Abstract
Lead-free ceramics (Bi0.5Na0.5)0.94-δBa0.06Ti1−xNbxO3 (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) were prepared via a solid-state sintering method. The ceramics exhibit pure perovskite structure as x ≤ 0.04, while the ceramic with x = 0.05 has a secondary phase. The rhombohedral (R)–tetragonal (T) morphotropic phase boundary exists in all the samples. The relative content of the T phase increases due to the Nb5+ doping. The ceramics show dense microstructures with mean grain sizes around 1–2 μm. The changes of dielectric constant and dielectric loss as a function of temperature for the unpoled and poled samples were compared. Two dielectric anomalies appear on the dielectric curves around the temperatures denoted as TRE and Tm. With the increase of Nb5+ amount, the values of TRE decrease and Tm increase. The Nb5+-doped ceramics exhibit better temperature stability of dielectric constant during a wide high-temperature window. The polarization hysteresis (P–E) loops change from typical ferroelectric loops for the sample with x = 0 to constricted P–E loops with increasing Nb5+ amount. The ceramic with x = 0.03 shows a maximum strain (Smax) of 0.55% and piezoelectric strain constant (d33*) of 675 pm/V.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
(Bi0.5Na0.5)0.94Ba0.06TiO3 (BNBT)-based piezoelectric ceramics, which have a tetragonal (T)–rhombohedral (R) morphotropic phase boundary (MPB), have been widely explored [1, 2]. Many studies have focused on the doping of ions into BNBT-based ceramics to improve their electrical properties. The dopants can greatly affect structure and electrical behavior of the BNBT-based ceramics. Li et al. reported that the doping of La3+ into the A-site of BNBT disrupts dominant long-range ferroelectric order of the ceramics and significantly weakens ferroelectric properties [3]. Yang et al. found that the doping of La3+ into BNBT causes a decrease in grain sizes of the ceramics and improves dielectric breakdown strength [4]. Wang et al. reported that the co-doping of Ce4+ and La3+ into BNBT greatly improves their piezoelectric and dielectric constants [5]. Jing et al. studied structure, dielectric, and piezoelectric properties of (Na0.47Bi0.47Ba0.06)0.95Ca0.05TiO3 and (Na0.47Bi0.47Ba0.06)0.95Sr0.05TiO3 ceramics, in which the Ca2+ and Sr2+ have the same electric charge and different radii, and found that the average ionic radii of the A-site cations in the ABO3 structure are important in affecting structure and electrical properties of BNBT-based ceramics [6]. The dopants into the B-site of BNBT are also important to tune electrical properties of the ceramics. Wang et al. reported that the doping of Zr4+ into (Na0.47Bi0.46Ba0.06K0.01)(Nb0.02Ti0.98)O3 causes a decrease in dielectric constant and decrement of ferroelectric properties of the ceramics [7]. The doping of Fe3+ into 0.94Bi0.5Na0.5TiO3-0.06BaTiO3 induces more obvious diffusion character of the phase transition around Curie temperature, which was suggested to be related to the formation of oxygen vacancies [8]. Wang et al. found that the Fe3+ doping into (Bi0.5Na0.4K0.1)Ti0.98Fe0.02O3-δ reduces dielectric constant and depolarization temperature of the ceramics [9]. The doping of Co3+ into 0.8Bi0.5Na0.5TiO3–0.2Bi0.5K0.5TiO3 promotes grain growth, increases electric conductivity, and weakens piezoelectric properties [10]. Oxygen vacancies can always be introduced into the lattice if the cations with the electric charge lower than that of Ti4+ are doped into the B-site (as commonly be called hard doping). The hard doping helps increasing electromechanical behavior of the piezoelectric materials. If the cations with the electric charge higher than + 4 are introduced into the B-site, the piezoelectricity and ferroelectricity properties of the ceramics should also be changed.
The radii of Nb5+ and Ti4+ are 0.64 Å and 0.605 Å in the case of coordination number (CN) 6, respectively [11]. The similar radii between Nb5+ and Ti4+ imply that Nb5+ can be doped into the Ti4+ site. Chauhan et al. reported that the substitution of Nb5+ for Ti4+ improves piezoelectricity and ferroelectricity of the Bi0.5Na0.25K0.25Nb0.015Ti0.975O3 ceramics [12]. Zhang et al. studied local lattice relaxation in Nb2O5-modified 0.94(Na0.5Bi0.5)TiO3-0.06BaTiO3 ferroelectrics and found that lattice relaxation destabilizes the field-induced ferroelectric phase [13]. Petnoi et al. reported that the transition temperature of Nb5+-doped (Bi0.5Na0.5)TiO3 shifts to lower temperature as the Nb5+ content increases [14]. Shen et al. reported that the doping of Nb5+ into BaTiO3-(Bi0.5Na0.5)TiO3 is beneficial to promote the formation of the core–shell structure and weakens dependence of dielectric properties on temperature [15]. The doping of Nb5+ will always introduce cation vacancies in the A-site because of higher electric charge of Nb5+, which can affect electrical performance of the ceramics.
