Abstract
This study deals with the habitat requirements and (meta)population ecology of the Birdwing Butterfly Troides aeacus in the Xiaolongshan forest area and the Baishuijiang Natural Reserve of Gansu Province, China. The more descriptive components mainly summarize the biology and habitat requirements of the species. A detailed account is given of 3-year presence/absence dynamics in a suspected metapopulation, which consists of ten habitat patches. By means of GLM a habitat model was developed which has shown that the abundance of Troides aeacus will increase with both the number of larval host plants and adult nectar plants, while it will decrease with denser forest canopy structure. The hierarchical partitioning of the explained variance indicated that the independent effects of the number of nectar plants and the forest canopy density are the most important factors, while the explanatory power of the number of host plants was minimal. Habitat loss and degradation are the most severe threats to Troides aeacus populations in the study area. These are mainly due to continuous human activities such as destruction of forest for reclamation, grazing, mine exploitation, and cutting of firewood, but also herbicide application and sometimes even certain types of afforestation. While the availability of host plants is a clear pre-requisite for the survival of the species, conservation should be most efficient through an increase in the abundance of nectar plants as well as through the avoidance of complete forest cover (through an appropriate cutting management which would also promote growth of the host plants). As environmental threats are quite similar in the entire Southern Gansu region, we expect that the implementation of such butterfly conservation measures should have positive impacts on many other components of biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Butterflies are generally recognized as a core indicator group for the state of the environment (Thomas and Clarke 2004; Thomas 2005). This is due to several features. In particular butterflies are well investigated and the state of knowledge is generally high. In addition, they have short life cycles and thus—compared to vertebrates, which are often used as indicators—a high number of generations and consequently react fast to environmental changes (Hanski and Gilpin 1997). However, research on butterflies has until now mainly focused on Europe (Settele et al. 2009) and Northern America (Paul et al. 1987; Boggs et al. 2003), while regions which harbor the majority of (endangered) species (New 1991; Koh 2007) have hitherto largely been neglected. Compared to temperate areas, in many of those regions loss and degeneration of habitats is an even greater threat to biodiversity in general and to butterflies in particular (Yang 1998; Yang et al. 2005).
For our study we have thus chosen China with its huge territory and very diverse and threatened ecosystems, where, in spite of some serious efforts (Biodiversity Committee of Chinese Academy of Sciences 1994), biodiversity research and conservation are still in their infancy. In China, possibly even more than in other countries, a fast economic development paired with huge human impact on the landscape leads to severe losses and fragmentation of ecosystems and thus habitats of butterflies (Biodiversity Committee of Chinese Academy of Sciences 1994). Furthermore, quite different from conditions in Europe and the US, the rise of butterfly trade and butterfly-based craftwork results in excessive capture of individuals from butterfly populations in the wild, which adds to the risk of extinction for many populations of the rarer species (Li, personal experience).
To examine present developments, we have chosen the Birdwing butterfly Troides aeacus ssp. aeacus (Felder & Felder). The species is one of the largest butterflies of China (see Fig. 1) and it probably requires a large habitat area, as is typical for large species (Chou 1994). This expectation combined with the high conservation relevance of the entire group (Collins and Morris 1985) and high status of Birdwings among collectors (Sands 2008) makes it a very suitable object for study. The adults of Troides aeacus, which are elegant and colorful, have high ornamental value, thus they became one of the main species for breeding in captivity and for the production of butterfly based artifacts. Due to its size, attractiveness and value the butterfly is frequently harvested from the wild (Li, personal experience).
In order to examine the population biology and survival chances of such a species, we conducted our study from 2001 to 2003 within a closed valley in the Baishuijiang Reserve and from 2005 to 2006 in 16 valleys in the South of Xiaolongshan forests area in the Gansu Province in China. The valley in the Baishuijiang Reserve encompasses one metapopulation of the species which is isolated from other populations as these are separated by at least 10 km. We develop first recommendations for the conservation of Birdwing butterflies in China—but in particular we also raise public awareness for the state of biodiversity in China and for the necessity to conserve these natural resources for the generations to come.
