Abstract
Background
Single-shot ablation has emerged as an effective technique for index atrial fibrillation (AF) ablation, with an advantage of short procedure time. Although recent guidelines recommend peri-procedural uninterrupted oral anticoagulants (OACs), the intra-procedural anticoagulation strategy remains uncertain under non-vitamin K OACs (NOACs). We investigated procedural safety of a single bolus administration of heparin without activated clotting time (ACT) measurement during cryoballoon ablation (CBA).
Methods
Two hundred patients (64.2 ± 10.0 years, 70% with non-paroxysmal AF) who underwent CBA with uninterrupted NOACs were randomly assigned to No-ACT group and ACT group. A bolus of heparin (100 U/kg) was routinely administered immediately after transseptal puncture. In the ACT group, an additional injection of heparin (30 U/kg) was administered if ACT at 30 min after the initial bolus was < 300 s.
Results
There were no differences in baseline characteristics including CHA2DS2-VASc score between the two groups. The left atrium indwelling and procedure times were 60.4 ± 13.1 min and 78.9 ± 13.9 min, respectively, and not significantly different between the two groups. The mean ACT was 335.2 ± 59.9 s in the ACT group. Any bleeding rate was 3.2% in all patients and there was no statistically significant difference in bleeding complications between the two groups. In the ACT group, groin hematoma, laryngopharyngeal bleeding, and hemoptysis occurred in 3, 1, and 1 patient, respectively. Cardiac tamponade occurred in 1 patient in the No-ACT group. No thromboembolic events occurred during the 30-day follow-up after CBA.
Conclusions
Single bolus administration of heparin without ACT measurement is a feasible anticoagulation strategy for CBA in patients with uninterrupted NOAC intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) is an effective rhythm control therapy for atrial fibrillation (AF) [1, 2]. However, procedure-related complications still cannot be ignored and thromboembolic complications remain a major limitation of AF ablation. Patients undergoing AF ablation are at a higher risk of intra- and post-procedural thromboembolic events; therefore, peri-procedural anticoagulation is necessary. Since non-vitamin K oral anticoagulants (NOACs) have been commonly used for stroke prevention of AF, recent studies examined the peri-procedural management of NOACs in patients scheduled to undergo radiofrequency catheter ablation (RFCA) for AF [3,4,5,6]. Based on these results, the performance of the ablation procedure without interruption of oral anticoagulant (OAC) is recommended for patients undergoing AF ablation who have been therapeutically anticoagulated with OAC including NOACs [7].
In terms of intra-procedural anticoagulation management, unfractionated heparin should be administered prior to or immediately following transseptal puncture and dose adjustment is recommended to achieve and maintain an activated clotting time (ACT) of at least 300 s [8]. However, this recommendation is based on results of studies conducted using vitamin K antagonists (VKA) in a setting of point-by-point AF ablation [9]. Moreover, the majority of recommendations were made based on results from studies evaluating the efficacy and safety of RFCA and not on diverse modalities for AF ablation. Several modalities use different energy sources to achieve PVI. Cryoballoon ablation (CBA), a balloon-based single shot technique for PVI, has shown the similar efficacy and safety profile with a relatively short learning curve compared to RFCA [10,11,12,13,14]. Previous randomized clinical trials (RCTs) demonstrated that CBA had a shorter procedure time and lower rates of re-hospitalization, cardioversion, and repeat ablation than RFCA. Therefore, the use has exponentially increased.
However, no data have been available in terms of anticoagulation strategy during CBA for patients who received uninterrupted NOACs. We aimed to investigate the safety of a simple anticoagulation strategy using a single bolus administration of heparin without ACT monitoring compared to that of the conventional approach using ACT-guided heparin administration in patients who underwent CBA with uninterrupted NOACs.
