All landscape and site variables, habitat type and location were tested for correlations using Spearman rank correlation for continuous variables (Crawley 2007) and Chi squared tests for categorical variables (Crawley 2007).
Effects on the abundance and species richness of bees and wasps were analysed separately for each month, using generalized linear mixed models. The glmmADMB package (Bolker et al. 2012; Skaug et al. 2012) was used to accommodate for zero inflated and overdispersed distribution of the abundance and species richness of bees and wasps (Crawley 2007). According to Crawley (2007) and Devroye (1986) a negative binomial distribution was used. Study sites were used as random factor and habitat types, habitat location, landscape and site explanatory variables as fixed effects.
Because most variables were correlated (Online Resource 1), separate models for each variable were used when testing bee and wasp abundance and richness per month. The variables habitat type, location and number of flowering plant species were not correlated with each other. These variables were then analysed together, to investigate if they play a role on bee and wasp abundance and species richness. This model was reduced to determine which variable showed the strongest influence. Statistical significance of model improvement compared to the null model was tested using restricted maximum likelihood tests (Crawley 2007). The best model fit was determined when the explanatory variable was significantly better than the intercept and the model significantly improved from the null model (Crawley 2007).
Results
Trap-nesting bee and wasp communities along the urban–rural gradient
In total, 3,842 nests were sampled with a total of 12,248 brood cells collected from 192 trap nests (occupancy of >13 %) over the entire study period. The samples included 20 bee species (8 111 brood cells) and 44 wasp species (4,137 brood cells), with bees comprising 66 % and wasps 33 % of the total number of brood cells. The bee community included 12 species of Megachilidae (leaf-cutting bees) and 8 species of Colletidae (plasterer bees) (Table 1). The wasp community included 20 species of Eumenidae (mason wasps), 22 species of Crabronidae (mud wasps) and 2 species of Pompilidae (spider wasps) (Table 1). The most abundant bee species was Osmia rufa Linnaeus, 1758 (70 %), followed by Heriades truncorum (Linnaeus, 1758) (18 %), Osmia caerulescens Linnaeus, 1758 (2.7 %) and Hylaeus communis Nylander, 1852 (2.5 %). The most abundant wasp species was Ancistrocerus nigricornis (Curtis, 1826) (26 %), followed by Passaloecus corniger Schuckard, 1837 (9 %) and Ancistrocerus gazella (Panzer, 1798) (4 %). The caterpillar-predating species (Eumeninae) comprised 62.12 % of the wasp individuals.
During the peak of the rapeseed bloom in May, bees were much more abundant in paired compared to isolated habitats, with over 73 times more bee brood cells in paired gardens than in isolated gardens and on average only one bee brood cell in isolated rapeseed sites (Fig. 2a; Table 2). Towards the end of the rapeseed bloom in June, bee abundance decreased in paired gardens to almost half the number of brood cells but was still significantly higher in paired habitats compared to isolated habitats. After the rapeseed blooming, in July and August, bee abundance was higher in paired gardens than in paired rapeseed fields (Table 2). After the disappearance of the rapeseed flower resources, bees rely solely on the resources of the paired gardens adjacent to the rapeseed fields. Towards the end of the season in September and October, bee abundance was similar in all habitat types and locations (Fig. 2a; Table 2).
During the peak of rapeseed bloom in May, wasp abundance was highest in paired gardens, but did not differ between paired and isolated rapeseed fields (Fig. 2b; Table 2). In July, August and September, wasp abundance was similar in all habitat types and locations (Fig. 2b; Table 2). In October, wasp abundance was lowest in isolated rapeseed fields (Table 2).
Bee species richness was overall low. In May, highest species numbers were observed in paired habitats (Table 2). In July, a peak in species richness was found in paired gardens. However, in the following months, bee species richness was similar in all habitat types (Fig. 2c; Table 2).
Wasp species richness was highest in paired gardens in June, but varied over the other months and different habitat types, with peaks of six or seven species in July in isolated gardens and October in paired gardens, respectively (Fig. 2d; Table 2).