Materials and methods
The study species
Troides aeacus is widely distributed in southern Chinese provinces including Shaanxi, South Gansu, Sichuan, Chongqing, Jiangxi, Zhejiang, Fujian, Guangdong, Guangxi, Hainan, Yunnan, and Tibet (Chou 1994). Outside China according to Chou (1994) it can, e.g., be found in Sikkim, Bhutan, Thailand, Vietnam, Burma, and India and according to D’Abrera (1982) also in Sri Lanka, Malaysia, Indonesia, and Papua New Guinea. It is a sub-tropical and tropical species (Chou 1994). Only few detailed studies on biological characteristics (Huang et al. 2002) and captive breading (Cai et al. 2003) have been conducted in China. Larval host plants belong to the genus Aristolochia and encompass Aristolochia tagala Champ, Aristolochia heterophylla Hemsl., Aristolochia mandshurensis Kom., Aristolochia contorta Bunge, Aristolochia debilis Sieb. & Zucc (Cai et al. 2003; Huang et al. 2002). In Guangzhou city, six to seven generations of Troides aeacus have been annually observed in captivity as well as in the wild. The adult butterfly’s lifespan in captivity is 9–14 days. Females produce 36–44 eggs, which are laid as singletons (Cai et al. 2003).
In Southern Gansu province, Troides aeacus is univoltine and mainly lives in Baishuijiang Natural Reserve and the Xiaolongshan forest area, which are the northernmost areas of the overall distribution. Due to a dense human population and frequent disturbances, habitats of Troides aeacus (see analysis further below for habitat definition) are seriously degraded and fragmented in these areas (own observations). Thus, only a limited number of patches with Troides aeacus can be found. Moreover, they often harbor only very small local populations which consequently face high extinction risks.
Study area and sites
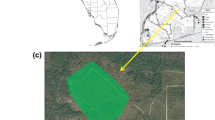
The research areas are (1) the Bifeng valley, which is located at the southeastern edge of the Baishuijiang natural reserve of Gansu Province (Eastern longitude 104°16′–105°27′, Northern latitude 32°16′–33°15′; see Fig. 2) and (2) the Xiaolongshan forests area, which belongs to the western section of the Qingling Mountains, situated in Southeastern Gansu (Eastern Longitude 104°22′–106°43′, Northern Latitude 33°30′–34°49′; see Fig. 2).
Bifeng valley has a South to North extension and a total area of about 30 km2. The altitude ranges from 600 to 1,800 m asl. The entire valley system is composed of one main valley and ten tributary valleys. All tributary valleys are isolated from each other by ridges. The altitudes of the two highest mountains delineating the valley from neighboring valley complexes are between 1,600 and 1,800 m. The climate is semi-tropical with an average annual temperature of 15.6°C and an average annual rainfall between 840 and 950 mm (Wu and Zhang 1997).
The entire valley originally was, and to quite some extent still is, a system of mixed evergreen and hardwood forests. The main trees species are Quercus engleriala, Q. liaotungensis, Q. acrodonta, Q. glandulifera, Q. spinosa, Pterocarya stenoptera, Phoebe chinense, Ph. neurantha, Cinnamomum inunctum, and Albizia julibrissin. The main shrub species are: Rhus chinensis, Lindera communis, Helwingia japonica, Vitex negunda, Elaeagnus lanceolata, Toddalia asiatica, Pyracantha fortuneana, Bauhinia glauca, Zanthoxylum armatum, and Z. dissitum. Plants of the understory include Aristolochia debilis, A. heterophylla, A. mandshurensis and Cordiocrinum giganteuum (Wu and Zhang 1997).
The deterioration of vegetation gradually declines from the mouth to the end of the valley. It mainly consists of farmland, scrubland, sparse forests, and dense forests. At the mouth of valley is the town of Bikou with several iron smelters with emissions which likely impact biodiversity (e.g., activity levels of all butterfly species; own observations). The butterflies mainly fly on the valley bottom and rarely move to other valleys. Butterfly diversity in the valley is very high with a total of more than 180 butterfly species (Li et al. 2006).
The Xiaolongshan forests cover an area of around 200 km from East to West and nearly 150 km from South to North. The area is separated by the ridges of the Qingling Mountains and crosses the Yellow River as well as the Yangtze River. It is part of the two water systems of Wei River and Jialingjiang River. On the northern slopes, the valleys are deep as are the tributary valley ridges. Southern slopes are smooth with most of the area on an elevation between 1,000 and 2,000 m. The cultivated areas cover 8,302 km2, the forests 6,424 km2. The biggest part of the Xiaolongshan forest area belongs to the area of temperate or subtropical continental monsoon climates. The annual average temperature in the area is in the range of 7–12°C. Annual precipitation ranges from 380 to 830 mm, and the period without frost lasts between 140 and 218 days (An 2002).
