Abstract
Purpose
The aim of this study was to investigate the efficacy and the safety of prophylactic use of protamine in a series of heparinized patients having undergone cryoballoon (CB) ablation for atrial fibrillation (AF).
Methods
From October 2013 to January 2014, 54 consecutive patients received protamine after CB ablation to neutralize unfractionated heparin (UFH) effects. They were prospectively included in this study and compared to a control group of 53 patients who underwent CB ablation without receiving protamine.
Results
A total of 54 consecutive patients (33 male, 61 %; mean age, 58 ± 12 years) were included. Twenty-one patients (39 %) presented with hypertension, 17 (31 %) with dyslipidemia, and 4 (7 %) with diabetes. Five patients (9 %) had a previous episode of ischemic stroke. Mean protamine dose was 68 ± 22 mg. No adverse reaction to protamine was observed. Among patients having received protamine, one (2 %) experienced a cardiac tamponade requiring non-surgical drainage. No patient having undergone protamine administration experienced vascular complications. Conversely, the group of patients not treated with protamine had a significantly higher incidence of vascular complications as compared to patients having undergone protamine infusion (11 vs 0 %, p = 0.01).
Conclusions
Reversing effects of UFH by the means of protamine administration appears to be safe after CB ablation for AF. It can allow in-laboratory sheath removal with potentially less vascular complications and no increase of thromboembolic risk. Larger randomized studies are needed in order to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ablation of atrial fibrillation (AF) by means of cryoballoon (CB) has been proven an effective procedure in achieving pulmonary vein isolation (PVI) [1–3]. Since then, CB ablation has become increasingly popular and is now widely being used for the treatment of symptomatic paroxysmal AF refractory to antiarrhythmic drugs (AADs) [4].
Patients undergoing catheter ablation of AF are at higher risk of intraprocedural and postprocedural thromboembolic (TE) events, including those identified as low-risk before ablation. Therefore, an adequate level of anticoagulation is needed throughout the entire procedural period. Current guidelines recommend intraprocedural administration of unfractionated heparin (UFH) prior to or immediately following transseptal puncture and dose adjustment to maintain an activated clotting time (ACT) above 300 s [4]. Moreover, systemic anticoagulation therapy with warfarin or direct thrombin or factor Xa inhibitors is recommended for at least 2 months following the ablation procedure. Periprocedural anticoagulation, although needful, might contribute to some of the most common procedural complications, including pericardial tamponade and vascular complications. In the setting of CB ablation, vascular complications might be increased due to the large 15-F sheath that is used in conjunction with the above-mentioned device. Thus, reversal of heparin-mediated anticoagulation effects following ablation procedure might be useful in allowing a quicker catheter removal while minimizing the risk of potentially severe procedural complications [5]. Protamine sulfate is the best agent to neutralize anticoagulant effects of UFH and has been safely used for decades in patients undergoing cardiothoracic surgery and percutaneous coronary interventions (PCI) [6, 7]. Moreover, it seems to be useful and safe even in heparinized patients after radiofrequency (RF) ablation procedures, reducing postoperative patient’s immobilization and discomfort with no increase of rate of thrombotic or bleeding complications [8]. To date, no specific data have been reported on protamine use after CB ablation. In the present study, we aim to assess the safety of prophylactic administration of protamine in a series of consecutive heparinized patients having undergone CB ablation for AF.
2 Methods
2.1 Study population
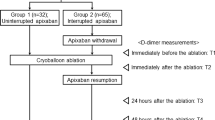
From October 2013 to January 2014, 54 consecutive patients received protamine after CB ablation to neutralize UFH effects and were prospectively included in this study. A retrospective control group was formed by the previous 53 consecutive patients who underwent CB ablation without receiving protamine from May to September 2013. All patients provided informed consent prior to the procedure. Patients were affected by drug-resistant paroxysmal AF. Paroxysmal AF was defined on the frequent occurrence of recurrent episodes of AF self-terminating within 7 days. Data collected included demographic characteristics such as age, sex, height, weight, comorbid conditions, current medications, and allergies. Baseline international normalized ratio (INR) value was obtained for all patients before the procedure. A transthoracic examination (TTE) enabling assessment of left ventricular ejection fraction and intracavitary dimensions as well as valve function was performed within 1 week prior to ablation. To exclude the presence of thrombi in the left atrial appendage, all patients underwent transesophageal echocardiogram (TEE) the day before the procedure. Moreover, all patients underwent a preprocedural computed tomography (CT) scan to assess left atrial anatomy. Periprocedural complications, including TE and bleeding events as well as cardiac tamponade, were collected for any patient. Cerebrovascular event (CVA), transient ischemic attack (TIA), pulmonary embolism (PE), deep vein thrombosis, and myocardial infarction were considered as TE complications. Occurrence of blood loss requiring transfusion or resulting in a 20 % or greater fall in hematocrit, groin hematoma requiring intervention, or intracranial hemorrhage were considered as major bleeding complications. Groin hematoma not requiring intervention was considered as minor vascular complication.