Comparisons of landscape and site variables
The number of flowering plant species and habitat type were independent from the habitat location (Online Resource 1). However, all other site and landscape variables were correlated.
Gardens and rapeseed fields in the urban–rural interface were similar in all landscape characteristics. Along the urban–rural gradient, isolated gardens have the highest percentage of sealed areas; paired gardens and rapeseed fields have similar intermediate values of sealed areas, whilst isolated rapeseed fields have the lowest percentages of sealed areas, within a 1 km radius (Table 3).
Isolated and paired gardens were similar regarding their age and the number of flowering plant species, but gardens in the urban–rural interface were on average three times as large as isolated gardens in the urban city centre. Gardens were significantly older than rapeseed fields but smaller in size, holding also a much higher richness of flowering plant species than rapeseed fields (Table 3).
Spillover and temporal effects on bee and wasp abundance and species richness
In May during the peak of rapeseed bloom, bee abundance was influenced by habitat location (paired compared to isolated) (Table 4), when highest bee abundances were found in paired habitats (see also Fig. 2a for a visualization). Towards the end of the rapeseed bloom in June, bee abundance was still significantly influenced by habitat location. After the rapeseed bloom in July and August, bee abundance decreased with increasing size of the sites (Table 4).
Temporal patterns of bee abundance (i.e. median number of brood cells) (a), bee species richness (i.e. median number of species) (c), wasp abundance (b) and wasp species richness (d) in two habitat types: garden and rapeseed field, in different habitat locations: paired and isolated, from April until October 2011. The shaded area represents the period of rapeseed flowering with the darker grey representing the peak of mass flowering. The tick marks at the x-axis represent the middle of the month. Given are median and median absolute deviations (MAD). Note that each graphic is given in a different scale
Wasp abundance increased from May to July but then decreased again, with low abundances in September (Fig. 2b). In May and July, the abundance of wasps was positively influenced by habitat location, which was highest in paired habitats. However, in June, wasp abundance was influenced by habitat type, being highest in gardens, compared to rapeseed fields (Table 4).
In May and June, bee species richness was highest in paired habitats, while in July, it was influenced by habitat type and habitat location, being highest in isolated and paired gardens and paired rapeseed fields (Table 4).
Wasp species richness was influenced by habitat location in May, which was highest in paired habitats and in June by habitat type and location, being highest in gardens and paired habitats (Table 4). Number of flowering plant species had a positive influence on wasp richness in July (Table 4).
Discussion
Trap-nesting bee and wasp communities along an urban–rural gradient
In comparison to other trap-nesting studies in cultural landscapes (Schüepp et al. 2011; Steffan-Dewenter 2002), our results showed considerably higher species richness and abundance of bees and wasps. Almost three times as many bee species and nearly twice as many wasp species were found compared to Schüepp et al. (2011), and nearly twice as many bee species and more than three times the number of wasp species observed by Steffan-Dewenter (2002). However, these studies were conducted in agricultural mosaic landscapes and did not include urban and suburban areas, where most of the species in the present study were found. In contrast to the studies in agricultural landscapes, Fetridge et al. (2008) observed 110 bee species in 21 residential gardens in New York City in a 3 years survey, where bee flower visitors were sampled using pan traps and hand collection. In our study, bee species numbers observed in isolated gardens in the urban city centre were not as high as observed by Fetridge et al. (2008), because we only sampled trap-nesting communities in a 1 year period. But the significant differences of species numbers between paired gardens and rapeseed fields we detected highlight the importance of residential gardens for bee and wasp diversity that was also shown by Fetridge et al. (2008) and Matteson and Langellotto (2011).
Even though rapeseed fields were considerably larger than garden sites, they provided only scarce provisioning resources for bees in the period after the rapeseed blooming. In contrast, gardens might provide different nesting and provisioning resources due to their heterogeneity (Goddard et al. 2010; Loram et al. 2011; Samnegård et al. 2011) over a long time period. Connected garden areas in the urban–rural interface were on average three times as large as connected garden areas in the urban landscape. Loram et al. (2008) showed that garden size is positively related to land-cover heterogeneity (including the number of trees and ponds), which might explain the differences in bee diversity we detected between gardens in urban areas and in urban–rural interface.