The Xiaolongshan forest area is rich in plant resources with more than 800 species of woody plants. The main forest types are temperate coniferous and broadleaved mixed forests. The main tree species include Pinus tabulaeformis, P. armandi, P. bungeana, Quercus aliena var. acuteserrata, Q. variabilis, Betula platphylla, B. albo sinensis, Fraxinus platypoda, Pterocatya macropter, Abiec chensiensis, and Picea asperata (the latter in higher elevations). Tree species used for afforestation include Larix leptolepis, Larix principis rupprechitii, Pinus tabulaeformis, P. armandi and Robinia pseudoacacia (An 2002; Lei et al. 2008).
The southern slopes of the west Qinling mountain in the Xiaolongshan forest area, which belong to the Hui County and the Liangdang County, have a subtropical climate and are rich in butterfly species (own data, unpublished). However, due to dense human populations on the smooth slopes, which led to a serious degeneration and fragmentation of suitable habitats, many species—including Troides aeacus—in this area are mostly restricted to forest reserves within the valley (Li, own observations).
The study sites have been selected through an intensive inventory of the entire research area. In Bifeng Valley all potentially suitable patches (those sites which had larval host plants as well as nectar plants within their vicinity; see Fig. 3; Table 4) have been selected. In the Xiaolongshan forest we have chosen transects which also contained at least one of the resources (see Table 2).
Methods
Life history characteristics
In early spring (March 2001) individuals of the host plant Aristolochia heterophylla were taken from the forest and transplanted in a 30 m2 garden, where free-living adults laid eggs on the undersides of the leaves. The plants were observed every day and when eggs were found, the egg-bearing leaf was labeled (label with number, date of oviposition, number of eggs, attached at branches of hostplant near to the eggs). The duration of the egg stage was quantified and larval growth observed throughout the different instars. In addition, the number of larval instars and the time of pupation have been assessed by indoor breeding. Also, the egg laying behavior of the adults, including the selection of the host plant species and the number of eggs laid per host plant were recorded within the habitat patch with the highest abundance of the butterfly (the patch named: “Pingshiban”). The behavior of adults (flight, mating, visits of nectar plants) has also been observed along the forest edges and near to nectar plants (Li et al. 2006).
Quantification and modeling of habitat requirements
The investigations of habitat requirements were conducted in five forestry farms (Gaoqiao, Yanping, Dahe, Yunping, and Zuojia) in the southern part of the Xiaolongshan forest, where 16 valleys within the distribution area of Troides aeacus have been selected (Table 2).
In each of the 16 valleys a 3 km transect was established at the valley bottom. For logistic reasons, each transect was visited only once during the peak flight period of Troides aeacus during sunny conditions. Potential shortcomings of low local precision are likely outweighed by the large number of valleys visited and the comparably long transects. Adult butterflies were counted within 2.5 m left and right of the transect path (Pollard 1977; Pollard and Yates 1993; Van Swaay et al. 2008). In addition to abundance data, we recorded density of nectar plants, density of larval host plants and coverage of forest canopy: The number of nectar plants Clerodendrum bungei and Albizzia julibrissin (An 2002, own observation) was counted on both sides of the valley, while the abundance of host plants was assessed by counting all plants in randomly selected units of 2 m width and 100 m length (within the same areas along the transects). Forest cover was assessed as proportion relative to the total area covered based on digital forest maps.
Habitat requirements of Troides aeacus were investigated by relating the number of observed adults to the three environmental variables: number of larval host plants (n/100 m2), number of nectar plants (n/transect), and forest canopy density. A generalized linear mixed effects model with a Poisson error distribution and a log-link function (Pinheiro and Bates 2000; Bolker et al. 2009) was developed in the lme4 package (Bates and Maechler 2008) of R software (R Development Core Team 2008) Data were considered to be nested within the five forestry farm areas and thus farm areas entered the model as a random effect. All three effects were significant and therefore no model simplification was needed. A hierarchical variance partitioning (ignoring the nested structure; Mac Nally 2000) was used to test for independent effects of nectar plants, forest canopy density and host plants. Hierarchical variance partitioning was performed in the hier.part package (Walsh and Mac Nally 2005) of R software.