2.2 Exclusion criteria
The exclusion criteria were the presence of left atrial thrombus, severe uncontrolled heart failure, contraindications to general anesthesia, and previous adverse reaction to protamine.
2.3 Cryoballoon ablation
The approach used for CB ablation of AF at our institution has been described in detail previously [9]. All procedures were performed under general anesthesia. Two right-sided femoral venous accesses were obtained. In case of accidental puncture of the femoral artery, a manual compression of the puncture site was performed for at least 10 min before a new puncture of the femoral vein was attempted. After having obtained venous accesses, a 6-Fr decapolar catheter was advanced in the coronary sinus. Then, a single transseptal puncture was carried out. A 0.32-Fr Emerald exchange wire (Cordis, Johnson and Johnson, Diamond Bar, CA, USA) was advanced in the left superior pulmonary vein (PV), and a steerable 15-F over-the-wire sheath (FlexCath Advance, CryoCath, Medtronic, MN, USA) was positioned in the left atrium (LA). A 20-mm in diameter inner lumen mapping catheter (ILMC) (Achieve, Medtronic, MN, USA) was then advanced in each PV ostium to obtain baseline electrical information. After withdrawing the mapping catheter, a 28-mm CB (Arctic Front, Medtronic, MN, USA) was advanced over the wire up to the LA, inflated, and positioned in the PV ostium of each vein. For each vein, CB ablation consisted of at least one application lasting 3 min. In order to avoid phrenic nerve palsy, a potential complication observed during right-sided PV cryoablation, diaphragmatic stimulation was achieved by pacing the ipsilateral phrenic nerve with a 1,000-ms cycle and 20-mA output. Isolation was verified 10 min after the last application. Subsequently, the 15-F sheath was retracted into the right atrium.
2.4 Intraprocedural anticoagulation
UFH was given immediately after having performed the transseptal puncture and having achieved left atrial access. A 100-U/kg bolus was given intravenously. Additional doses of UFH were supplied, if needed, to maintain a target ACT of 300 to 400 s throughout the procedure. Baseline and maximum achieved ACT was determined in all patients. ACT was measured at 15-min intervals until therapeutic anticoagulation was achieved and then 30-min intervals for the duration of the procedure.
2.5 Protamine administration
Protamine was given at the end of the procedure, after having assessed persistence of PV electrical isolation after ablation. Protamine dosage was determined on the basis of the amount of UFH received during the procedure using 1 mg of protamine per 100 units of heparin received. The drug was administered slowly over a time period of 5 min. ACT was measured before and after protamine infusion. Blood pressure was monitored during and after the infusion.
2.6 Sheath removal and mobilization
The 6-F and 15-F femoral sheaths were removed at the end of protamine administration. After the intervention, all patients were transferred to the intensive care unit for observation. In patients who did not undergo protamine administration, sheaths were removed in the intensive care unit when ACT declined to 150 s. Sheaths were removed by the electrophysiologist in charge of the procedure in all patients, despite the use of protamine. Venous bleeding was stopped by means of manual compression lasting at least 10 min. Once the hemostasis was achieved, a period of 10 h of bed rest and groin compression bandage was indicated. No hemostasis devices, such as sandbags, other weights, or fem-o-stops, were used.