In this work, the (Bi0.5Na0.5)0.94-δBa0.06Ti1−xNbxO3 ceramics were obtained via solid-state sintering. Effects of Nb5+ doping on microstructure, phase structure, dielectric, piezoelectric, and ferroelectric behavior were studied.
2 Experimental procedure
The ceramics (Bi0.5Na0.5)0.94-δBa0.06Ti1−xNbxO3 (denoted as BNBTN/x, x = 0, 0.01, 0.02, 0.03, 0.04, 0.05) were prepared by means of a solid-state sintering method. The raw powders are BaCO3 (≥ 99.0%), Na2CO3 (≥ 99.8%), Bi2O3 (≥ 98.9%), TiO2 (≥ 98.0%), and Nb2O5 (≥ 99.99%). After being baked at 120 °C for 1 day, the powders were weighed according to the compositions. The dried powders were ball milled for 1 day in a planetary milling apparatus. Then, the milled powders were calcined at 900 °C for 180 min and then ball milled again. 5 wt% PVA was added into the powders, and then green pellets with diameter 10 mm and thickness approximate 1.5 mm were pressed at 200 MPa. The PVA was burned out at 500 °C for 120 min. The sintering temperature, dwelling time, and heating/cooling rates are 1170 °C, 180 min, and 3 °C/min, respectively.
Bulk densities of the ceramics were obtained via the Archimedes method. The method for calculating weight loss of the samples during the sintering process has been clarified in the previous work [16]. Phase structure was measured via a Rigaku D/Max 2550 X-ray diffraction (XRD) apparatus. Raman spectra were obtained via an Almega-dispersive Raman spectrometer. Scanning electron microscope (SEM) images for the thermally etched ceramics were measured via a Quanta 200 SEM. The ceramics were poled at 5 kV/mm for 30 min at room temperature. Piezoelectric constant d33 was measured via a quasistatic d33 meter (ZJ-4A). Ferroelectric behavior was measured via Precision Premier II ferroelectric testing system. Dielectric properties were measured on a dielectric spectrometer (Novocontrol Concept 80).
3 Result and discussion
Figure 1 shows the SEM images of the surfaces of the thermally etched BNBTN/x ceramics. All the ceramics exhibit dense microstructures with average grain sizes around 1–2 μm. The grains and grain boundaries are clear. Almost no pores were found in the samples. The dense microstructures correspond to their high densities. The bulk densities of the BNBTN/x samples are larger than 5.75 g/cm3 (Fig. 2). All the samples exhibit relative densities higher than 96% (theoretical density of (Na0.5Bi0.5)0.94Ba0.06TiO3 5.98 g/cm−3 roughly used for all the compositions [17]), implying that the ceramics were well sintered at 1170 °C. Weight loss of the ceramics during sintering is shown in Fig. 2. It is found that there appears an increase in the weight loss with the increase of Nb5+ content. As mentioned above, all the samples with the different compositions were prepared using the same synthesis parameters, such as sintering temperature, dwelling time, and heating/cooling rates. So, the difference in weight loss of the samples should be caused by the difference in the Nb5+ amounts. It is well known that the doping of Nb5+ into the B-site will cause cation vacancies in the A-site, as shown by the Kröger–Vink equation:
According to the equation, more cation vacancies including VBi/// and VNa/ will be introduced as more Nb5+ cations are doped into the Ti4+ sites, which should be responsible for the increase in weight loss of the samples. In addition, the sample with x = 0.05 shows a slight decrease in weight loss, which may be related to the formation of a secondary phase in the sample, as shown in Fig. 3a.
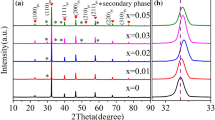
The XRD curves of the ceramics are shown in Fig. 3a. The samples exhibit typical diffraction peaks of the perovskite structure. For the sample with x = 0.05, there appears another peak around 29°, implying a secondary phase exists in the ceramic. The peak position of the secondary phase is in accordance to that of the XRD peak with the largest intensity for Nb2O5 (International Centre for Diffraction Data, No. 32-0710). It is reasonable to infer that the secondary phase is related to the excess Nb2O5. It is well known that the rhombohedral–tetragonal MPB exists in the ceramics with the composition of (Bi0.5Na0.5)0.94Ba0.06TiO3 [18, 19]. For the MPB composition, three XRD peaks exist around 46° corresponding to the diffraction planes (002)T, (200)T, and (200)R in which R and T represent the rhombohedral phase and tetragonal phase, respectively [20]. The detail of the XRD peaks in the 2θ range from 46° to 47° is exhibited in Fig. 3b. The XRD peaks around 46° of the x = 0 and x = 0.01 ceramics are asymmetric obviously. With further increasing the Nb5+ amount, the peaks split remarkably. The peaks can be well fitted into three peaks. Relative amount of the tetragonal phase (T%) can be estimated via the equation:
in which S(200)R, S(002)T, and S(200)T are area values of the (200)R, (002)T, and (200)T diffraction peaks, respectively. The obtained T% values are 45%, 48%, 50%, 52%, 54%, and 57% for the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. Compared to BNBTN/0, the Nb5+ doping induces an increment of T%. In addition, the XRD peaks move toward lower 2 theta direction. For example, the 2θ values of the (200)R peaks for the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05 are 117°, 103°, 101°, 100°, 98°, and 97°, respectively. It is known that the radius of Nb5+ with CN = 6 is 0.64 Å, and that of Ti4+ in the case of CN = 6 is 0.605 Å [11]. The substitution of Nb5+ for Ti4+ causes enlargements of the unit cell because of the larger radius of Nb5+, contributing to the shift of the peaks. In addition, the doping of Nb5+ into the B-site will cause cation vacancies in the A-site (Eq. 1). The formation of point defects in the ceramics induces lattice distortion, also contributing to the shift of the XRD peaks.