Survey of butterfly population and patch dynamics
For the metapopulation study, presence and absence of the butterfly was investigated in Bifeng valley in 3 years (2001–2003). When, during the peak flight period of adults (late June to early July), butterflies (at least one female during a 2 h survey) were found in a patch it was regarded as being colonized. The survey was repeated twice (per generation) if no adults were found in the first or second survey, respectively. Searches for eggs were not conducted as these are much harder to find than the adults. In 2001 data were collected on topography, nectar and host plants (see Table 4). The highest elevations of the occurrence of the host plants and Troides aeacus adults were used to define the elevational boundary of potential patch locations in order to delineate and assess the size of the patches and the total habitat area. All patches were mapped (Fig. 3). During the investigation we paid particular attention to areas of sparse forest with shrubs and hosts as well as nectar plants, which (as a result of or own pre-analyses) in principle have been regarded as suitable patches.
The habitat model based on detailed survey data from Xiaolongshan forest was used to calculate a habitat suitability index for the patches in Bifeng Valley. Therefore, expected abundance data were predicted by the model for environmental conditions in the patches and taken as an index of habitat suitability. Together with patch area, the habitat suitability index was then used to explain patterns in patch consistency, extinction and re-colonization. In a generalized linear mixed effects model with a Poisson error distribution and a log-link function habitat suitability, patch area and their interaction were related to the number of years in which a patch was occupied. This model was subsequently simplified based on the minimum Akaike’s information criterion (AIC).
Results
Life history
Observed characteristics of the different stages
Eggs: Half-ball form, saffron yellow, high lubricity, diameter 2.4 mm (own results).
Larvae: five instars; the body color of newly hatched larvae is light yellow, and length is 5.8 mm; from the third instar onwards larvae are dark red with black head capsules. An odorous horn-shaped, yellowish organ (an osmaterium; own observation) is used as a defense response to natural enemies. From the third instar onwards the spines on the body become very large (see Fig. 4). Larval food plants in the study area are Aristolochia heterophylla and A. mandshuriens in Baishuijiang Nature Reserve, while in Xiaolongshan forests Aristolochia contorta and Aristolochia heterophylla were used. Newly hatched larvae eat buds and new leaves, with older larvae feeding on older leaves. The larvae feed on the underside of the leaves. More mature larvae also feed on stems of Aristolochia (possibly in order to get fibrin; see Li et al. 2006). Shortly before pupation larvae climb up shrubs or tree branches where they pupate.
Pupae: girdled; length 39–40 mm; the pupa color is green (see Fig. 5) to/or brown and resembles the background vegetation color.
Phenology
Our observations on the transplanted hostplants revealed that Troides aeacus is univoltine in Southern Gansu province. The adults eclose in late May and reach their peak in population density in June, while the flight period ends towards the end of july. The adults lay eggs from early June until July. The egg stage lasts for about 20 days. Eggs begin to hatch in late June. The total duration of the larval stage is 50–55 days from late June to mid September. By mid August the first larvae are mature and begin to pupate. The peak of pupation is in early September. The pre-pupae last for 2 days, while the entire duration of the pupal stage is about 9 months (Table 1).
Observed adult behavior
In late May first mainly male adults eclose (protandry; own data), while females start eclosing in early June. Eclosion is strongly influenced by weather; it does not take place during hot and dry periods, but seems to be highest during sunny weather after rainfall events (personal observation). After eclosion on the valley slopes, the males fly to the valley bottom to forage and mate. During the search for mates, the males show patrolling behavior (see also Yuan et al. 1998). Males are strong and fast flyers, fly high above the ground covering larger distances and can fly far above the canopy (own observations). They are easily disturbed. In particular, they show this behavior after they have been captured (no matter whether they have been marked or not). Thus they represent a good example of a “trap shy” butterfly (compare Casula and Nichols 2003), which may explain why during our MRR study we had only few recaptures, and thus we had no reasonable data for analysis. An alternative explanation is that they have a very large habitat area—as suggested earlier—and that we might have sampled only parts of it. After eclosion, females visit flowers (if these are present) and mate near to their natal place (after being successfully searched for by males). But if near to their natal place these flowers are absent, they also fly to the valley bottom (where the suitable flowers mainly grow) for nectar uptake and mating. Afterwards, for oviposition they return to the natal patch or other patches in the vicinity where the host plants grow. Compared to males, they do not move much within the valleys (own observations). During flower visitation they fly and react rather slowly, thus, while in low abundance, they are relatively easy to capture.
