Abstract
We analysed the habitat preferences of adult stages and oviposition electivity of Melitaea aurelia in calcareous grasslands in the Diemel Valley (central Germany) to assess the key factors for successful management. Egg-laying and adult habitats of M. aurelia were more or less congruent. Oviposition electivity at the host plant (Plantago media) was best explained by a combination of host plant quantity and vegetation structure. Habitat quality, isolation and patch area explained 86% of the current patch occupancy of M. aurelia. With M. aurelia preferentially inhabiting transitional vegetation types, management requires a balance between abandonment and disturbance. Disturbances provide open soil that facilitates germination of the host plant Plantago media. On the other hand, immature and adult stages of M. aurelia perform best on calcareous grasslands with a high amount of host plants and low disturbance intensity. Traditional rough grazing regimes seem to be the most favourable tool for developing the necessary spatial and temporal heterogeneity in patches. The best results may be achieved by rotational grazing where only a subset of inhabited patches is grazed intensively each year. Our analysis of patch occupancy indicates that it would be desirable to restore patches in close proximity to occupied sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The losses of butterflies in Europe exceed those of many other animal groups or vascular plants, presumably because they respond particularly rapidly to environmental changes. Therefore, butterflies are well established as sensitive indicators in conservation policies (Thomas and Clarke 2004; Thomas et al. 2004; Thomas 2005). Among the butterflies, checkerspots (Ehrlich and Hanski 2004) provide one of the most established model groups in animal ecology and conservation.
Based on extensive research of this group, habitat quality within sites, habitat patch size, and patch isolation have been identified as the most critical parameters determining butterfly persistence in cultivated landscapes (Dennis and Eales 1997; Moilanen and Hanski 1998; Thomas et al. 2001; Fleishman et al. 2002; Fred and Brommer 2003; Anthes et al. 2003b; WallisDeVries 2004). Numerous metapopulation studies during the past decade preferentially addressed these three parameters, whereas the importance of the landscape matrix between habitat patches for the survival and conservation of butterfly populations has only recently been recognised (Ricketts 2001; Chardon et al. 2003; Dennis et al. 2003, 2006; Shreeve et al. 2004).
A general definition of habitat quality is lacking so far. Dennis et al. (2003, 2006) postulate a resource-based definition of habitat and suggest viewing the landscape as a continuum of overlapping resources that may be differentially suitable for different purposes (such as feeding, migrating, ovipositing, sun-basking etc.). Habitats and resources perfectly match only where small patches of semi-natural vegetation (habitat) are situated within intensively used agricultural landscapes (non-habitat).
Most studies ascertain habitat quality on the basis of the requirements of the immature stages (e.g. oviposition sites), because they are more specific than those of the adults (Thomas 1991; Clarke et al. 1997; Thomas et al. 1998, 2001; Bourn and Thomas 2002; Fartmann 2004; García-Barros and Fartmann submitted). This is due to the low or absent mobility as well as the longer life time of the immature stages (Fartmann 2004). A patch with suitable host plants for a butterfly is generally only a fraction of the total host plant population in an area and the core habitats of immature and adult stages usually only partly overlap (Dennis et al. 2006).
Nickerl’s fritillary (Melitaea aurelia) is restricted to calcareous grasslands in Central Europe (Ebert and Rennwald 1991; Seifert 1994; Leopold 2001; Fartmann 2004) and has recently expanded its range in northwestern Germany (Fartmann 2004). Nevertheless, it is listed as ‘vulnerable’ in Germany (Pretscher 1998) and in Europe as a whole (van Swaay and Warren 1999). Due to frequent confusion with other co-occurring Melitaea species such as M. athalia, M. britomartis or M. parthenoides, the knowledge about the ecology and conservation of this species is still poor (Ebert and Rennwald 1991). Although some studies provide information on phenology and nectar resources as well as adult and larval habitats (Ebert and Rennwald 1991; Seifert 1994; Fartmann 2004), comprehensive studies considering the whole life cycle of M. aurelia as the basis for successful management of the populations are lacking so far.
In this paper, we determine, for the first time, the conditions that promote the persistence of M. aurelia in calcareous grasslands at its northwestern range limit in central Europe. We place a particular emphasis on oviposition site electivity at the microhabitat level and to patch occupancy at the landscape level. Finally, we also use these data to derive management recommendations for the conservation of Melitaea aurelia.
Materials and methods
Study species
M. aurelia occurs from northeastern France to central Asia and from southern Sweden to the Balkans (Bink 1992; van Swaay and Warren 1999; Kudrna 2002). In Germany, it is predominantly found in chalk and limestone areas in the south (Ebert and Rennwald 1991; BLfU 2001). In the Diemel Valley, M. aurelia was first recorded in 1990, and numerous occupied calcareous grasslands have since been discovered. The Diemel Valley is currently the northwestern distribution border in Europe (Fartmann 2004).