The majority of studies on solitary bees and wasps in urban areas were focused on highly urbanized landscapes (Matteson et al. 2008; McFrederick and Le Buhn 2006; McIntyre and Hostetler 2001; Saure 1996; Smith et al. 2006a, b; Zanette et al. 2005) whereas the urban–rural interface is still not well investigated (but see Fetridge et al. 2008, Samnegård et al. 2011). Gardens in the urban–rural interface are unique in their combination of structural and resource heterogeneity at the local scale (within the gardens) (Goddard et al. 2010; Loram et al. 2008) and also at the landscape level (Holzschuh et al. 2010; Samnegård et al. 2011). This combination of structural and resources heterogeneity at local and landscape levels describe residential gardens in the urban–rural interface as important for biodiversity of beneficial insects (Goulson et al. 2002).
Another aspect that might greatly influence the structure of the gardens and hence the abundance and diversity of bees and wasps is the social status of the garden owners. Urban gardens in this study were owned by people who were mostly full-time students or employees and the gardens were usually less cared for, not well maintained and exhibited lower plant species diversity and fewer structural elements such as trees, shrubs or hedges. In contrast, most gardens in the urban–rural interface were larger than urban gardens and were owned by retirees who dedicated more time to maintaining their gardens, making it a daily task. Therefore, gardens in the urban–rural interface exhibited higher plant species richness, more structural features like trees, hedges, shrubs or pond vegetation, providing more resources for bees and wasps (Pereira-Peixoto, personal communication).
In the urban–rural interface, gardens seemed to be a status symbol, confirming Gross and Lane (2007) and reflecting a personal relationship with nature, corroborating Goddard et al. (2010).
Spillover of bees and the role of mass flowering crops in the urban–rural interface
Our findings indicate a spillover of bees between paired gardens and rapeseed fields during the mass flowering of rapeseed, but not at other times in the study period, with exceptionally high bee abundance across the urban–rural interface. The overall abundance of bees in the urban–rural interface (paired gardens and rapeseed fields) indicates that bees from paired gardens are attracted to the adjacent mass-flowering resource and build their nests in the paired rapeseed field, resulting in an increase of the local bee population in the field. Therefore, bees benefit from the diversity of plant and nesting resources in gardens and the mass-flowering resource from the rapeseed in the blooming period. This highlights that in paired habitats, spillover is mediated by an overabundance of feeding resources in one habitat (i.e. rapeseed fields), benefiting bee populations in the other habitat without this feeding resource (i.e. garden). In turn, gardens contain a much higher diversity of plants and structural elements, therefore providing a high abundance of nesting resources compared to the rapeseed fields in the habitat pairs. Furthermore, the trap nests in the gardens might even supplement garden nesting resources. It is important to point out that we provided artificial nests which were not in the rapeseed fields before. Usually bees would not nest in the rapeseed fields (due to a lack of available cavities) but in the gardens. However, they would still spill over to the rapeseed fields for feeding resources and would return to their nesting site. By providing nesting resources in the rapeseed field, we captured a part of the pollinator community that had not started building nests in the garden but might originate from there. The increased pollinator populations in both adjacent habitat types can enhance pollination in the nearby paired rapeseed fields (Samnegård et al. 2011; Stanley et al. 2013), potentially leading to higher yield in some rapeseed varieties (Hudewenz et al. 2013).
After the rapeseed blooming, bee abundance considerably decreased in paired gardens and rapeseed fields but maintained the same levels in isolated gardens and isolated rapeseed fields. The strong decline in the paired habitats followed the disappearance of the overabundance of pollen and nectar provided by the mass flowering rapeseed field. Even though rapeseed flowering also ceased in the isolated rapeseed fields, population levels did not drop significantly because they were already at low levels. The resources still being provided by other plants (e.g. weeds or herbs in the surrounding semi-natural habitat) might have been sufficient to maintain the existing bee populations.