The adults are active from about 8:30 until 19:00 h local time, with an activity peak between 10:00 and 13:00 h. Their preferred nectar plant is Albizzia julibrissi in the first days after eclosion, but then seem to prefer Bauhinia glauca around mid-June, and finally Clerodendrum bungei after early July. For egg laying, females first fly very low above the ground, close to the shrub and grass layer to search for host plants. If there are no open areas, they search for larval host plants in sparse forests. Dense forests seem unsuitable (butterflies were never observed to enter dense forests). After the host plant has been located, females normally lay eggs on the underside of the leaves (in exceptional cases also on the upper side). Eggs are laid as singletons—one egg per leaf.
Habitat requirements and habitat modeling of Troides aeacus
The main host plant of Troides aeacus is Aristolochia heterophylla (own observations). In Bifeng Valley it is distributed in an elevational range from 820 to 1,680 m (with most observed egg-laying incidences within a range from 1,200 to 1,500 m). The plants mainly grow along forested paths and forest edges with a canopy cover below 80%. The host plants are rarely found when forest canopy cover exceeds 90%.
The most suitable habitat patch for Aristolochia heterophylla as well as for Troides aeacus in the research area was “Pingshiban” (4). Its elevation range is 1,200–1,400 m. This patch originally was covered by dense forest, which was cut in 1999. In 2000 it was re-afforested with young trees of Juglans regia. Thus, at the time of the study only a few tall trees and a large number of shrubs grew there together with grasses and Aristolochia heterophylla. During the transect investigations we found 25 plants of Aristolochia heterophylla. As this place is rather open, females can easily find the host plants to lay eggs. There are many flowers in this patch and open water is nearby.
In the forestry farms Yunping and Yanping of the southern Xiaolongshan area (in a distance of ca. 150 km from the area shown in Fig. 3), Aristolochia heterophylla and A. mandshuriens occur within an elevation range from 700–1,300 m. Forest canopies have a relatively high coverage. Troides aeacus also has populations in this area, which is dominated by human and livestock activities. There are many villages and all land with a slope under 30° is under use (e.g., for cattle grazing or wheat growth). Vegetation is heavily impacted as cattle also enters forest areas and feeds on and/or destroys the host plants.
Relating the number of adult butterflies to the three environmental variables (see Table 2) by means of GLM revealed that the abundance of Troides aeacus will increase with both the number of larval host plants and adult nectar plants, while it will decrease with denser forest canopy structure (Table 3). The hierarchical partitioning of the explained variance indicated that the independent effects of the number of nectar plants and the forest canopy density are the most important factors in determining the abundance of Troides aeacus, while the explanatory power of the number of host plants was low (Fig. 6). This indicates that adults of Troides aeacus also utilize other patches than breeding habitats, and that especially forest canopy structure and nectar plant density significantly add to the importance of the larval host plant in determining overall butterfly abundance.
Metapopulation structure and patch dynamics of Troides aeacus in Bifeng valley
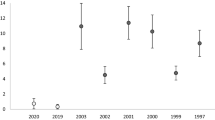
Habitat suitability according to forest canopy density, and amount of nectar and larval host plants differed substantially among the potentially suitable habitat patches in Bifeng Valley as did patch area (Table 4). However, when the habitat suitability index and patch area were related to the number of years a patch was occupied by Troides aeacus, only patch area remained important in a minimum adequate model (deviance based coefficient of determination, D 2 = 0.67). This means that only in sufficiently large areas (above a threshold of 0.45 km2; regression tree analysis) constant occupancy occurred; while local extinction and re-colonization was the case in smaller patches (see also Table 5). Interestingly, even high densities of nectar and/or host plants or suitable canopy structure did not counterbalance the negative effect of small areas, which was indicated by a lack of interaction effects.
Consequently, we assume that Troides aeacus in Bifeng Valley lives as a metapopulation (source-sink population) with ten patches (one of them permanently unoccupied; see Table 5), which is lower than minimum amount of suitable habitat (MASH) of 20, where metapopulations are expected to show a long-term survival (Hanski et al. 1995). Thus, we theoretically should expect a relatively high extinction risk. In the two (sub-)patches of Shanwangmiao (1) and Xigou (3) individuals of Troides aeacus showed the highest abundance (data only available for 2001, see Table 4). They are connected through the Laizitan (sub-)patch (2), which can thus be regarded as a corridor patch. The latter is an open area with generally high nectaring and patrolling activities of Troides aeacus. The large patch (which consists of the subpatches Shanwangmiao (1), Xigou (3), and Laizitan (2); compare Fig. 3) can be regarded as a source patch due to the large area of 2.5 km2 with high habitat quality and high abundance of the species (see Table 4). Pingshiban patch (4) with an area of 0.6 km2 has 1.5 km distance from Laizitan patch (2). It should be easily reached by individuals dispersing from Shanwangmiao (1) and Xigou (3). It has a high habitat quality and a relatively large populationFootnote 1 of the butterfly. The latter is also the case for the Dapingshan patch (7) with an area of 0.7 km2. Patches (4) and (7) have relatively large populations, while populations in other patches (tributary valleys), including Guanyingmiao (5), Xuefangli (6), Jingdongpo (8), and Zhubatan (10) all show typical characteristics of sink populations with small areas between 0.2 and 0.4 km2. Their vegetation also is severely impacted by man, with only small subpopulations of the butterfly and thus with a high extinction risk. Patch Yawan (9) only has a very small area of 0.1 km2 and only contains nectar plants but had no larval host plants during the investigation period. No adults of Troides aeacus have been observed there within 3 years (see Table 5).