2.7 Postprocedural management
All patients were dismissed the day following ablation if they had no complication. After the intervention, patients were continuously monitored with ECG telemetry for at least 18 h. Postprocedural clinical evaluation consisted in a physical examination after 6 h from the procedure and before discharge. The groin was examined for local puncture complications after sheath removal, before mobilization, and at discharge the next day. Moreover, a TTE was performed in all individuals in order to exclude postprocedural pericardial effusion. Oral anticoagulation (OAC) was started the evening of ablation and continued for at least 3 months even in patients who were not on anticoagulants before the procedure. OAC was discontinued after this period in patients with CHA2DS2-VASc score below 2 as recommended by guidelines [4]. Therapy with warfarin was started the same day following ablation, and low molecular weight heparin (LMWH) was used as a bridge to resumption of a target INR of 2–3. Alternatively, novel oral anticoagulants were administered. The choice of the anticoagulant drug was driven by preprocedural patient’s therapy. If warfarin was not interrupted before ablation, use of LMWH was avoided. Antiarrhythmic therapy was administered for 1 month following the procedure and discontinued if the patient was free of AF relapse. Patients who experienced minor vascular complications, such as groin hematomas, were followed up by means of clinical visit at 1 week, 1 month, and 3 months after discharge.
2.8 Statistical analysis
Data are presented as mean ± standard deviation (SD) or as absolute values and percentages where appropriate. The χ 2 test and Fisher’s exact test were used to compare categorical variables. Continuous variables between two groups were analyzed using the unpaired or paired Student’s t test or the Mann-Whitney test as appropriate. A p value less than 0.05 was considered statistically significant. Statistical analyses were conducted using the SPSS software (SPSS v21, IL, USA).
3 Results
3.1 Baseline characteristics
A total of 54 consecutive patients (33 male, 61 %; mean age, 60 ± 11 years) were included. Baseline clinical characteristics of the study population are detailed in Table 1. All patients presented with paroxysmal AF and failed ≥1 class I or III antiarrhythmic drug (AAD). Mean CHA2DS2-Vasc score was 1.6 ± 1.3 and HAS-BLED score was 1.0 ± 0.9. Preprocedural oral anticoagulation therapy consisted of warfarin in 31 patients (57 %) and novel OAC agents in 9 patients (17 %). The remaining 14 patients (26 %) received preprocedural therapy with aspirin. Twenty-seven patients of 31 (87.1 %) in the protamine group and 28 out of 32 (87.5 %) in the control group did not interrupt warfarin before the procedure. Mean INR value was 1.6 ± 0.5. A total of 216 veins were depicted on the preprocedural CT scan.
3.2 Procedural characteristics
Mean procedural and fluoroscopy times were 93 ± 8 and 14 ± 6 min, respectively. All patients underwent the procedure using the 28-mm CB. Femoral artery was accidentally punctured in two patients (4 %). Mean minimal temperatures were −52 ± 7 °C in the left superior PV, −49 ± 5 °C in the left inferior PV, −51 ± 7 °C in the right superior PV, and −49 ± 4 °C in the right inferior PV. All 216 PVs (100 %) could be isolated with the CB only. During procedures, transient phrenic nerve palsy was observed in three patients (5 %). Diaphragmatic contraction resumed before the end of the procedure in all. Procedural characteristics of the study population are shown in Table 2.
3.3 Heparin and protamine administration
The mean dose of UFH administered was 8,900 ± 2,500 U. Initial UFH bolus dose was 8,000 ± 1,700 U. All patients achieved during the procedure an ACT value higher than 300 s. Eleven patients (20 %) received an additional dose of UFH. The average protamine dose was 68 ± 22 mg. Mean ACT value during the procedure was 325 ± 23 s. Mean ACT value before and after protamine infusion was 285 ± 40 and 166 ± 22 s, respectively.
3.4 Postprocedural management
No significant difference was found in time to hemostasis between patients having received protamine and patients in whom protamine was not administered. Conversely, there was a significant reduction in the intensive care monitoring time in patients having undergone protamine as compared to patients in whom protamine was not administered (78 ± 34 vs 167 ± 56 min, p < 0.01). Moreover, no significant difference was found in the time from sheath removal to mobilization between the two groups (687 ± 224 vs 725 ± 301 min, p = 0.46).
3.5 Complications
No adverse reaction to protamine was observed. Among patients having received protamine, one (2 %) experienced a major periprocedural complication: a late cardiac tamponade requiring non-surgical drainage. No vascular complication occurred in the group of patients having undergone protamine infusion. On the other hand, among control group patients, six subjects (11 %) experienced a vascular complication. Five patients had a minor vascular complication which consisted in groin hematoma that did not require any acute intervention. They were followed up with clinical visit at 1 week, 1 month, and 3 months after discharge, and groin hematoma spontaneously resolved in all of them. The other patient experienced a femoral pseudoaneurysm which was treated with thrombin injection with no further vascular sequelae. Of note, the group of patients not treated with protamine had a significantly higher incidence of vascular complications as compared to patients having undergone protamine administration (11 vs 0 %, p = 0.01).