A spillover of wasps across the urban–rural interface in response to the mass flowering of rapeseed fields was not observed. In the paired habitats, wasp abundance was only high in gardens and did not extend across the urban–rural interface into the rapeseed fields. Solitary wasps are known to cover distances of up to 750 m (Krewenka et al. 2010). Thus, even though wasps might benefit from feeding resources in the neighbouring rapeseed fields, they may prefer nesting in more heterogenic habitats such as gardens. In addition, wasps do not collect pollen for feeding their offspring, therefore they do not depend on the mass pollen production of rapeseed fields. However, because of the wasps’ flight capacity, gardens in the urban–rural interface could still be a potential source of predator wasps for rapeseed fields in the urban–rural interface, which might promote natural pest control in crops (Matteson and Langellotto 2011; Thies et al. 2005). Wasp species diversity was positively influenced by flowering plant species richness in July, which was provided by gardens rather than rapeseed fields. Most of the wasp species we identified feed on herbivorous insects and herbivores may rely on more diverse plant resources compared to monocultures (Scherber et al. 2006).
Our results show that bees spill over from gardens to adjacent rapeseed fields during rapeseed bloom and from rapeseed fields to adjacent gardens for flower and nesting resources after the period of rapeseed blooming. These results also support Goulson et al. (2010), who show that crops cultivated within 1 km of gardens are likely to receive more visits from bumblebees, whose nests are in these gardens. Some site characteristics (age of the sites and number of flowering plant species) were more similar in similarly managed habitats (i.e. gardens versus rapeseed fields), whereas landscape characteristics (percentage of arable fields, percentage of sealed areas and percentage of forests) were more similar in paired than in isolated habitats. Hennig and Ghazoul (2012) found that at the landscape scale in urban areas, spatial factors like edge density and the extent of green area had a positive impact on bee diversity, potentially influencing their foraging behaviour and therefore potential services to plant species within the urban environment. At the local scale, plant diversity and flower abundance influenced diversity and visits of bees. The influence of habitat location on bee and wasp abundance and richness might therefore be an indirect combined effect of landscape and site characteristics (Loram et al. 2008; Wojcik and McBride 2011).
In July, the period after blooming, one isolated rapeseed field site showed a peak in wasp abundance. Most wasp species we identified from trap nests in this field were wasps feeding on caterpillars. This particular field site was located close to a small semi-natural area of trees, shrubs and grassland, which might have provided abundant prey and nest conditions, explaining the high wasp abundance.
Our study shows that gardens and rapeseed fields in the urban–rural interface both serve as source of nesting and provisioning resources for bees at different times. For wasps, rapeseed fields were of minor importance as nesting and provisioning resource, compared to gardens. We highlight the occurrence of spillover of bees in the urban–rural interface due to the mismatch of nesting and feeding resources during the mass flowering of an agricultural crop. Bee populations in the urban–rural interface benefit from the mass flowering in rapeseed fields as well as the nesting resources provided from gardens paired with rapeseed fields.
Gardens, as heterogeneous habitat, support crop pollinators especially after the harvest of the crop fields. Hence, gardens in the urban–rural interface play an invaluable role for biodiversity and also provide important ecosystem services like pollination or natural pest control, benefiting not only the garden owner but also nearby agricultural crops.