In Germany, M. aurelia is a characteristic species of extensively used calcareous grasslands, classified as the Gentiano-Koelerietum vegetation type (cf. Rennwald 2000) and typically occurring on south-facing slopes (Seifert 1994; Leopold 2001; Fartmann 2004). Little is known about population patterns and dynamics, but available observations indicate that M. aurelia currently occurs in metapopulations (Fartmann 2004).
The adults are frequent and non-discriminating visitors to nectaring plants and are on the wing from the beginning of June until the end of July. In the Diemel Valley, flight activity peaks at the end of June (median = 22/06, Fartmann 2004). Females lay the eggs in clusters on the underside of the host plant leaves (mostly Plantago spp.). In general, two clutches are laid, the first with 150–200 eggs and the second with 50–150 eggs, on average (Bink 1992). The larvae hatch after 18 days and live gregariously in a web on the host plant. Hibernation takes place in a smaller silken web at the base of the host plant. In April or May, the larvae pupate and adults hatch after 18 days (Bink 1992).
Plantago media has been recognised as the only larval host plant in the Diemel Valley (Fartmann 2004). The plant is common and widely distributed in unimproved semi-dry grassland in Germany (Haeck 1992; Peintinger and Philippi 1996). The seeds of P. media are wind-dispersed and the plant behaves as a pioneer coloniser of disturbed bare ground patches, typically created by grazing. P. media has flat rosettes and grows on base-rich soils with moderate nutrient supply. At undisturbed sites, the plants become quickly overgrown by tall grasses (van der Aart and Vulto 1992; Peintinger and Philippi 1996).
Study area
The study area covers about 130 km² of the Middle and Lower Diemel Valley (Fartmann, 2004, 2006) along the border between the federal states of North Rhine-Westphalia and Hesse (central Germany; 51°32′N/9°00′E and 51°38′N/9°25′E) at an elevation of 140–300 m a.s.l. (Fig. 1a). The climate is suboceanic with an average annual temperature of 9°C. Mountain ranges west of the Diemel Valley shield the region from some of the incoming oceanic moisture, resulting in a comparably low annual precipitation of less than 700 mm (Müller-Westermeier 1999).
Until the mid-19th century, the landscape of the Diemel Valley consisted of nutrient-poor arable fields, large sheep pastures, and open woodlands (Brökel 1984; Lucan and Eger 1996). Since then, the extent of sheep pastures has continuously decreased. Following World War II, many of the formerly sheep-grazed calcareous grasslands were abandoned and/or afforested (Hozak and Meyer 1998; Fartmann 2004), which is in line with the development described for other parts of Europe (e.g. WallisDeVries et al. 2002). Nowadays, grasslands cover approximately 660 ha, or ca. 5% of the study area. The most abundant vegetation type of the grasslands is the Gentiano-Koelerietum (Fartmann 2004).
Large parts of the Diemel Valley are proposed Sites of Community Interest (pSCI) (E. Schröder, German Federal Agency for Nature Conservation, pers. comm.) and the prime butterfly area ‘Diemeltal’ is part of the study area (van Swaay and Warren 2003).
Methods
Oviposition microhabitat
For microhabitat analyses, we chose 16 sites that contained M. aurelia populations in high density. The search for egg-batches started in July when the likelihood to find them was highest. Within each site, we systematically checked a randomly chosen subset of host plants for egg-batches (91 in total) in all potential parts of the site. To contrast the characteristics of these occupied host plant individuals with a random distribution of egg clutches across host plants, we further recorded the available habitat structures at 25 randomly chosen host plant individuals (the nearest plant to a randomly thrown stick was taken; Anthes et al. 2003b). To contrast habitat structures around potential host plants at occupied habitat patches with those at unoccupied patches, we performed a similar characterisation of available host plant individuals in 13 randomly chosen uncolonised patches. For each analysed host plant, environmental parameters were recorded in a 1 m × 1 m quadrat with the plant as the centre (Table 1). These parameters were then compared between occupied and vacant host plant individuals.
Patch occupancy
Habitat preferences of adult M. aurelia in the Diemel Valley were assessed at all 55 sites (patches) that were considered particularly suitable for the butterfly, irrespective of whether or not they were currently occupied. Calcareous grassland habitats were considered “particularly suitable” when they contained the host plant. A patch was defined as contiguous suitable habitat if it was isolated from the nearest neighbouring suitable habitat by over 100 m of woodland, improved grassland, or arable fields. From the end of June to the end of July, each site was visited twice to assess the presence or absence of the species. As there are no co-occurring Melitaea species in the area, all stages of M. aurelia were easy to identify. All sites were systematically searched for adults, and host plants were checked for eggs. At each site, several environmental parameters were examined in representative quadrates of 1 m × 1 m (Table 1). These variables were then compared between occupied and vacant patches.