4 Discussion
To our knowledge, this is the first study assessing the periprocedural outcome of protamine administration in heparinized patients having undergone CB ablation for recurrent paroxysmal AF. The main finding of our experience underlines the safety of using protamine to reverse the effects of heparin after CB ablation.
It has been reported that patients undergoing RF catheter ablation have an increased risk of TE events when ablation is performed in the left heart (1.8 to 2 %) [10]. Due to the potentially severe consequences of experiencing a periprocedural TE event, aggressive anticoagulation with UFH is required during catheter ablation of AF and has become a cornerstone of the procedural management of these patients [4]. To reduce the risk of bleeding, ACT must return towards normal range (<180 s) prior to the removal of the femoral sheaths. Due to the 90-min half-life of UFH, this process can take several hours and the patient can experience discomfort for the prolonged immobilization period and for the 15-F femoral sheath placed in the femoral vein. Most importantly, the presence of a large sheath in the femoral vein for prolonged time could increase the risk of vascular complications.
Current guidelines state that administration of protamine should be considered in patients having undergone AF ablation [4]. Protamine is a complex of polycationic peptides. It has been demonstrated in vitro to decrease thrombin activity, decrease activation of factor VII by tissue factor, enhance tissue-type plasminogen activator-mediated fibrinolysis, decrease factor V activation by activated factor X or thrombin, and attenuate platelet function by inhibition of glycoprotein Ib-von Willebrand factor activity [11–15]. Because protamine is positively charged, it forms a stable complex with the negatively charged heparin, a heterogeneous compound with alternating residues of iduronic acid and glucosamine. The protamine-heparin complex is devoid of anticoagulant activities. Protamine can shorten the amount of time from the end of the procedure to when the femoral sheaths can be removed and the patient allowed to mobilize. Additionally, in-laboratory removal of the femoral sheath can reduce groin complications and can shorten the postprocedural period of bed rest and hospital stay after an invasive procedure [5, 7]. It has been shown that antagonizing heparin with protamine enables immediate removal of an 8-F sheath from the femoral artery in patients having undergone PCI with no increased risk of TE complications [6]. Moreover, protamine has been used to treat bleeding complications following PCI. In fact, the majority of episodes of cardiac tamponade can be successfully managed by immediate percutaneous drainage and reversal of anticoagulation with protamine.
The impact of protamine neutralization of UFH on TE and bleeding complications has been previously evaluated in the setting of RF ablation of atrial and ventricular arrhythmias by a retrospective study by Patel et al., and no significant difference in complications was found in patients treated with protamine as compared to those not receiving protamine [8]. Similarly, in our study, TE or bleeding complications were not increased in the group of patients treated with protamine as compared to the control group. One patient experienced a late cardiac tamponade after CB ablation which did not require surgical intervention and was solved by percutaneous drainage and facilitated by protamine administration. Of note, in our study the incidence of vascular complications, including groin hematomas and pseudoaneurysms, was significantly lower in the group of patients having undergone protamine administration as compared to those not having received protamine.
Although unfrequently, use of protamine can be occasionally associated with potentially severe adverse reactions including hypotension, anaphylactic responses, and pulmonary vasoconstriction [16]. In our study, no adverse reaction to protamine occurred. Episodes of abrupt hypotension during protamine infusion have been reported in 1.2 % of patients undergoing catheter ablation of AF [17]. Although rapid administration of protamine can be the cause of abrupt onset of hypotension, its specific mechanism is still not well understood. Appropriate interventions for these cases should include maintenance of the airway with 100 % oxygen and rapid intravascular volume expansion. Moreover, epinephrine can be used in patients with severe hypotension not responding to fluid resuscitation.
5 Limitations
Our study has a certain number of limitations. First of all, it is a single-center non-randomized study conducted in a limited number of patients. Furthermore, the calculated dose of protamine based on the total amount of heparin administered does not take into consideration heparin elimination and could have resulted in excess circulating protamine.
6 Conclusions
Reversing effects of heparin by means of protamine administration appears to be safe after CB ablation for AF. It can allow in-laboratory sheath removal with potentially less vascular complications and no increase of thromboembolic risk. Larger randomized studies are needed in order to confirm our findings.