2 Methods
2.1 Study population
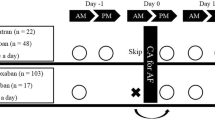
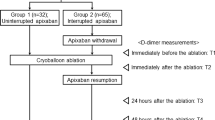
This trial was a randomized, open-label, single-center study that included 200 patients undergoing first-time CBA for drug-refractory AF who had been taking once-daily rivaroxaban (n = 100) or edoxaban (n = 100) between July 2019 and June 2020. Paroxysmal AF (PAF) was defined as a self-terminating AF that lasted ≤ 7 days. The exclusion criteria were patients younger than 18 years, mechanical prosthetic valve or moderate-severe mitral stenosis, a history of AF ablation or cardiac surgery, chronic kidney disease (creatinine clearance rate [CCr] < 30 mL/min or dialysis), an acute stroke within 6 months, and a refusal to take NOACs in the long-term period. The study protocol was approved by the institutional review board of each institute and complied with the principles of the Declaration of Helsinki. Written informed consent was obtained from all enrolled patients.
2.2 Anticoagulation
Figure 1 shows a flow diagram of the study population. Of the enrolled participants, we excluded 6 and 4 patients taking rivaroxaban and edoxaban, respectively, because they did not have continuous NOACs; therefore, 190 patients completed the study. During the study period, all enrolled patients took their scheduled dose of rivaroxaban 20 mg (15 mg if CCr was < 50 mL/min) or edoxaban 60 mg (30 mg if they met one or more dose reduction criteria) [15] at night (recommended hora somni).
Patients with or without additional peri-procedural ACT-guided heparin administration were randomly assigned in a 1:1 ratio to an ACT or a No-ACT group, respectively. Systemic anticoagulation with NOACs has been initiated at least 3 weeks before the procedure, during the procedure, and at least 2 months post-ablation. All patients received intra-procedural anticoagulation with intravenous unfractionated heparin. A bolus of heparin (100 U/kg) was routinely administered immediately after transseptal puncture in all patients. In the ACT group, an additional injection of heparin (30 U/kg) was administered if the ACT at 30 min after the initial bolus administration was < 300 s. In the No-ACT group, ACT was not measured and no additional heparin was administered.
2.3 Ablation procedure
Deep sedation was performed with a continuous infusion of propofol and fentanyl. A 9-French (Fr) long sheath, a 7-Fr long sheath, and an 8.5-Fr long sheath (SL-1) were introduced through the right femoral vein. Intracardiac echocardiography (ICE; ViewFlex™, Abbott, Inc., USA) was used to identify the absence of thrombi in the left atrium (LA) or LA appendage before transseptal puncture and to guide the appropriate transseptal puncture for CBA in all patients. After a single transseptal puncture, the SL-1 sheath was exchanged with a 15-Fr steerable sheath (FlexCath™, Medtronic, USA). The cryoballoon (CB) catheter (CB2; Arctic Front AdvanceTM, Medtronic), assembled with an intraluminal mapping catheter (20 mm, Achieve™, Medtronic), was gently advanced through the steerable sheath until it reached the distal CB shaft marker to the entrance of the steerable sheath. The CB catheter was advanced over the intraluminal mapping catheter inserted into each PV and inflated. After advancing the inflated CB to each PV antrum, Doppler ICE imaging was used to identify the complete occlusion of each PV. With best-fit occlusion, the mapping catheter was used to obtain PV potential recordings for real-time monitoring of PVI. The dosing regimen was different depending on the time to isolation (TTI, time from freeze initiation until loss of PV potentials). An initial freeze was delivered for 3 min if the TTI was ≤ 30 s. Otherwise, the initial freeze was delivered for 4 min. The freeze stopped and reposition of the CB catheter was performed if the TTI was not achieved or the temperature did not reach − 40 °C for 60-s freezing. After PVI, a bonus freeze was applied for 2 min at the more antral side of each PV to create a wide antral circumferential ablation. To avoid phrenic nerve palsy, diaphragmatic stimulation was achieved by pacing the ipsilateral phrenic nerve with a 900-ms cycle and a 20-mA output during right-sided CBA. A fisherman’s knot suture closure without protamine injection was performed to obtain femoral venous hemostasis following sheath removal. After 6 h of immobilization, puncture sites were inspected. NOAC was taken at the scheduled time on the day of the procedure after confirming complete hemostasis or the absence of bleeding complications.