Statistical analysis
To test for patch occupancy and oviposition preferences, binary stepwise-forward logistic regression was used to recognise those parameters with the highest explanatory power on occurrence. Several parameters showed a right-skewed distribution and were log10-transformed to achieve normality. Differences in habitat quality between occupied and unoccupied host plants were assessed with Student’s t-test. Differences between observed and expected frequencies in nominal and ordinal variables were analysed using χ²-test. Because χ²-tests do not allow empty categories, frequencies of 0 were conservatively set to 0.1. All analyses were performed with statistical package SPSS 13.
Results
Oviposition microhabitat
In total, we found 91 clutches of Melitaea aurelia on the undersides of Plantago media leaves. Almost half of them (44%, N = 40) were deposited in the middle of the centre rib at an average height of 2.7 cm (±1.0 cm, ranging from 1.1 to 6.2 cm) above ground. The average clutch size was 110 eggs, ranging from 3 to 330.
The egg-laying sites of M. aurelia were well exposed to the sun with 11.9 h (±2.0 h) of potential daily sunshine in June, and were mostly on rather steep slopes (15.8 ± 7.0°) in SSE aspects (165.8 ± 45.4°) (Table 2). The predominant vegetation type was the Gentiano-Koelerietum typicum (59%, N = 54), followed by the Gentiano-Koelerietum cladonietosum (59%, N = 54) and the Trifolio-Agrimonietum (12%, N = 11) (Table 3). M. aurelia used grazed or mown as well as abandoned sites for egg-laying. Managed sites containing clutches typically had not been grazed or mown for more than 4 weeks (63%, N = 35). The majority of the sites were subject to a rough grazing regime. Most of the oviposition sites showed evidence of low management pressure. Therefore, the coverage of mosses and lichens was very high with a mean of 40.3% (±30.4%). In conjunction with the cover of the first herb layer (61.7% ± 21.9%) and a certain amount of litter (9.9% ± 6.8%), this resulted in an almost complete coverage of the ground by living or dead plant biomass. Especially in the first 5 cm above the ground, the vegetation was dense with a horizontal coverage of 39.0% (±26.5%). Due to the short swards (7.0 cm ± 2.1 cm), vegetation cover further up was more or less negligible. The average occupied host plant rosette was characterised by a height of 4.7 cm (±1.6 cm), a diameter of 13.3 cm (±2.8 cm), and 7.2 leaves (±2.2). In a perimeter of 20 cm around the host plant, there were on average two more potential host plants (2.3 ± 1.4).
Host plant height and the number of adjacent Plantago plants were significantly higher for occupied plants than for unoccupied plants (Table 2). Plant communities surrounding a potential host plant differed significantly between occupied and available individuals: the Gentiano-Koelerietum cladonietosum was disproportionally used for oviposition, whereas the Gentiano-Koelerietum typicum was under-represented (Table 3). Host plant growth form differed significantly between used and available host plants, with prominent plants being over represented.
In a stepwise regression model, the oviposition pattern on Plantago media was best explained by a combination of host plant quantity and vegetation structure parameters (Table 4): the likelihood of a host plant being accepted for oviposition increased with host plant height and the number of Plantago individuals in close proximity, but decreased with vertical coverage at 10 cm height, coverage of the first herb layer, and the height of the second herb layer. Accordingly, P. media plants were three times more often occupied when the plant protruded over the first herb layer.
Patch occupancy
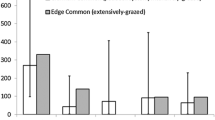
Out of 55 studied sites, 25 were occupied by Melitaea aurelia. The average size of occupied patches was 6.9 ha (±6.3 ha) (Table 5). The geometric mean of the distance to the next three populated patches was 1.5 km (±3.0 km). The predominant vegetation type of the M. aurelia patches was the Gentiano-Koelerietum (84%, N = 21). More than half (60%, N = 15) of the occupied sites were abandoned (Table 6). Of the 10 grazed or mown sites, 7 (70%) had not been used for more than 4 weeks. Almost all sites were south-facing (92%, N = 23), predominantly SW and SSW (56%, N = 14).
M. aurelia sites were characterised as calcareous grasslands with a low coverage of shrubs, a low turf height with a mean of 7.3 cm (±3.1 cm), and a sparsely covering first herb layer (47.5% ± 17.4%) (Table 5). Due to the low grazing pressure on most sites, the coverage of the moss/litter layer was very high (60.0% ± 25.6%) and bare ground was rare (6.6% ± 6.9% coverage). The average occupied site had 12.2 (±9.6) potential host plants per m² that covered 4.1% (±2.9%) of the ground surface. At those sites, the mean Plantago media plant was 2.6 cm (±1.1 cm) high and had a diameter of 10.6 cm (±2.1 cm).
Occupied sites had a significantly lower coverage of the first herb layer and were significantly less isolated than unoccupied available sites (Table 5). Furthermore, P. media plants on occupied sites were significantly higher than those on vacant sites.
