Abstract
Background
Heparin dosing of patients anticoagulated with direct oral anticoagulants (DOACs) undergoing atrial fibrillation (AF) ablation can be challenging as they require more heparin than those on warfarin therapy. We sought to compare periprocedural activated clotting times (ACTs) of patients on warfarin vs. DOAC and determine an optimal weight-based heparin dosage strategy.
Methods
Patients who underwent AF ablation over 28 months were reviewed for type of anticoagulation, intraprocedural heparin dosing, ACTs, and adverse outcomes. A heparin dosing strategy was then tested in a prospective validation cohort.
Results
There were 89 patients in the DOAC group and 43 in the warfarin group. Demographics, comorbidities, and complication rates were similar. Mean ACT and percentage of therapeutic ACTs were lower in the DOAC group, most significantly in those with a weight > 90 kg. In DOAC patients, a higher initial heparin bolus ≥ 150 units/kg yielded a higher percentage of therapeutic intraprocedural ACTs (49% ± 10 vs. 29% ± 7, p = 0.0008). In a prospective validation cohort of 25 patients administered an initial heparin bolus ≥ 150 units/kg, the mean ACT was 295 ± 33 and 49% of the ACTs collected were therapeutic, similar to findings of our high-dose retrospective subgroup.
Conclusion
Patients on DOACs require more heparin during AF ablation to achieve therapeutic ACT. We suggest an initial heparin dose of at least 150 units/kg in this subset of patients, particularly in those with a weight > 90 kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Periprocedural management of oral anticoagulation therapy for atrial fibrillation has evolved. Initial strategies called for discontinuation of oral anticoagulation prior to ablation due to a perceived increased bleeding risk with the administration of intraprocedural heparin in patients on long-term oral anticoagulation. Subsequent studies have shown the safety of heparin during ablation in the setting of uninterrupted warfarin therapy [1, 2]. Similarly, studies have shown that uninterrupted, or minimally interrupted, direct oral anticoagulants (DOACs) are equally safe as warfarin with low bleeding and thromboembolic rates [3,4,5,6]. Current guidelines reflect the findings of these studies, with recommendations for uninterrupted oral anticoagulation with intraprocedural administration of heparin prior to or immediately after transseptal puncture to achieve consistent intraprocedural activated clotting times (ACTs) of > 300 s [7]. Current guidelines also suggest the continuation of oral anticoagulation for at least 2 months post-ablation regardless of their stroke risk.
Despite an approximate decade of experience with periprocedural management of DOACs, intraprocedural dosing of heparin remains challenging, even with weight-based dosing [13,14,15,16,17,18,19]. In addition, it has been widely reported that patients on DOACs require more heparin and take longer to achieve therapeutic ACT [8,9,10,11,12,13,14]. There is little evidence showing the impact of weight, body mass index (BMI), or obesity on heparin dosing for patients on a DOAC. The primary aims of this study were to compare periprocedural ACT between patients on warfarin and patients on a DOAC and to determine a baseline weight-based heparin dosage to achieve therapeutic ACT during AF ablation for patients on a DOAC.
2 Methods
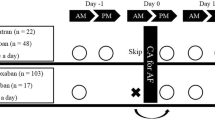
In this retrospective, observational single-center study, charts of consecutive patients who underwent AF ablation by a single operator from September 01, 2015, to January 31, 2018, were reviewed after institutional review board approval. Data collected included patient demographics, comorbidities, periprocedural oral anticoagulation use, intraprocedural dosing of heparin, and intraprocedural ACTs. Patients were divided and analyzed into two groups, those on warfarin and those on DOAC.
All patients underwent a similar protocol for atrial fibrillation ablation under general anesthesia by a single operator. Techniques for transspetal access, RF ablation, venous access, electroanatomical mapping, and hemostasis were consistent throughout the study. All patients received 4 h of bedrest post-procedure.
Patients on warfarin continued the medication uninterrupted through the ablation procedure. DOAC use was minimally interrupted, as patients were instructed to hold the medication for 12–24 h prior to the procedure. Baseline ACT was obtained regardless of OAC (oral anticoagulant) type. Intraprocedural anticoagulation was performed per guidelines with an initial weight-based dose of at least 120 units/kilogram (kg) intravenous unfractionated heparin bolus [7]. A repeat ACT was drawn every 30 min with intervals maintained by a timer function of the electrophysiology lab recording system and measured by a Hemochron® system (Accriva Diagnostics). Additional heparin boluses were administered as required with a goal ACT of ≥ 300 s.
Major bleeding events were reported as defined by the International Society on Thrombosis and Hemostasis (ISTH) [15]. Major bleeding events were reported for up to 8 weeks after ablation, as also stroke, transient ischemic attacks, and systemic embolism. Minor bleeding events were defined as clinical bleeding events that did not fulfill ISTH criteria for major bleeding events.