References
Ascher JS, Pickering J (2014) Discover life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). http://www.discoverlife.org/mp/20q?guide=Apoidea_species. Accessed 16 February 2014
Bellmann H (2005) Bienen, Wespen, Ameisen: Hautflügler Mitteleuropas. Kosmos Verlags-GmbH, Stuttgart
Bianchi FJJA, Goedhart PW, Baveco JM (2008) Enhanced pest control in cabbage crops near forest in The Netherlands. Landsc Ecol 23:595–602. doi:10.1007/s10980-008-9219-6
Blitzer EJ, Dormann CF, Holzschuh A, Klein AM, Rand TA, Tscharntke T (2012) Spillover of functionally important organisms between managed and natural habitats. Agric Ecosyst Environ 146:34–43. doi:10.1016/j.agee.2011.09.005
Bolker B, Skaug H, Magnusson A, Nielsen A (2012) Getting started with the glmmADMB package: http://glmmadmb.r-forge.r-project.org/glmmADMB.html. Accessed 12 February 2014
Crawley MJ (2007) The R Book. Wiley, Chichester
Croci S, Butet A, Clergeau P (2008) Does urbanization filter birds on the basis of their biological traits? The Condor 110:223–240
Devroye L (1986) Non-Uniform Random Variate Generation. Springer, New York
Environmental Survey Research Institute (ESRI) (2011) ArcGIS Desktop: release 10. Environmental Systems Research Institute, Redlands
Fetridge ED, Ascher JS, Langellotto GA (2008) The bee fauna of residential gardens in a suburb of New York City (Hymenoptera: Apoidea). Ann Entomol Soc Am 101:1067–1077. doi:10.1603/0013-8746-101.6.1067
Garibaldi LA, Steffan-Dewenter I, Kremen C, Morales JM, Bommarco R, Cunningham SA, Carvalheiro LG, Chacoff NP, Dudenhöffer JH, Greenleaf SS (2011) Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol Lett 14:1062–1072. doi:10.1111/j.1461-0248.2011.01669.x
Gathmann A, Tscharntke T (1999) Landschaftsbewertung mit Bienen und Wespen in Nisthilfen: Artenspektrum, Interaktionen und Bestimmungsschlüssel (Biomonitoring using trap-nesting bees and wasps: community structure, interactions and identification keys). Naturschutz und Landschaftspflege Baden-Württ 73:277–305
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Animl Ecol 71:757–764. doi:10.1046/j.1365-2656.2002.00641.x
Giraudoux P (2012) Data analysis in ecology: package ‘pgirmess’. Repository Cran, version 1.5.4. R package version 0.7.2.12
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98. doi:10.1016/j.tree.2009.07.016
Goulson D, Hughes WOH, Derwent LC, Stout JC (2002) Colony growth of the bumblebee, Bombus terrestris in improved and conventional agricultural and suburban habitats. Oecologia 130:267–273
Goulson D, Lepais O, O’Connor S, Osborne JL, Sanderson RA, Cussans J, Goffe L, Darvill B (2010) Effects of land use at a landscape scale on bumblebee nest density and survival. J App Ecol 46:1207–1215. doi:10.1111/j.1365-2664.2010.01872.x
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760. doi:10.1126/science.1150195
Gross H, Lane N (2007) Landscapes of the lifespan: exploring accounts of own gardens and gardening. J Environ Psychol 27:225–241
Hennig EI, Ghazoul J (2012) Pollinating animals in the urban environment. Urban Ecosyst 15:149–166. doi:10.1007/s11252-011-0202-7
Holzschuh A, Steffan-Dewenter I, Tscharntke T (2010) How do landscape composition and configuration, organic farming and fallow strips affect the diversity of bees, wasps and their parasitoids? J Anim Ecol 79:491–500. doi:10.1111/j.1365-2656.2009.01642.x
Hoyle M, Hayter K, Cresswell JE (2007) Effect of pollinator abundance on self-fertilization and gene flow: application to GM canola. Ecol Appl 17:2123–2135
Hudewenz A, Pufal G, Bögeholz AL, Klein AM (2013) Cross-pollination benefits differ among oilseed rape varieties. J Agric Sci. doi:10.1017/S0021859613000440
Kadlec T, Benes J, Jarosik V, Konvicka M (2008) Revisiting urban refuges: changes of butterfly and burnet fauna in Prague reserves over three decades. Landsc Urban Plan 85:1–11