The logistic-regression model provided an 86% correct classification of current patch occupancy (Table 7). Patch occupancy was negatively correlated with isolation and positively correlated with patch area, the height of the moss/litter layer, and host plant coverage. This suggests that the likelihood of occupancy is best in cases of low distances between patches and large patch areas (Fig. 1). Patch occupancy also increased with a high moss/litter layer height, indicating a preference for late successional stages of the Gentiano-Koelerietum. At least, the increasing coverage of the larval host plant P. media highly promotes colonisation.
The comparison of studies between 1998 and 2000 (Fartmann 2004) and the data presented here from 2004 show the colonisation of two new patches (1.3 and 8.0 km away from the next populated patch) and the extinction of three formerly occupied patches (between 0.4 and 2.7 km away from the next populated patch) (Fig. 1a).
Discussion
Oviposition microhabitat
The oviposition habitat requirements of Melitaea aurelia were best explained by a combination of host plant quantity and vegetation structure. In terms of host plant quantity, tall P. media with many adjacent potential host plants were preferentially used for egg-laying. A sufficient amount of food is essential for the survival of the larvae, in particular in cluster building species with gregarious caterpillars (García-Barros and Fartmann submitted) such as M. aurelia. Accordingly, preferences for large and prominent plants have been observed in many other butterflies (e.g. Wiklund 1984; Porter 1992; Dennis 1995; Küer and Fartmann 2005) including checkerspots (Anthes et al. 2003a, b). For larval groups of Melitaea cinxia, food shortage occurs frequently (Nieminen et al. 2004). Plantago media typically grows in dense and conspicuous groups, facilitating their location by ovipositing females of M. aurelia and movement by larvae to nearby plants in the case of food shortage (Fig. 2).
In terms of vegetation structure, oviposition occurred preferentially in patches characterised by a low coverage of the first herb layer within 10 cm above the ground, surrounded by few tall grasses in the second herb layer. At typical oviposition sites, nearly all of the ground was covered by plant biomass in a proportion of two thirds herbs/grasses and one third mosses/lichens. The average sward was very short (7.0 ± 2.1 cm) and the higher growing second vegetation layer was sparsely developed. In summary, the architecture of egg-laying sites can be described as host plants in a dense and very short turf with some higher growing plants around. Due to the preferred southern slope aspect and the short turf, the microclimate is most likely to be warm to hot.
Although M. aurelia prefers well developed herb layers, host plants usually remain easily accessible because lower densities and horizontal coverage of the lower herb layer support a better accessibility while searching for a host plant. In the logistic regression model, only 35% of the predicted suitable host plants were used. This shows that there are more suitable host plants available than M. aurelia can use (Warren and Stephens 1989; Konvička et al. 2003). Therefore, the availability of Plantago media is not as restrictive as the specific vegetation structure, providing the specific microclimate described above (Hermann and Steiner 1997; Thomas et al. 2001; Anthes et al. 2003b; Konvička et al. 2003; Fartmann and Mattes 2003; Fartmann 2004, 2006).
M. aurelia prefers extensively used or recently abandoned sites with Gentiano-Koelerietum vegetation for oviposition. Ongoing succession continuously reduces host plant quantity, because Plantago media rosettes become overgrown (van der Aart and Vulto 1992; Peintinger and Philippi 1996) and less accessible for the ovipositing butterfly. Additionally, in advanced successional stages, shading by taller vegetation deteriorates the presumably required microclimatic conditions. On the other hand, open and initial grassland sites with sparser vegetation, a high proportion of bare ground, and small growing P. media plants may not provide enough biomass to satisfy the feeding demands of the larvae even if there are many individuals of the food plant. Furthermore, the microclimate near the soil surface is probably too dry and hot under such open conditions. Thus, the optimal larval habitat conditions depend on a fragile balance between a low growing but densely closed vegetation structure near the soil surface and the vital performance of the host plant. Both essential requisites are provided by counteracting processes: whereas the required vegetation structure and microclimate is optimally provided by the absence or a very low intensity of disturbances, the regeneration and the vitality of the host plant is strongly enhanced by regularly occurring higher levels of disturbance. This seems to be not only a local phenomenon since our findings are in line with qualitative habitat descriptions from other parts of central Europe (e.g. Leopold 2001).
Patch occupancy
As with many other butterfly species (e.g. Dennis and Eales 1997; Osborne and Redak 2000; Thomas et al. 2001; see introduction), patch occupancy of Melitaea aurelia in the Diemel Valley was best explained by isolation, patch area, and habitat quality. Fartmann (2004) reported in a more qualitative way that the chance of colonisation is best in the case of short distances between patches and a large patch area. The most important parameters explaining habitat quality in the patch occupancy model were the height of the moss/litter layer and the host plant coverage.