2.4 Study endpoint
The study endpoint was the safety outcomes including thromboembolic events and bleeding complications during the procedure and 30 days following CBA. Thromboembolism was diagnosed by a combination of both the clinical situation and radiologic studies. Thromboembolic events were defined as stroke, transient ischemic attack (TIA), or systemic embolism (SE). Bleeding complications were defined as any procedure-related bleeding.
2.5 Statistical analysis
The sample size was arbitrary adopted based on previous study conducted by catheter ablation [3] because no data was available in terms of anticoagulation strategy during CBA for patients who received uninterrupted NOACs. Central randomization at a 1:1 ratio was performed using computer-generated random permutation sequences to avoid any potential bias. All normally distributed continuous variables are expressed as mean and standard deviation (SD) and were compared using Student’s t-test. Categorical variables were expressed as numbers and percentages. Categorical variables are reported as counts and proportions and were analyzed using Pearson’s chi-square or Fisher’s exact tests, as appropriate. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA).
3 Results
3.1 Baseline characteristics
Baseline characteristics of enrolled patients are shown in Table 1. The mean age was 64.2 ± 10.0 years. The majority of patients were male (80.0%) and had non-PAF (70.0%). The mean CHA2DS2-VASc score was 2.6 ± 1.7, and CCr was 76.2 ± 24.4 mL/min. There were no significant differences in the medical history and echocardiographic parameters between the two groups, except for vascular disease (P = 0.035). Compared to the ACT group, concomitant use of anti-platelet agent was higher in the No-ACT group, but was not statistically different between the two groups.
3.2 Procedural outcomes
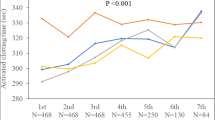
The procedural data are presented in Table 2. Successful PVI was achieved in all enrolled patients. The average total procedure and LA indwelling times were 78.9 ± 13.9 min and 60.4 ± 13 min, respectively. The mean ablation and fluoroscopy times were 27.7 ± 9.4 min and 3.3 ± 3.5 min, respectively. In the ACT group, the mean ACT at 30 min after the initial bolus administration of heparin (100 U/kg) was 335.2 ± 59.9 s, and additional heparin injection was performed in 31 patients (33%). There were no significant differences in the total procedure time, LA indwelling time, ablation time, fluoroscopy time, and initial heparin dose between the two groups, except for the total heparin dose (P < 0.001). Figure 2 shows the mean heparin dose and mean ACT between patients taking rivaroxaban and those taking edoxaban in the ACT group. The mean ACT of patients taking rivaroxaban was significantly lower than that of patients taking edoxaban (316.9 ± 46.0 s vs. 353.1 ± 66.6 s, P = 0.003); nonetheless, the initial heparin dose was significantly higher in patients taking rivaroxaban (P < 0.001).
3.3 Safety outcomes
Table 3 shows procedure-related complications. During the 30-day period following CBA, none of the enrolled patients had stroke, TIA, or SE events. Any bleeding events occurred in 6 patients, 5 in the ACT group and 1 in the No-ACT group, but there was no significant difference between the two groups. In the ACT group, groin hematoma developed in 3 patients, and laryngopharyngeal bleeding and hemoptysis developed in each 1 patient. In the No-ACT group, cardiac tamponade requiring urgent pericardial drainage developed in 1 patient during linear ablation at the cavo-tricuspid isthmus. None of the enrolled patients developed fatal complications. Table 4 shows bleeding events in patients with or without an additional administration of heparin. Any bleeding event rate was higher in patients with an additional heparin injection (9.7%) compared to those without (1.9%), but there was no statistical difference between the groups (P = 0.056).
4 Discussion
To the best of our knowledge, our study is the first prospective RCT to evaluate the safety of a single bolus administration of heparin (100 U/kg) without ACT monitoring under the condition of uninterrupted NOACs in patients with AF undergoing CBA. No thromboembolic or fatal events occurred during the 30-day follow-up period following CBA.