Klein AM, Steffan-Dewenter I, Tscharntke T (2006) Rainforest promotes trophic interactions and diversity of trap-nesting Hymenoptera in adjacent agroforestry. J Anim Ecol 75:315–323. doi:10.1111/j.1365-2656.2006.01042.x
Kleyer M, Bekker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, Poschlod P, Van Groenendael JM, Klimeš L, Klimešová J, Klotz S, Rusch GM, Hermy M, Adriaens D, Boedeltje G, Bossuyt B, Dannemann A, Endels P, Götzenberger L, Hodgson JG, Jacke A-K, Kühn I, Kunzmann D, Ozinga WA, Römermann C, Stadler M, Schlegelmilch J, Steendam HJ, Tackenberg O, Wilmann B, Cornelissen JHC, Eriksson O, Garnier E, Peco B (2008) The LEDA traitbase: a database of life-history traits of the Northwest European flora. J Ecol 96:1266–1274
Krewenka KM, Holzschuh A, Tscharntke T, Dormann CF (2010) Landscape elements as potential barriers and corridors for bees, wasps and parasitoids. Biol Conserv 144:1816–1825. doi:10.1016/j.biocon.2011.03.014
Landesbetrieb für Statistik und Kommunikationstechnologie Niedersachsen: Bevölkerungsfortschreibung. http://www.lskn.niedersachsen.de. Accessed 25 January 2014
Liow LH, Sodhi NS, Elmqvist T (2001) Bee diversity along a disturbance gradient in tropical lowland forests of south-east Asia. J Appl Ecol 38:180–192. doi:10.1046/j.1365-2664.2001.00582.x
Loram A, Warren P, Gaston K (2008) Urban domestic gardens (XIV): the characteristics of gardens in five cities. Environ Manag 42:361–376. doi:10.1007/s00267-008-9097-3
Loram A, Warren P, Thompson K, Gaston K (2011) Urban domestic gardens: the effects of human interventions on garden composition. Environ Manag 48:808–824. doi:10.1007/s00267-011-9723-3
Magura T, Lövei GL, Tóthmérész B (2009) Does urbanization decrease diversity in ground beetle (Carabidae) assemblages? Glob Ecol Biogeogr 19:16–26. doi:10.1111/j.1466-8238.2009.00499.x
Matteson KC, Langellotto GA (2011) Small scale additions of native plants fail to increase beneficial insect richness in urban gardens. Insect Conserv Divers 4:89–98. doi:10.1111/j.1752-4598.2010.00103.x
Matteson KC, Ascher JS, Langellotto GA (2008) Bee richness and abundance in New York City urban gardens. Ann Entomol Soc Am 101:140–150
McFrederick QS, Le Buhn G (2006) Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol Conserv 129:372–382. doi:10.1016/j.biocon.2005.11.004
McIntyre NE, Hostetler ME (2001) Effects of urban land use on pollinator (Hymenoptera: Apoidea) communities in a desert metropolis. Basic Appl Ecol 2:209–218. doi:10.1078/1439-1791-00051
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Müller A, Krebs A, Amiet F (1997) Bienen: Mitteleuropäische Gattungen, Lebensweise. Beobachtung, Naturbuch
Pawelek JC, Frankie GW, Thorp RW, Przybylski M (2009) Modification of a community garden to attract native bee pollinators in urban San Luis Obispo, California. Cities Environ 2:1–20
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0: http://www.R-project.org/
Rand TA, Louda SM (2006) Spillover of agriculturally subsidized predators as a potential threat to native insect herbivores in fragmented landscapes. Conserv Biol 20:1720–1729. doi:10.1111/j.1523-1739.2006.00507.x
Rand TA, Tylianakis JM, Tscharntke T (2006) Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol Lett 9:603–614. doi:10.1111/j.1461-0248.2006.00911.x
Samnegård U, Person AS, Smith HG (2011) Gardens benefit bees and enhance pollination in intensively managed farmland. Biol Conserv 144:2602–2606. doi:10.1016/j.biocon.2011.07.008
Saure C (1996) Urban habitats for bees: the example of the city of Berlin. In: Westrich P, Williams IH, Matheson A, Buchmann SL, O’Toole C (eds) Linnean society of London and the international bee research association. The conservation of bees, London, pp 47–53