One of the major topics of research in checkerspot butterflies is the spatial structure of their populations (Murphy et al. 2004). In the Diemel Valley, M. aurelia has an obvious metapopulation structure. The local populations breed in clearly separated habitat patches. For some patches, extinction and colonisation events were documented. However, a number of remote and edge patches are not yet colonised. As in Euphydryas aurinia (Warren 1994; Thomas 1995; Lewis and Hurford 1997; Wahlberg et al. 2002; Anthes et al. 2003b), the population structure of M. aurelia resembles mostly the ‘mainland-island’ or ‘source-sink’ type. In contrast, other Melitaea species such as M. cinxia build a classical metapopulation of the Levins-type consisting of many small and highly connected patches with a high turnover rate (Hanski et al. 1994; Bourn and Warren 1997; Hanski 1999; Nieminen et al. 2004).
Spatial arrangement of immature and adult habitats
Oviposition and adult habitats of Melitaea aurelia in the Diemel Valley are more or less congruent: both the within-patch microhabitat analysis as well as the landscape-level patch occupancy analysis showed a clear preference for south-facing slopes. On average, the slopes are quite steep (15°) and sun-exposed (12 h of possible daily sunshine in June, measured only in the microhabitat study). In most cases, M. aurelia uses the Gentiano-Koelerietum typicum (more than 60% of the occupied sites in both study parts) followed by the Gentiano-Koelerietum cladonietosum. Regarding management, it appears that M. aurelia has a preference for infrequently managed or recently abandoned sites. At the grazed or mown sites, M. aurelia tends to use patches and host plants where the last grazing or mowing occurred more than 4 weeks ago (more than 60% of the used sites in both study parts). Sites with a higher amount of host plants have a higher likelihood of being used for oviposition and as an adult habitat.
All the stages (egg, larvae, pupae, adult) of a species need specific resources. These resources may be spatially separated and overlap only partly (Dennis et al. 2006). Although adults of M. aurelia are good flyers, the adult habitats closely match those of the immature stages. In contrast, the related Melitaea athalia has spatially separated adult feeding and larval breeding habitats (Schwarzwälder et al. 1997). As found for other species, larval habitat quality represented mainly by host plant availability and vegetation structure/microclimate is usually the primary restricting key factor for patch occupancy (Dennis and Eales 1997; Osborne and Redak 2000; Thomas et al. 2001; Anthes et al. 2003b; Fred and Brommer 2003; Fartmann 2004, 2006).
Management
Creating suitable habitats for Melitaea aurelia requires finding the right balance between abandonment and disturbance. Disturbance creates the gaps for the germination of the host plant Plantago media. In contrast, immature and adult stages of M. aurelia need calcareous grasslands with a high amount of host plants and low disturbance intensity, typically found under light grazing conditions or on young fallows. A possible solution to solve this apparent contradiction in the long run could be infrequent, spatially and temporarily heterogeneous grazing (Balmer and Erhardt 2000; Anthes et al. 2003b; Dennis et al. 2004). As for many other butterflies of calcareous grasslands, traditional rough grazing seems to be the most favourable tool (BUTT 1986; Fartmann 2004, 2006). The best results may be achieved by rotational grazing systems using only a selection of patches at a given time (Warren and Stephens 1989; Dolek 1994; Kleyer et al. 2007). A short term effect of such management schemes will decrease the suitable area for M. aurelia, but in the long run, spatially restricted, heavier disturbances will promote the existence of optimal Plantago media stands, which are of crucial importance (see also Moilanen and Hanski 1998). Leaving parts ungrazed provides enough space within the patch for the butterfly to hide. In addition, heterogeneity has proven to be beneficial in unfavourable climatic years by offering possibilities to shift habitats on a small scale level (Weiss et al. 1988; Fartmann 2006). Intensive grazing of the whole patch is only preferable if enough unmanaged patches within a short distance can act as hideaways (Gerken and Meyer 1994; Michels and Woike 1994). In the following years, the managed patch should be left fallow to allow regeneration of suitable vegetation structures. In some cases, M. aurelia seem to be robust towards higher grazing intensity.
At the metapopulation level, it would be advisable to restore patches, especially near colonised sites (Schultz 2001). Many of the former calcareous grasslands in the Diemel Valley are now abandoned and invaded by shrubs and trees. Restoration priority should be given to sites that were formerly covered with calcareous grassland vegetation and/or still hold some remnants of it and are not further than 1.5 km away from known breeding sites (geometric mean of the next three populated patches). Thomas et al. (1992) and Maes et al. (2004) proved stepping-stone habitats to be more efficient than corridors. Because of numerous and patchy distributed calcareous grasslands in the Diemel Valley, installing step-stone habitats seems to be the most promising way to restore a healthy metapopulation structure.