The results of the presence/absence investigations of the adult butterflies are summarized in Table 5.
Discussion
Landscape scale dynamics of Troides aeacus
The current study contributes to more in-depth knowledge about the biology and ecology of Troides aeacus, and may directly guide management actions for this representative of a conservation flagship taxon of birdwing butterflies.
Through the study on presence/absence dynamics in Bifeng Valley (see Fig. 3), we have a first indication of a metapopulation structure for a birdwing butterfly. The particular situation there gives a good indication for a mainland-island metapopulation structure, which consists of ten patches of which one was permanently unoccupied. This patch number is far below the minimum amount of suitable habitat (MASH) of 20, for which according to a rule of thumb by Hanski et al. (1995) one can expect a long-term survival of metapopulations. Consequently, the risk of extinction of the Troides aeacus populations under study should be relatively high. Particularly in small patches presence of the species might also often just be a short term event, where due to the high potential mobility of adults these might easily leave such patches again shortly after arrival. Our observations may therefore not necessarily have been the documentation of colonization or extinction events, but the detection of dispersal, use of a stepping-stone, flight within a larger territory, or temporary resource exploitation. But for sure such constellations contribute to a high level of dynamics on the landscape scale, where extinction rather seems to occur in patches smaller than around 0.5 km2 (sink populations), while more stable conditions are to be observed in patches of 0.6–0.7 km2 and of course in the core or mainland population with its 2.5 km2.
However, we have to be very cautious about these interpretations. Firstly, as the minimum of 20 patches (Hanski et al. 1995) is only a rule of thumb, the concrete situation might be quite different as it, e.g., depends in part on stochastic processes, population size, mobility and patch connectivity patterns. Secondly, the study only lasted for 3 years, a much too short period for the analysis of turnover within patches (Schtickzelle and Baguette 2009). Thirdly, we used the habitat model developed on the detailed inventory data from Xiaolongshan to predict abundance in the patches of Bifeng, but the abundance data must not be taken as reliable estimates of real abundance due to differences in vegetation surveys (potentially false estimate of the intercept leading to slightly negative abundance estimates), but they may serve as a reliable habitat suitability index. Fourthly, also the interpretation of the relationships between extinction and patch size is only based on some first indications and thus rather has to be regarded as a start for hypotheses for future in-depth research, which then would be accompanied by an extinction probability analysis. Then we might be able to answer the questions, whether here patch size really is the most important factor (as it seems after the habitat suitability index analysis, where according to a regression tree analysis only in sufficiently large areas above a threshold of 0.45 km2 constant occupancy occurred), or whether extinction risk could relate more to hostplant and nectar density or absolute abundance. In particular we would like to emphasize that constructing future metapopulation research around questions such as those addressed by Schtickzelle and Baguette (2009) might guide the way to create further insights also in so far under-researched regions like China.
As we have no long-term data on the population dynamics of Troides aeacus in the area, we do not know any hard facts about trends and turnovers. However, if we consider all what we know from land use changes in the area in the last decade, we can speculate that we might eventually witness an extinction process. But independent from whether this is the case or not, increasing the amount of suitable habitat patches as well as expanding the area of the existing patches, combined with the establishment of corridor or stepping-stone elements in the center of metapopulations, should increase individual exchanges between the patches and contribute to a longer-term survival.