Scherber C, Mwangi PN, Temperton VM, Roscher C, Schumacher J, Schmid B, Weisser WW (2006) Effects of plant diversity on invertebrate herbivory in experimental grassland. Oecologia 147:489–500. doi:10.1007/s00442-005-0281-3
Schmid-Egger C (2004) Bestimmungsschlüssel für die deutschen Arten der solitären Faltenwespen (Hymenoptera: Eumeninae). Deutscher Jugendbund für Naturbeobachtung, Hamburg
Schüepp C, Herrmann JD, Herzog F, Schmidt-Entling MH (2011) Differential effects of habitat isolation and landscape composition on wasps, bees, and their enemies. Oecologia 165:713–721. doi:10.1007/s00442-010-1746-6
Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B (2012) Generalized linear mixed models using AD model builder. R package version 0.7.2.12
Smith RM, Gaston KJ, Warren PH, Thompson K (2006a) Urban domestic gardens (VIII): environmental correlates of invertebrate abundance. Biodivers Conserv 15:2515–2545. doi:10.1007/s10531-005-2784-y
Smith RM, Warren PH, Thompson K, Gaston KJ (2006b) Urban domestic gardens (VI): environmental correlates of invertebrate species richness. Biodivers Conserv 15:2415–2438. doi:10.1007/s10531-004-5014-0
Staniforth RJ (2002) Effects of urbanization on bird populations in the Canadian Central. Arctic 55:87–93
Stanley DA, Gunning D, Stout JC (2013) Pollinators and pollination of oilseed rape crops (Brassica napus L.) in Ireland: ecological and economic incentives for pollinator conservation. J Insect Conserv 17:1181–1189
Steffan-Dewenter I (2002) Landscape context affects trap-nesting bees, wasps, and their natural enemies. Ecol Entomol 27:631–637
Steffan-Dewenter I, Leschke K (2003) Effects of habitat management on vegetation and above-ground nesting bees and wasps of orchard meadows in Central Europe. Biodivers Conserv 12:1953–1968. doi:10.1023/A:1024199513365
Steffan-Dewenter I, Schiele S (2008) Do resources or natural enemies drive bee population dynamics in fragmented habitats? Ecology 89:1375–1387
Thies C, Roschewitz I, Tscharntke T (2005) The landscape context of cereal aphid-parasitoid interactions. Proc R Soc 272:203–210. doi:10.1098/rspb.2004.2902
Tscharntke T, Rand TA, Bianchi FJJA (2005) The landscape context of trophic interactions: insect spillover across the crop-noncrop interface. Ann Zool Fenn 42:421–432
Watling JI, Orrock JL (2010) Measuring edge contrast using biotic criteria helps define edge effects on the density of an invasive plant. Landsc Ecol 25:69–78. doi:10.1007/s10980-009-9416-y
Westphal C, Steffan-Dewenter I, Tscharntke T (2009) Mass flowering oilseed rape improves early colony growth but not sexual reproduction of bumblebees. J Appl Ecol 46:187–193. doi:10.1111/j.1365-2664.2008.01580.x
Westrich P (2011) Wildbienen: Die anderen Bienen. Verlag Dr. Friedrich Pfeil, München
Wojcik VA, McBride JR (2011) Common factors influence bee foraging in urban and wildland landscapes. Urban Ecosyst. doi:10.1007/s11252-011-0211-6
Zanette LRS, Martins RP, Ribeiro SP (2005) Effects of urbanization on Neotropical wasp and bee assemblages in a Brazilian metropolis. Landsc Urban Plan 71:105–121. doi:10.1016/j.landurbplan.2004.02.003
Acknowledgments
We thank the land and garden owners who allowed us to use their properties for this study. We acknowledge the help of Dr. Thomas Niemeyer with site selection and fieldwork, Isabel Siemers with plant species identification, Dr. Paul Westrich for reassurance of some bee species identities and numerous colleagues with fieldwork support. We thank the DAAD–Deutscher Akademischer Austauschdienst, Leuphana University Lüneburg and CAPES–Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for funding this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pereira-Peixoto, M.H., Pufal, G., Martins, C.F. et al. Spillover of trap-nesting bees and wasps in an urban–rural interface. J Insect Conserv 18, 815–826 (2014). https://doi.org/10.1007/s10841-014-9688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-014-9688-7