Recommendations for the size of restored patches are still in discussion: according to Thomas et al. (2001), every patch, irrespective of its size, is beneficial for the species. In contrast, Fred and Brommer (2003) report low immigration rates of wandering adults to small patches due to a low likelihood of finding them. In addition, emigration rates seem to be higher from small patches because the borders of the patch are more often reached by the butterflies (Crone and Schultz 2003). Due to the aforementioned and the realised metapopulation type of M. aurelia we recommend that the bigger the sites the better. Based on our results, a patch area of 7 ha (the average area of colonised sites) or more seems to be of special relevance, although patch area seems to be clearly of minor importance relative to patch connectivity.
References
Anthes N, Fartmann T, Hermann G (2003a) Wie lässt sich der Rückgang des Goldenen Scheckenfalters (Euphydryas aurinia) in Mitteleuropa stoppen? Erkenntnisse aus populationsökologischen Studien in voralpinen Niedermoorgebieten und der Arealentwicklung in Deutschland. Naturschutz und Landschaftsplanung 35:279–287
Anthes N, Fartmann T, Hermann G, Kaule G (2003b) Combining larval habitat quality and metapopulation structure – the key for successful management of prealpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Balmer O, Erhardt A (2000) Consequences of succession on extensively grazed grasslands for Central European butterfly communities: rethinking conservation practices. Conserv Biol 14:746–757
Bink FA (1992) Ecologische Atlas van de Dagvlinders van Nordwest-Europa. Schuyt & Co, Haarlem, The Netherlands
BLfU (Bayerisches Landesamt für Umweltschutz) (2001) Artenschutzkartierung Bayern: Arbeitsatlas Tagfalter. Bayerisches Landesamt für Umweltschutz, Augsburg, Germany
Bourn NAD, Thomas JA (2002) The challenge of conserving grassland insects at the margins of their range in Europe. Biol Conserv 104:285–292
Bourn NAD, Warren MS (1997) Species action pan—Glanville Fritillary Melitaea cinxia. Butterfly Conservation, Dorset
Brökel G (1984) Erlinghausen, eine Dorfgeschichte. Selbstverlag, Gemeinde Erlinghausen, Germany
BUTT (Butterflies Under Threat Team) (1986) The management of chalk grassland for butterflies. Focus on Nature Conserv 17:1–80
Chardon JP, Adriaensen F, Matthysen E (2003) Incorporating landscape elements into a connectivity measure: a case study for the speckled wood butterfly (Pararge aegeria L.). Landscape Ecol 18:561–573
Clarke RT, Thomas JA, Elmes GW, Hochberg ME (1997) The effects of spatial patterns in habitat quality on community dynamics within a site. Proc R Soc Lond, Ser B: Biol Sci 264:347–354
Crone EE, Schultz CB (2003) Movement behavior and minimum patch size for butterfly population persistence. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies. Ecology and evolution taking flight. The University of Chicago Press, Chicago, pp 561–576
Dennis RLH (1995) Euchloe ausonia (Hübner) (Lepidoptera: Pieridae) oviposition on Brassica nigra (L.) Koch (Cruciferae): big immature plants are preferred. Entomol Gaz 46:253–255
Dennis RLH, Eales HT (1997) Patch occupancy in Coenonympha tullia (Müller, 1764) (Lepidoptera: Satyrinae): habitat quality matters as much as patch size and isolation. J Insect Conserv 1:167–176
Dennis RLH, Hodgson JG, Grenyer R, Shreeve TG, Roy DB (2004) Host plants and butterfly biology. Do host-plant strategies drive butterfly status? Ecol Entomol 29:1–16
Dennis RLH, Shreeve TG, Dyck H van (2003) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102:417–426
Dennis RLH, Shreeve TG, Dyck H van (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966
Dolek M (1994) Der Einfluss der Schafbeweidung von Kalkmagerrasen in der Südlichen Frankenalb auf die Insektenfauna (Tagfalter, Heuschrecken). Agrarökologie 10:1–126
Ebert G, Rennwald E (1991) Die Schmetterlinge Baden-Württembergs. Band 1, Tagfalter I. Verlag Eugen Ulmer, Stuttgart, Germany
Ehrlich PR, Hanski I (eds) (2004) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford
Fartmann T (2004) Die Schmetterlingsgemeinschaften der Halbtrockenrasen-Komplexe des Diemeltales. Biozönologie von Tagfaltern und Widderchen in einer alten Hudelandschaft. Abh Westf Mus Naturkde 66:1–256
Fartmann T (2006) Oviposition preferences, adjacency of old woodland and isolation explain the distribution of the Duke of Burgundy butterfly (Hamearis lucina) in calcareous grasslands in central Germany. Ann Zool Fenn 43:335–347