Impacts on Troides aeacus populations and possibilities for conservation management
Generally, from what we experienced in the area, habitat loss and degeneration are the biggest threats to Troides aeacus populations. Continuously, human activities such as destruction of forest for reclamation, grazing and cutting firewood, result in loss and fragmentation of habitats. Moreover, in Xiaolongshan forest area, mine exploitation also leads to forest destruction. In the research area “Pingshiban” (4), disturbances (e.g., mowing) became more frequent. In the course of the recent afforestation activities Aristolochia heterophylla was often either cut directly or exposed to direct sunshine which limits the plant’s growth. Furthermore, for weed control herbicides were used quite regularly, which leads to wilting and death of Aristolochia heterophylla and thus impacts the survival of the plants and consequently the butterfly larvae.
Insight of how to improve habitat conditions can be derived from our habitat model. While the availability of host plants is a clear pre-requisite for the survival of the species, increasing the abundance of nectar plants as well as limiting the degree of forest cover (through an appropriate cutting management which also would promote growth of the host plants) seem to be very appropriate measures which should and probably could be implemented immediately. However, it has to be stressed that in principle the species needs an overall high forest canopy density. If forest canopy density is under 50%, then habitats are hardly occupied.
When developing prescriptions for conservation, the geographical setting should also be kept in mind. For example the difference of the elevational distribution range of host plants, as observed between Xiaolongshan and Bifeng valley, have to be considered. These can be expected to be due to the fact that Xiaolongshan is located further north and thus is cooler than the Bifeng valley, which is already in a subtropical setting. In our northern area Aristolochia contorta (the main foodplant there) was mainly distributed within the geographical range of farmland, situated at the edge of forests (elevation 900–1,100 m). These facts (climate and land use) also might explain why Troides aeacus in this area—which is near to the border of its distribution—only has small populations.
1100 people live in the Bifeng valley (state: 2002; village major, personal communication). Increasing environmental deterioration, due to difficult economic conditions, frequent human disturbances, and severe destruction of vegetation can be observed in the valley. These seriously impact the habitats and many species are under severe threat. Destruction of vegetation and human disturbance is quite similar in several neighboring valleys of the same reserve. Therefore, the development of butterfly conservation research in this area could have quite some positive impact on biodiversity in general.
Notes
Within mark-release-recapture studies in Bifeng valley from June to July in 2002 and 2003 we captured a total of 75 Troides aeacus individuals (16 females and 59 males). Due to the low number of recaptures we could not make any population estimates. However, as we could capture many of the butterflies we have seen (females easier than males, thus much less females present than males), the study population can be assumed to be extremely small and surely below the population size often observed as MVP for butterflies (Schtickzelle and Baguette 2009).
References
An DG (2002) Xiaolong Mountain high plants flora of Gansu province (in Chinese). Race Publisher, Gansu
Bates D, Mechler M (2008) Linear mixed effects models using S4 classes, ver 0.999375-27. http://lme4.r-forge.r-project.org
Biodiversity Committee of Chinese Academy of Sciences (1994) Biodiversity studies series no. 1. Principles and methodologies of biodiversity studies. Chinese Scientific and Technological Press, Beijing
Boggs CL, Watt WB, Ehrlich PR (2003) Butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Cai YX, Liao ST, Wu FQ (2003) Manual breeding observation of Troides aeacus and Troides helena. Guangdong Agric Sci 5:51–53
Casula P, Nichols D (2003) Temporal variability of local abundance, sex ratio and activity in the Sardinian Chalk hill blue butterfly. Oecologia 136:374–382
Chou I (1994) Monographia Rhopalocerorum Sinensium. Henan Scientific and Technological Publishing House, Zhengzhou
Collins NM, Morris MG (1985) Threatened swallowtail butterflies of the world. The IUCN red data book. IUCN, Gland
D’Abrera B (1982) Butterflies of the oriental region part 1. Hill House, Victoria
Hanski I, Gilpin M (1997) Metapopulation biology. Academic Press, San Diego
Hanski I, Pakkala T, Kuussaari M, Lei GC (1995) Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 72:21–28