Twenty-five consecutive patients anticoagulated with DOAC for were then enrolled in a prospective validation cohort using an initial heparin bolus of 150 units/kg. The procedural technique for pulmonary vein isolation, protocol for periprocedural anticoagulation management, intraprocedural heparin administration after an initial bolus, and patient information collected were identical to the retrospective study as detailed above. Results were compared with patients in the retrospective DOAC group.
All data was analyzed using Stata 13 software (StataCorp, Texas, USA). Categorical variables were presented as frequencies and percentages while continuous variables were presented as mean ± standard deviation (SD) if normally distributed, or median with interquartile range (IQR) if not. The Shapiro-Wilk normality test was used to assess the distribution of continuous variables. Continuous variables with normal distribution were compared using Student’s t test and linear regression. The Wilcoxon rank-sum test was used to compare continuous variables with non-parametric distribution. Categorical variables were compared using chi-square and Fischer’s exact tests. All significance tests were 2-sided, and the results were considered statistically significant if the p value was lower than 0.05.
3 Results
A total of 132 patients underwent ablation in the 28-month period and were included in our analysis. There were 89 patients in the DOAC group (group A) and 43 patients in the warfarin group (group B). In group A, there were 11 patients on dabigatran, 39 on apixaban, 36 on rivaroxaban, and 3 on edoxaban. Patient demographics including age, gender, weight, renal function, and comorbidities were similar between the two groups (Table 1). There was no difference in mean procedural duration between the two groups (228 ± 64 min in group A vs. 242 ± 79 min in group B, p = 0.06). While mean procedural ACT was not related to procedural duration (p = 0.48), total heparin dose was proportional to procedural duration.
International normalized ratio (INR) on the day of procedure and baseline ACT were significantly higher in group B as expected (2.01 ± 0.4 vs. 1.10 ± 0.3, p < 0.001 and 164.6 ± 23.8 vs. 124.45 ± 26, p < 0.001, respectively). Mean ACT and the percentage of ACTs within therapeutic range (> 300 s) were higher in group B despite higher initial and total heparin doses in group A (Table 2).
There were no significant differences in complications between the two groups. One pericardial effusion occurred in group A that required pericardiocentesis. There were 2 thromboembolic events in group A (1 stroke on apixaban at 4 weeks, and 1 pulmonary embolism on dabigatran on the second post-operative day). There were 2 minor bleeds, small hematomas, in group A and 1 in group B. A small AV fistula also occurred in group B, which was managed conservatively.
3.1 Weight
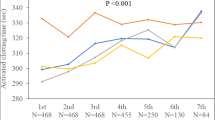
For ease of analysis, patients were divided into quartiles based on weight. On comparison between the two anticoagulation groups within the weight quartiles, mean procedural ACT was similar in quartile 1 (weight ≤ 89.65 kg). There was, however, a significant difference between the two groups in quartiles 2–4, with weights > 89.5 kg, with higher mean ACTs in group B (Fig. 1). Similarly, the percentage of procedural ACTs within therapeutic range was comparable between the two groups for weights ≤ 89.65 kg but were significantly higher in group B in weight quartiles 2–4 (weights > 89.5 kg) (Fig. 2).
3.2 Determination of optimal heparin dosage
To determine an optimal initial heparin dose for patients anticoagulated with DOACs, we retrospectively divided patients in group A into two subgroups: a high-dose cohort who received a dose ≥ 150 units/kg (n = 28 patients), and a low-dose cohort who received a dose < 150 units/kg (n = 61). This value was derived from the cutoff of the highest quartile of initial heparin boluses. While the percentage of 1st ACT within therapeutic range was similar between the two groups (23% in the low-dose group vs. 20% in the high-dose group), there was a significant difference in the total percentage of therapeutic ACTs (49% in the high dose vs. 29% in the low dose, p = 0.0008). Additionally, the mean procedural ACT was > 300 s in the high-dose cohort, compared with 282 s in the low-dose cohort (Table 3).
3.3 Prospective validation cohort with initial heparin dosage ≥ 150 units/kg
All 25 patients in the prospective validation cohort were anticoagulated with either apixaban or rivaroxaban, held for 1 dose prior to AF ablation. Demographic data including age, weight, gender, and co-morbidities were similar to the retrospective group A (Table 4). The initial heparin bolus dose was more tightly regulated in the validation, with mean dose 160.1 ± 12.5 units/kg therefore being lower than the corresponding retrospective DOAC high-dose heparin cohort. The validation cohort also had lower total heparin dose per weight. The mean procedural ACT was just below therapeutic range (295.45 ± 32.84) but not statistically different compared with the retrospective group. Although the percentage of 1st ACT within therapeutic range was lower in the validation cohort, the percentage of 2nd ACT within therapeutic range was similar (Table 4).