Fartmann T, Mattes H (2003) Störungen als ökologischer Schlüsselfaktor beim Komma-Dickkopffalter (Hesperia comma). Abh Westf Mus Naturkde 65:131–148
Fleishman E, Ray C, Sjörgen-Gulve P, Boggs CL, Murphy DD (2002) Assessing the roles of patch quality, area and isolation in predicting metapopulation dynamics. Conserv Biol 16:706–716
Fred MS, Brommer JE (2003) Influence of habitat quality and patch size on occupancy and persistence in two populations of the Apollo butterfly (Parnassius apollo). J Insect Conserv 7:85–98
García-Barros E, Fartmann T (accepted) Oviposition sites. In: Settele J, Konvička M, Shreeve TG, Dyck H van (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge
Gerken B, Meyer C (1994) Kalkmagerrasen in Ostwestfalen. Über Pflege und Entwicklung der Kalkmagerrasen in Ostwestfalen – Kreise Höxter, Paderborn und Lippe. LÖBF-Mitteilungen 3:32–40
Haeck J (1992) Phytosociology of Plantago habitats in the Netherlands and the relation with habitat characteristics. In: Kuiper PJC, Bos M (eds) Plantago: A multidisciplinary study. Ecol. Stud. 89:20–29
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Kuussaari M, Nieminen M (1994) Metapopulation structure and migration in the butterfly Melitaea cinxia. Ecology 75:747–762
Hermann G, Steiner R (1997) Eiablage- und Larvalhabitat des Komma-Dickkopffalters (Hesperia comma Linné, 1758) in Baden-Württemberg (Lepidoptera, Hesperiidae). Carolinea 55:35–42
Hozak R, Meyer C (1998) Konzepte zur Wiederbelebung der Hüteschäferei auf Kalkmagerrasen und Heiden. LÖBF-Mitteilungen 4:22–28
Kleyer M, Biedermann R, Henle K, Obermaier E, Poethke H-J, Poschlod P, Schröder B, Settele J, Vetterlein D (2007) Mosaic cycles in agricultural landscapes of Northwest Europe. Basic Appl. Ecol. 8:295–309
Konvička M, Hula V, Fric Z (2003) Habitat of pre-hibernating larvae of the endangered butterfly Euphydryas aurinia (Lepidoptera: Nymphalidae): What can be learned from vegetation composition and architecture? Eur J Entomol 100:313–322
Kudrna O (2002) The distribution atlas of European butterflies. Oedippus 20:1–342
Küer A, Fartmann T (2005) Prominent shoots are preferred: microhabitat preferences of the Alcon Blue (Maculinea alcon) in Northern Germany (Lycaenidae). Nota Lepidopterol 27:309–319
Leopold P (2001) Schmetterlingszönosen ausgewählter Kalk-Magerrasen im Saale-Unstrut-Gebiet (Sachsen-Anhalt) unter besonderer Berücksichtigung der Habitate des Segelfalters und der Berghexe. Unpublished Diploma Thesis, Institute of Landscape Ecology, University of Münster, Münster, Germany
Lewis OT, Hurford C (1997) Assessing the status of the marsh fritillary butterfly (Eurodryas aurinia): an example from Glamorgan, UK. J Insect Conserv 1:159–166
Lucan V, Eger W (1996) Der Einfluss des Menschen auf die Pflanzendecke. In: Becker W, Frede A, Lehmann W (eds) Pflanzenwelt zwischen Eder und Diemel. Flora des Landkreises Waldeck-Frankenberg mit Verbreitungsatlas. Naturschutz in Waldeck-Frankenberg 5:46–53
Maes D, Vanreusel W, Talloen W, Dyck H van (2004) Functional conservation units for the endangered alcon blue butterfly Maculinea alcon in Belgium (Lepidoptera: Lycaenidae). Biol Conserv 120:229–241
Michels C, Woike M (1994) Schafbeweidung und Naturschutz. Pflege von Heiden, Mooren, Kalkmagerrasen und Grünlandflächen. LÖBF-Mitteilungen 3:16–25
Moilanen A, Hanski I (1998) Metapopulation dynamics: effects of habitat quality and landscape structure. Ecology 79:2503–2515
Müller-Westermeier G (1999) Klimaatlas der Bundesrepublik Deutschland. Teil 1. Lufttemperatur, Niederschlag, Sonnenscheindauer. Deutscher Wetterdienst, Offenbach, Germany
Murphy DD, Wahlberg N, Hanski I, Ehrlich PR (2004) Introducing checkerspots: taxonomy and ecology. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a model system for population biology. Oxford University Press, Oxford, pp 17–33
Nieminen M, Siljander M, Hanski I (2004) Structure and dynamics of Melitaea cinxia metapopulations. In: Ehrlich PR, Hanski I (eds) On the wings of checkerspots: a Model system for population biology. Oxford University Press, Oxford, pp 63–91
Osborne KH, Redak RA (2000) Microhabitat conditions associated with the distribution of postdiapause larvae of Euphydryas editha quino (Lepidoptera: Nymphalidae). Ann Entomol Soc Am 93:110–114
Peintinger H, Philippi G (1996) Plantaginaceae, Wegerichartige. In: Sebald O, Seybold S, Philippi G, Wörz A (eds) Die Farn- und Blütenpflanzen Baden-Württembergs. Band 5: Spezieller Teil (Spermatophyta, Unterklasse Asteridae). Buddlejaceae bis Caprifoliaceae. Eugen Ulmer, Stuttgart, pp 247–255