Huang GD, Yan QK, Zhou W (2002) Biology observation of Troides helena. Entomol Knowl 39:224–226
Koh LP (2007) Impacts of land use change on South-east Asian forest butterflies: a review. J Appl Ecol 44:703–713
Kühn I, Sykes MT, Berry PM, Thuiller W, Piper JM, Nigmann U, Araújo MB, Balletto E, Bonelli S, Cabeza M, Guisan A, Hickler T, Klotz S, Metzger M, Midgley G, Musche M, Olofsson J, Paterson JS, Penev L, Rickebusch S, Rounsevell MDAR, Schweiger O, Wilson E, Settele J (2008) MACIS: minimisation of and adaptation to climate change impacts on biodiversity. GAIA-Ecol Perspect Sci Soc 17:393–395
Lei JP, Jiang ZP, Yuan SY (2008) Research on the multi-objective management of the secondary forest in Xiaolong Mountains. J Northwest For Univ 23(6):182–186
Li XS, Zhang YL, Luo YQ, Settele J (2006) Study on life history, life table, habitat and conservation of Byasa impediens (Lepidoptera: Papilionidae). Acta Ecol Sin 10:3184–3197
Mac Nally R (2000) Regression and model-building in conservation biology, biogeography and ecology: the distinction between—and reconciliation of—‘predictive’ and ‘explanatory’ models. Biodivers Conserv 9:655–671
New TR (1991) Butterfly conservation. Oxford University Press, Oxford
Paul E, Dennis R, Murphy D (1987) Conservation lessens from long-term studies of checkerspot butterflies. Conserv Biol 1:122–131
Pinheiro J, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pollard E (1977) A method for assessing change in the abundance of butterflies. Biol Conserv 12:115–132
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation: the British Butterfly Monitoring Scheme. Conservation biology series No. 1. Chapman & Hall, London
Sands D (2008) Conserving the Richmond Birdwing Butterfly over two decades: where to next? Ecol Manag Restor 9:4–16
Schtickzelle N, Baguette M (2009) (Meta)population viability analysis: a crystal ball for the conservation of endangered butterflies? In: Settele J, Shreeve T, Konvička M, Van Dyck H (eds) Ecology of Butterflies in Europe. Cambridge University Press, Cambridge, pp 339–352
Settele J, Hammen V, Hulme P, Karlson U, Klotz S, Kotarac M, Kunin W, Marion G, O’Connor M, Petanidou T, Peterson K, Potts S, Pritchard H, Pysek P, Rounsevell M, Spangenberg J, Steffan-Dewenter I, Sykes M, Vighi M, Zobel M, Kühn I (2005) ALARM–assessing large-scale environmental risks for biodiversity with tested methods. GAIA-Ecol Perspect Sci Soc 14:69–72
Settele J, Shreeve T, Konvicka M, Van Dyck H (eds) (2009) Ecology of Butterflies in Europe. Cambridge University Press, Cambridge
R Development Core Team (2008) The R foundation for Statistical Computing, ver 2.8. http://www.r-project.org/
Thomas JA (2005) Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Philos Trans R Soc B 360:339–357
Thomas JA, Clarke RT (2004) Extinction rates and butterflies—response. Science 305:1563–1565
Van Swaay CAM, Nowicki P, Settele J, van Strien AJ (2008) Butterfly monitoring in Europe—methods, applications and perspectives. Biodivers Conserv 17:3455–3469
Walsh C, Mac Nally R (2005) hier.part: hierarchical partitioning. R package version 1.0-1. URL http://www.r-project.org
Wu GH, Zhang KR (1997) General investigation report in Gansu Baishuijiang national natural reserve (in Chinese). Gansu Science and Technology Press, Lanzhou
Yang DR (1998) Studies on the structure of the butterfly community and diversity in the fragmentary tropical rainforest of Xishuangbanna, China. Acta Entomol Sin 41:48–55
Yang P, Deng HL, Qi B, Liu Q (2005) The occupied rate of microhabitats, sampled percentage of species and relative abundance of butterfly community in the Three Gorge Reservoir Area of Yangtze River. Acta Ecol Sin vol 25, No. 3
Yuan DC, Mai GQ, Xue DY (1998) The habitat, biology and conservation status of Luehdorfia chinensis (Lepidoptera: Papilionidae). Chin Biodivers 6:105–115
Acknowledgments
In process of this research we have received intensive support from Mr. Wang Hongjian, who works for the management bureau of the Baishuijiang natural reserve, Gansu province and from Mr. Yuan Shiyun who works for the Xiaolongshan Forestry experimental bureau, Gansu province. Support for X. L. also was generously received from the scientific enterprise project of Gansu province Qs022-C31-069. O. S. and J. S. have received support from the FP6 projects ALARM (www.alarmproject.net; GOCE-CT-2003-506675; Settele et al. 2005) and MACIS (www.macis-project.net; 044399 (SSPI); Kühn et al. 2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Xs., Luo, Yq., Zhang, Yl. et al. On the conservation biology of a Chinese population of the birdwing Troides aeacus (Lepidoptera: Papilionidae). J Insect Conserv 14, 257–268 (2010). https://doi.org/10.1007/s10841-009-9254-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-009-9254-x