References
Van Belle, Y., Janse, P., Rivero-Ayerza, M. J., Thornton, A. S., Jessurun, E. R., Theuns, D., et al. (2007). Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. European Heart Journal, 28, 2231–2237.
Packer, D. L., Kowal, R. C., Wheelan, K. R., Irwin, J. M., Champagne, J., Guerra, P. G., et al. (2013). Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. Journal of the American College of Cardiology, 61(16), 1713–1723.
Andrade, J. G., Khairy, P., Guerra, P. G., Deyell, M. W., Rivard, L., Macle, L., et al. (2011). Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm, 8(9), 1444–1451.
Calkins, H., Kuck, K. H., Cappato, R., Brugada, J., Camm, A. J., Chen, S. A., et al. (2012). HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Journal of Interventional Cardiac Electrophysiology, 2012, 171–257
Pan, M., Suárez de Lezo, J., Medina, A., Romero, M., Hernández, E., Segura, J., et al. (1997). In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. American Journal of Cardiology, 80(10), 1336–1338.
Briguori, C., Di Mario, C., De Gregorio, J., Sheiban, I., Vaghetti, M., & Colombo, A. (1999). Administration of protamine after coronary stent deployment. American Heart Journal, 138(1 Pt 1), 64–68.
Kuiper, K. K., & Nordrehaug, J. E. (2000). Early mobilization after protamine reversal of heparin following implantation of phosphorylcholine-coated stents in totally occluded coronary arteries. American Journal of Cardiology, 85(6), 698–702.
Patel, A. A., Clyne, C. A., Henyan, N. N., White, C. M., Zembrowski, B. F., Migeed, M., et al. (2007). The use of protamine after radiofrequency catheter ablation: a pilot study. Journal of Interventional Cardiac Electrophysiology, 18(2), 155–158.
Chierchia, G. B., Di Giovanni, G., Sieira-Moret, J., de Asmundis, C., Conte, G., Rodriguez-Mañero, M., et al. (2014). Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. Journal of Interventional Cardiac Electrophysiology, 39(2), 145–151.
Zhou, L., Keane, D., Reed, G., & Ruskin, J. (1999). Thromboembolic complications of cardiac radiofrequency catheter ablation: a review of the reported incidence, pathogenesis and current research directions. Journal of Cardiovascular Electrophysiology, 10(4), 611–620.
Cobel-Geard, R. J., & Hassouna, H. I. (1983). Interaction of protamine sulfate with thrombin. American Journal of Hematology, 14, 227–233.
Chu, A. J., Wang, Z., Raicu, M., Beydoun, S., & Ramos, N. (2001). Protamine inhibits tissue factor-initiated extrinsic coagulation. British Journal of Haematology, 115, 392–399.
Nielsen, V. G. (2006). Protamine enhances fibrinolysis by decreasing clot strength: role of tissue factor-initiated thrombin generation. Annals of Thoracic Surgery, 81, 1720–1727.
Ni Ainle, F., Preston, R. J. S., Jenkins, P. V., Nel, H. J., Johnson, J. A., Smith, O. P., et al. (2009). Protamine sulfate down-regulates thrombin generation by inhibiting factor V activation. Blood, 114, 1658–1665.
Barstad, R. M., Stephens, R. W., Hamers, M. J., & Sakariassen, K. S. (2000). Protamine sulfate inhibits platelet membrane glycoprotein Ib-von Willebrand factor activity. Thrombosis & Haemostasis, 83, 334–337.
Horrow, J. C. (1985). Protamine: a review of its toxicity. Anesthesia and Analgesia, 64(3), 348–361.
Chilukuri, K., Henrikson, C. A., Dalal, D., Scherr, D., MacPherson, E. C., Cheng, A., et al. (2009). Incidence and outcomes of protamine reactions in patients undergoing catheter ablation of atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 25(3), 175–181.
Conflict of interest
GBC and CdA have received compensation for teaching and proctoring services for AF solutions as well as speaker fees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gian-Battista Chierchia and Pedro Brugada contributed equally as senior authors.
Rights and permissions
About this article
Cite this article
Conte, G., de Asmundis, C., Baltogiannis, G. et al. Periprocedural outcomes of prophylactic protamine administration for reversal of heparin after cryoballoon ablation of atrial fibrillation. J Interv Card Electrophysiol 41, 129–134 (2014). https://doi.org/10.1007/s10840-014-9922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-014-9922-y