Porter K (1992) Eggs and egg-laying. In: Dennis R (eds) The ecology of butterflies in Britain. Oxford University Press, Oxford, pp 46–72
Pretscher P (1998) Rote Liste der Großschmetterlinge (Macrolepidoptera). Schriftenr Landschaftspfl Naturschutz 55:87–11
Rennwald E (ed) (2002) Verzeichnis und Rote Liste der Pflanzengesellschaften Deutschlands – mit Datenservice auf CD-Rom. – Schriftenr. Vegetationskde 35:1–800
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Schultz CB (2001) Restoring resources for an endangered butterfly. J Appl Ecol 38:1007–1019
Schwarzwälder B, Lörtscher M, Erhardt A, Zettel J (1997) Habitat utilization by the Heath Fritillary butterfly, Mellicta athalia ssp. celadussa (Rott.) (Lepidoptera: Nymphalidae) in montane grasslands of different management. Biol Conserv 82:157–165
Seifert C (1994) Biozönologische Untersuchungen an tagaktiven Schmetterlingen in Nordosthessen. Tuexenia 14:455–478
Shreeve TG, Dennis RLH, Dyck H van (2004) Resources, habitats and metapopulations – whither reality? Oikos 106:404–408
Swaay C van, Warren M (1999) Red data book of European butterflies (Rhopalocera). Nat Environ 99:1–260
Swaay C van, Warren M (eds) (2003) Prime butterfly areas in Europe: priority sites for conservation. National Reference Centre for Agriculture, Nature and Fisheries, Ministry of Agriculture, Nature Management and Fisheries, Wageningen, The Netherlands
Thomas CD (1995) Ecology and conservation of butterfly metapopulations in the fragmented British landscape. In: Pullin AS (ed) Ecology and conservation of butterflies. Chapman & Hall, London, pp 46–68
Thomas CD, Thomas JA, Warren MS (1992) Distribution of occupied and vacant butterfly habitats in fragmented landscapes. Oecologia 92:563–567
Thomas JA (1991) Rare species conservation: case studies of European butterflies. In: Spellerberg IF, Goldsmith FB, Morris MG (eds) The scientific management of temperate communities for conservation. Blackwell Scientific, Oxford, pp 149–197
Thomas JA (2005) Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos Trans R Soc Lond, Ser B: Biol Sci 360:339–357
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc R Soc Lond, Ser B: Biol Sci 268:1791–1796
Thomas JA, Clarke RT (2004) Extinction rates and butterflies. Science 305:1563–1564
Thomas JA, Simcox DJ, Wardlaw JC, Elmes GW, Hochberg ME, Clarke RT (1998) Effects of latitude, altitude and climate on the habitat and conservation of the endangered butterfly Maculinea arion and its Myrmica ant hosts. J Insect Conserv 2:39–46
Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH (2004) Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303:1879–1881
Tonne F (1954) Besser Bauen mit Besonnungs- und Tageslicht-Planung. Hofmann, Schorndorf, Germany
van der Aart PJM, Vulto JC (1992) General ecology. In: Kuiper PJC, Bos M (eds) Plantago: A multidisciplinary Study. Ecol. Stud. 89:6
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232
WallisDeVries MF (2004) A quantitative conservation approach for the endangered butterfly Maculinea alcon. Conserv Biol 18:489–499
WallisDeVries MF, Poschlod P, Willems JW (2002) Challenges for the conservation of calcareous grasslands in Northwestern Europe: integrating the requirements of flora and fauna. Biol Conserv 104:265–273
Warren MS (1994) The UK status and suspected metapopulation structure of a threatened European butterfly, the marsh fritillary (Eurodryas aurinia). Biol Conserv 67:239–249
Warren MS, Stephens DEA (1989) Habitat design and management for butterflies. The Entomologist 108:123–134
Weiss SB, Murphy DD, White RR (1988) Sun, slope and butterflies: topographic determination of habitat quality for Euphydryas editha. Ecology 69:1486–1496
Wiklund C (1984) Egg-laying patterns in butterflies in relation to their phenology and the visual apparency and abundance of their host plants. Oecologia 63:23–29
Acknowledgements
Our grateful thanks to Nils Anthes, Gabriel Hermann, Norbert Hölzel, and Martin Konvička for helpful comments on an earlier version of this paper. The study was partly funded by the Akademie für ökologische Landeserforschung in Westfalen (AÖL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eichel, S., Fartmann, T. Management of calcareous grasslands for Nickerl’s fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation, and patch area. J Insect Conserv 12, 677–688 (2008). https://doi.org/10.1007/s10841-007-9110-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-007-9110-9