4 Discussion
Our results corroborate other studies that found that more heparin is required to achieve a therapeutic ACT during AF ablation in patients on DOACS [8,9,10,11,12,13,14]. Only one previous study has shown weight to be a factor influencing heparin dosage in this patient population. In this study of 417 patients comparing patients on uninterrupted rivaroxaban to uninterrupted warfarin, body weight was found to be a strong predictor of total heparin dose on multivariable analysis with platelet count and INR being weaker predictors. Based on their data, an initial heparin dose of 120 mg/kg was used in a small (n = 25) prospective validation study, with 88% achieving a therapeutic ACT (> 300 s) prior to septal puncture [12]. This recommendation is concordant with current guidelines suggesting the same initial bolus for patients who are taking a DOAC and have held one or two doses [7]. However, our data suggests that a dose of 120 units/kg is insufficient, with equivalent and insufficient mean procedural ACT, total percentage ACT ≥ 300 s, and percentage of 1st and 2nd ACT within therapeutic range compared with the < 120 units/kg group (Table 5). Even with our proposed higher initial bolus, the percentage of 1st and overall ACT values within therapeutic range was suboptimal, as evidenced by our findings in the validation cohort using initial heparin dosage > 150 units/kg. Regardless, an initial bolus of at least 150 units/kg heparin appears to be a more appropriate starting point to achieve therapeutic ACT during ablation, with particular consideration for patients weighing ≥ 89.65 kg. It is possible that an even higher initial heparin weight-based bolus might be required with increasing weight.
Achieving therapeutic intra-operative ACT during AF ablation is of paramount importance. Although a low occurrence of stroke (< 1%) has been reported even in patients who are sub-optimally anticoagulated, stroke can be debilitating with a risk that can be easily modulated with heparin administration [16, 17]. An ACT > 300 has been associated with lower risk of thromboembolic events regardless of oral anticoagulant type [10, 18]. As DOACS are being used more readily, it is important to note the differences in heparin dosing requirements. As we now show, this is especially important in patients who also have weight ≥ 90 kg.
Although the trend of patients on DOACS requiring more heparin to achieve therapeutic ACT during AF ablation than patients on warfarin is well established, little literature exists as to why this occurs. Warfarin acts upon multiple enzymes in the clotting cascade including elements in the intrinsic, extrinsic, and common pathways while DOACs are more specific in their actions, working more downstream in the common pathway. Dabigatran is a direct thrombin inhibitor while the other DOACs are direct factor Xa inhibitors. It would be expected that DOACs would have a similar effect on ACT, which is primarily an evaluation of the intrinsic clotting cascade; however, different variations in ACT values have been reported. It has been suggested that this may be a function of variable ACT sensitivity [19, 20]. In our study, the number of patients on dabigatran was small (n = 11) and there were no differences otherwise between the ACT values of the patients on different DOAC medications.
In addition, although its use is widespread, ACT may not be the best modality to access the level of anticoagulation. There is more than one ACT testing system, and there are reports of a decline in correlation and reproducibility with higher doses of heparin [21, 22]. The use of alternative modalities such as anti-Xa and thromboelastogram (TEG) testing is limited by turnaround time and lack of proximity to the procedural theater. Advancements in these or other technologies are needed to replace ACT.
5 Limitations
The primary limitation of this study is its retrospective and non-randomized nature, which can lead to potential selection bias. External validity may be limited by a single-center, single-operator study. Our results may also not be generalizable to all patients on a DOAC as dabigatran and edoxaban were under-represented; however, this is consistent with general prescription trends of DOACs with apixaban and rivaroxaban being more commonly prescribed [23]. Additionally, pre-procedure DOAC usage was non-uniform due to differences in half-lives and labeled dosing. This may have contributed to ACT variability within this group of patients.
Baseline ACT and INR were not used in our analysis of optimal heparin dosage or our validation cohort. While these additional variables have been used to predictably achieve therapeutic levels in patients anticoagulated with warfarin [24], their significance is less understood in patients anticoagulated with a DOAC. Further studies should consider the determination of the initial heparin dose using a combined formula to accommodate both weight, baseline INR, and baseline ACT in these patients.
6 Conclusion
Patients on direct oral anticoagulants require significantly higher procedural heparin dosage during ablation for atrial fibrillation to achieve a therapeutic ACT of > 300 s in patients weighing ≥ 89.5 kg. We suggest an initial heparin dose of at least 150 units/kg in this subset of patients to achieve an initial therapeutic ACT of > 300 s.