Notwithstanding the low CHA2DS2-VASc scores, patients undergoing AF ablation have the greatest risk for intra- and post-procedural thromboembolism development. An appropriate anticoagulation management is, therefore, essential throughout the entire procedural period. Current guidelines recommend uninterrupted systemic OAC therapy for at least 3 weeks before and 2 months after AF ablation, regardless of the CHA2DS2-VASc score. Moreover, intra-procedural administration of unfractionated heparin prior to or immediately after transseptal puncture as well as additional dose adjustment every 15–30 min to maintain ACT above 300 s is recommended. However, this recommendation about intra-procedural heparin administration was based on the results of studies using VKA in patients with AF undergoing RFCA.
In terms of the initial loading dose of heparin, previous studies revealed that the administration of a higher dose of heparin was required to achieve the target ACT in AF patients with uninterrupted NOACs therapy than in those with uninterrupted VKA therapy [16,17,18,19]. In addition, Yamaji et al. [20] reported that an adequate loading dose of heparin was different for each NOAC because NOACs have different mechanisms of action. In the present study, although the average ACT in the ACT group was over 300 s (335.2 ± 59.9), there was significant difference in mean ACT between patients who took rivaroxaban and those who took edoxaban (P = 0.003). This finding is in line with that reported in previous studies.
A previous study demonstrated that radiofrequency (RF) energy was significantly more thrombogenic than cryoenergy, with a higher incidence of thrombus formation and larger thrombus volumes [21]. Compared to cryoablation, RF ablation resulted in a > fivefold increased risk of thrombus formation with larger thrombus volumes for endocardial ablation lesions of equal size in equivalent cardiac chambers. Specifically, the thrombus volume was positively correlated with the extent of hyperthermic tissue injury, whereas it was not related to the degree of hypothermic tissue injury. They suggested that these results probably reflected the histological observation that cryoablation resulted in well-delineated and discrete lesions with preservation of tissue ultrastructure, including the endothelial cell layer. In contrast, RF energy–induced lesions had serrated edges with extensive endothelial cell destruction.
However, several studies evaluating the incidence of acute silent cerebral lesions after PVI using magnetic resonance imaging (MRI) have demonstrated that CBA may not be associated with a lower risk of thromboembolic cerebral events than RFCA [22,23,24]. These results underline the hypothesis that thrombus formation originating from an extended destruction of the endothelium surface is probably not the only cause of acute cerebral lesions. Although the definite cause of micro-cerebral infarctions associated with AF ablation has not been fully elucidated, several possible embolic sources are considered, such as gaseous emboli or microthrombi entrapped within the sheath during catheter insertion or extraction, platelet-rich microthrombi formed at the endothelial injury site, gaseous emboli formed during blood heating, and microparticles (char) as a result of rapid hyperthermal tissue damage. Among these possible embolic sources, the first can be the presumable cause of microthromboembolism associated with CBA. Therefore, the systemic use of intravenous heparin does not appear to fully eliminate the CBA-related microthromboembolic risk. Instead, intermittent flushing with heparinized saline through the central lumen of the steerable sheath during the entire CBA procedure appears to be more effective in preventing the formation of gaseous emboli or microthrombi at the gap portion between the steerable sheath and CB shaft [25].
A recent meta-analysis demonstrated a significantly shorter procedure time in CBA compared to RFCA [26]. The total procedural time in previous studies has been reported from 73.5 ± 16 to 192.9 ± 44 min during CBA and from 118.5 ± 15 to 283.7 ± 78.0 min during RFCA [27]. In the present study, the total procedure and LA indwelling times were 79.0 ± 14.3 min and 60.4 ± 13.2 min, respectively. If the LA indwelling time is less than 60 min, additional administration of heparin would not be necessary, especially in patients with ACT of above 300 s at 30 min after transseptal puncture. Peripheral vascular complications are cumulatively reported in approximately 1–2% of cases, and cardiac perforation was noted in 0.1–3.2% of cases [28,29,30]. The incidence of stroke or TIA has been reported to be lower than 1% despite a relatively high rate of silent cerebral embolic lesions [31]. In the present study, no patient developed thromboembolic events and fatal complications. Any bleeding rate was 3.2% in all enrolled patients. Non-fatal bleeding complications including groin hematoma, laryngopharyngeal bleeding, and hemoptysis were only observed in the ACT group. Also, additional heparin injection showed a tendency for increasing bleeding event (P = 0.056). The incidence of any bleeding complications was similar to other studies [8], but no statistical difference between the two groups was found which may be due to small sample size.