References
Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of Coumadin in preventing thromboembolism in atrial fibrillation (AF) patients undergoing catheter ablation (COMPARE) randomized trial. Circulation. 2014;129:2638–44.
Gautam S, John RM, Stevenson WG, et al. Effect of therapeutic INR on activated clotting times, heparin dosage, and bleeding risk during ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:248–54.
Calkins H, Gerstenfeld EP, Schilling R, Verma A, Willems S. RE-CIRCUIT study-randomized evaluation of dabigatran etexilate compared to warfarin in pulmonary vein ablation: assessment of an uninterrupted periprocedural anticoagulation strategy. Am J Cardiol. 2015;115:154–5.
Di Biase L, Lakkireddy D, Trivedi C, et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm. 2015;12:1162–8.
Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2014;63:982–8.
Reynolds MR, Allison JS, Natale A, Weisberg IL, Ellenbogen KA, Richards M, et al. A prospective randomized trial of apixaban dosing during atrial fibrillation ablation: the AEIOU trial. JACC Clin Electrophysiol. 2018;4:580–8.
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444.
Armbruster HL, Lindsley JP, Moranville MP, Habibi M, Khurram IM, Spragg DD, et al. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann Pharmacother. 2015;49:278–84.
Bin Abdulhak AA, Kennedy KF, Gupta S, Giocondo M, Ramza B, Wimmer AP. Effect of pre-procedural interrupted apixaban on heparin anticoagulation during catheter ablation for atrial fibrillation: a prospective observational study. J Interv Cardiac Electrophysiol : an international journal of arrhythmias and pacing. 2015;44:91–6.
Briceno DF, Villablanca PA, Lupercio F, et al. Clinical impact of heparin kinetics during catheter ablation of atrial fibrillation: meta-analysis and meta-regression. J Cardiovasc Electrophysiol. 2016;27:683–93.
Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805–11.
Enriquez AD, Churchill T, Gautam S, et al. Determinants of heparin dosing and complications in patients undergoing left atrial ablation on uninterrupted rivaroxaban. Pacing Clin Electrophysiol : Pace. 2017;40:183–90.
Kuwahara T, Abe M, Yamaki M, et al. Apixaban versus warfarin for the prevention of periprocedural cerebral thromboembolism in atrial fibrillation ablation: multicenter prospective randomized study. J Cardiovasc Electrophysiol. 2016;27:549–54.
Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M, et al. Differences in activated clotting time and initial heparin dosage during atrial fibrillation ablation for patients with edoxaban compared with warfarin. J Cardiovasc Electrophysiol. 2018;29:835–43.
Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost : JTH. 2005;3:692–4.
Cardoso R, Knijnik L, Bhonsale A, Miller J, Nasi G, Rivera M, et al. An updated meta-analysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Heart Rhythm. 2018;15:107–15.
Haeusler KG, Kirchhof P, Endres M. Left atrial catheter ablation and ischemic stroke. Stroke. 2012;43:265–70.
Ren JF, Marchlinski FE, Callans DJ, et al. Increased intensity of anticoagulation may reduce risk of thrombus during atrial fibrillation ablation procedures in patients with spontaneous echo contrast. J Cardiovasc Electrophysiol. 2005;16:474–7.
Nagao T, Inden Y, Yanagisawa S, Kato H, Ishikawa S, Okumura S, et al. Differences in activated clotting time among uninterrupted anticoagulants during the periprocedural period of atrial fibrillation ablation. Heart Rhythm. 2015;12:1972–8.
Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M, et al. Adequate initial heparin dosage for atrial fibrillation ablation in patients receiving non-vitamin K antagonist oral anticoagulants. Clin Drug Invest. 2016;36:837–48.
Doherty TM, Shavelle RM, French WJ. Reproducibility and variability of activated clotting time measurements in the cardiac catheterization laboratory. Catheter Cardiovasc Interv: official journal of the Society for Cardiac Angiography & Interventions. 2005;65:330–7.
Maslow A, Chambers A, Cheves T, Sweeney J. Assessment of heparin anticoagulation measured using i-STAT and Hemochron activated clotting time. J Cardiothorac Vasc Anesth. 2018;32:1603–8.
Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy. 2018;38:907–20.
Hamam I, Daoud EG, Zhang J, Kalbfleisch SJ, Augostini R, Winner M, et al. Impact of international normalized ratio and activated clotting time on unfractionated heparin dosing during ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:491–6.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This was a retrospective, observational study conducted after institutional review board approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Payne, J.E., Koerber, S.M., Bickel, T. et al. Higher initial weight-based heparin dosing is required with direct oral anticoagulants during catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 58, 185–191 (2020). https://doi.org/10.1007/s10840-019-00579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-019-00579-y