As VKA and NOACs have different effects on the intrinsic and/or extrinsic blood coagulation cascade, the same intra-procedural anticoagulation strategy could not be applied in patients with uninterrupted VKA and NOACs intake. However, the recommendation about intra-procedural anticoagulation regimens has not been changed, even though different ablation modalities such as CBA are widely used in clinical practice. Indeed, intra-procedural anticoagulation with unfractionated heparin might cause minor or major bleeding complications, which could be increased in patients undergoing CBA for whom a 15-Fr sheath is used. Therefore, the results of the present study may be meaningful for reappraising the intra-procedural anticoagulation strategy under the condition of uninterrupted NOACs at timely manner. In addition, a single bolus administration of heparin strategy without ACT measurements can be beneficial through cost and labor savings that can be incurred by repeated measures to reach the target ACT.
This study had several limitations. First, the sample size was not large enough and the number of events was small. Thus, it might be insufficient to identify a significant difference in clinical outcomes depending on whether or not an additional dose of heparin was administered. Furthermore, highly experienced operators performed the procedure in the present study, which could reduce the sensitivity of the results. Second, only two NOACs were evaluated in this study. In order to maintain a similar inhibitory effect of endogenous factor Xa between the two groups, we enrolled only patients who had been taking once-daily NOACs (rivaroxaban and edoxaban) at night, and the maximum interval between the last pre-ablation NOAC dose and the ablation procedure was 12 h. Therefore, the results could not be extrapolated to all NOACs. Third, silent stroke was not reflected in thromboembolic events because routine MRI before and after CBA was not performed in this study. Therefore, further RCT including brain imaging study should be needed to generalize this strategy. Lastly, we could not measure the peak level of ACT because we measured ACT at 30 min after the initial bolus administration of heparin. However, it might be unnecessary to assess the peak ACT level because the LA indwelling time was relatively short.
5 Conclusions
The present study demonstrated that a single bolus injection of heparin (100 U/kg) without ACT measurement was an effective and safe intra-procedural anticoagulation regimen in patients with uninterrupted NOACs undergoing CBA, which had a relatively short LA indwelling time. With the current increase in NOAC administration and CBA, our results would be helpful in determining the peri-procedural anticoagulation strategy. Further large-scaled RCTs are required to clarify the uncertainty about an optimal intra-procedural anticoagulation management under the condition of uninterrupted NOACs.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66.
Khan AR, Khan S, Sheikh MA, Khuder S, Grubb B, Moukarbel GV. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation: systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2014;7(5):853–60.
Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ, et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36(28):1805–11.
Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376(17):1627–36.
Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J. 2018;39(32):2942–55.
Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L, et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J. 2019;40(36):3013–21.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020. https://doi.org/10.1093/eurheartj/ehaa612
Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–444.
Biase LD, Gaita F, Toso E, Santangeli P, Mohanty P, Rutledge N, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014;11(5):791–8.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–45.
Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37(38):2858–65.
Aryana A, Singh SM, Kowalski M, Pujara DK, Cohen AI, Singh SK, et al. Acute and long-term outcomes of catheter ablation of atrial fibrillation using the second-generation cryoballoon versus open-irrigated radiofrequency: a multicenter experience. J Cardiovasc Electrophysiol. 2015;26(8):832–9.
Squara F, Zhao A, Marijon E, Latcu DG, Providencia R, Di Giovanni G, et al. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace. 2015;17(5):718–24.
Ciconte G, Baltogiannis G, de Asmundis C, Sieira J, Conte G, Di Giovanni G, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace. 2015;17(4):559–65.
Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
Briceno DF, Villablanca PA, Lupercio F, Kargoli F, Jagannath A, Londono A, et al. Clinical impact of heparin kinetics during catheter ablation of atrial fibrillation: meta-analysis and meta-regression. J Cardiovasc Electrophysiol. 2016;27(6):683–93.
Payne JE, Koerber SM, Bickel T, Ghadban R, Flaker G, Gautam S. Higher initial weight-based heparin dosing is required with direct oral anticoagulants during catheter ablation for atrial fibrillation. J Interv Card Electrophysiol. 2020;58(2):185–91.
Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M, et al. Differences in activated clotting time and initial heparin dosage during atrial fibrillation ablation for patients with edoxaban compared with warfarin. J Cardiovasc Electrophysiol. 2018;29(6):835–43.
Armbruster HL, Lindsley JP, Moranville MP, Habibi M, Khurram IM, Spragg DD, et al. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann Pharmacother. 2015;49(3):278–84.
Yamaji H, Murakami T, Hina K, Higashiya S, Kawamura H, Murakami M, et al. Adequate initial heparin dosage for atrial fibrillation ablation in patients receiving non-vitamin K antagonist oral anticoagulants. Clin Drug Investig. 2016;36(10):837–48.
Khairy P, Chauvet P, Lehmann J, Lambert J, Macle L, Tanguay JF, et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107(15):2045–50.
Neumann T, Kuniss M, Conradi G, Janin S, Berkowitsch A, Wojcik M, et al. MEDAFI-trial (micro-embolization during ablation of atrial fibrillation): comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace. 2011;13(1):37–44.
Herrera Siklody C, Deneke T, Hocini M, Lehrmann H, Shin DI, Miyazaki S, et al. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J Am Coll Cardiol. 2011;58(7):681–8.
Nakamura T, Okishige K, Kanazawa T, Yamashita M, Kawaguchi N, Kato N, et al. Incidence of silent cerebral infarctions after catheter ablation of atrial fibrillation utilizing the second-generation cryoballoon. Europace. 2017;19(10):1681–8.
Su W, Kowal R, Kowalski M, Metzner A, Svinarich JT, Wheelan K, et al. Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3000 procedures. Heart Rhythm. 2015;12(7):1658–66.
Fortuni F, Casula M, Sanzo A, Angelini F, Cornara S, Somaschini A, et al. Meta-analysis comparing cryoballoon versus radiofrequency as first ablation procedure for atrial fibrillation. Am J Cardiol. 2020;125(8):1170–9.
Jin ES, Wang PJ. Cryoballoon ablation for atrial fibrillation: a comprehensive review and practice guide. Korean Circ J. 2018;48(2):114–23.
Cardoso R, Mendirichaga R, Fernandes G, Healy C, Lambrakos LK, Viles-Gonzalez JF, et al. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2016;27(10):1151–9.
Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014;129(25):2638–44.
De Ponti R, Cappato R, Curnis A, Della Bella P, Padeletti L, Raviele A, et al. Trans-septal catheterization in the electrophysiology laboratory: data from a multicenter survey spanning 12 years. J Am Coll Cardiol. 2006;47(5):1037–42.
Mugnai G, Irfan G, de Asmundis C, Ciconte G, Saitoh Y, Hunuk B, et al. Complications in the setting of percutaneous atrial fibrillation ablation using radiofrequency and cryoballoon techniques: a single-center study in a large cohort of patients. Int J Cardiol. 2015;196:42–9.
Funding
This work was supported by the Medtronic Korea, Ltd. (H20190710).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study protocol was approved by Hallym University Sacred Heart Hospital Institutional Review Board (HALLYM2019-04025).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, D.G., Lee, M., Ahn, J. et al. Safety of a single bolus administration of heparin without the measurement of activated clotting time during cryoballoon ablation: a prospective randomized controlled trial. J Interv Card Electrophysiol 66, 463–470 (2023). https://doi.org/10.1007/s10840-022-01349-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01349-z