Figure 4 shows the result of Raman spectroscopy. All the samples show similar curves. The curves can be divided into four regions, i.e., Region I, Region II, Region III, and Region IV, which are associated with the vibration modes of A-site, Ti–O bond, TiO6 octahedra, and A1+E overlapping band, respectively [21]. According to the eight Gaussian–Lorentzian modes [6], spectral deconvolution in the Raman spectroscopy was performed, and the typical positions of the Raman vibration modes corresponding to Ti–O bond and TiO6 octahedra are shown in Fig. 4b. It is obvious that two peaks exist in Region II. The splitting of the peaks locating in Region II is often regarded as a sign of the coexistence of rhombohedral and tetragonal phases [22]. The two peaks corresponding to Ti–O bond imply coexistence of the rhombohedral and tetragonal phases, which are in accordance with the XRD result (Fig. 3b). The positions of the two peaks corresponding to Ti–O bond shift toward lower wavenumber slightly with the increase of Nb5+ amount (Fig. 4b), suggesting the variation in B–O bond (B = Ti/Nb) due to the doping of Nb5+. For TiO6 octahedra vibration mode, the Nb5+-doped ceramics exhibit higher wavenumber value compared with the ceramic with x = 0. In addition, the positions of the two peaks around 500 cm−1 and 620 cm−1 tend to shift together with the increase of x. For example, the distance between the two peaks for the ceramic with x = 0 is 61 cm−1, while that for the ceramic with x = 0.05 is 43 cm−1. The substitution of Nb5+ for Ti4+ causes lattice distortion, inducing change of TiO6 octahedra vibration mode. The Raman result further demonstrates the entrance of Nb5+ into the BNBT lattice.
Raman spectra of the ceramics (a); the typical result of spectral deconvolution for the ceramic with x = 0.05 according to the Gaussian–Lorentzian modes via a best-fitting algorithm is shown in the bottom in a. Typical positions of the Raman vibration modes corresponding to Ti–O bond and TiO6 octahedra (b)
Figure 5 shows variation of dielectric loss (tanδ) and dielectric constant (εr) of the unpoled samples with changing temperature (T). There are two dielectric anomalies for all the samples, which are denoted as TRE and Tm, respectively. TRE is the temperature at which εr values at various frequencies begin to merge together. When the temperature is lower than TRE, εr has an obvious dependence on frequency, and εr at high frequency is less than that at low frequency. When temperature is higher than TRE, the εr values at different frequencies are very close, and the frequency dispersion tends to disappear. The temperature corresponding to the maximum permittivity (εm) is denoted as Tm. The similar dielectric behavior was always observed in BNBT-based ceramics [6, 8, 20]. The dielectric anomalies around TRE and Tm have been suggested to be associated with thermal evolution of polar nanoregions with the different symmetries [23, 24]. The values of TRE, Tm, and εm of the ceramics are shown in Table 1. Here, the TRE values were determined via the temperatures at which the difference in dielectric constant at 1 kHz and 100 kHz, i.e., Δεr = εr (1 kHz) − εr (100 kHz), is minimum [25]. As the Nb5+ amount increases from x = 0 to x = 0.05, TRE decreases from 217 to 171 °C, Tm changes from 272 to 297 °C, and εm changes from 6282 to 2880. The broadening of temperature window (ΔT = Tm − TRE) between TRE and Tm as well as decrease in εm improves high-temperature stability of εr. The εr values at TRE are 5655, 3551, 3114, 3110, 2656, and 2441 for the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. Those at Tm are 6282, 4529, 3852, 3836, 3248, and 2880 for BNBTN/0, BNBTN/0.01, BNBTN/0.02, BNBTN/0.03, BNBTN/0.04, and BNBTN/0.05, respectively. The differences in εr values at the temperatures between TRE and Tm (labeled as Δεr) are 627, 978, 738, 726, 592, and 439 for the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively (Table 1). In addition, the temperature coefficient of capacitance (TCC) is also applied to evaluate the temperature window for temperature stability of εr. Here, TCC is defined as
in which εr,L is dielectric constant at the temperature TRE and εr,H is dielectric constant at a given higher temperature TH. For the temperature TH, the TCC value is equal to 15%. So, a higher TH corresponds to a wider temperature window with TCC ≤ 15%, implying better temperature stability of εr. The temperature window for TCC ≤ 15% is also depicted in Fig. 5. The window values between TH and TRE are 55 °C, 42 °C, 50 °C, 65 °C, 74 °C, and 80 °C for the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. The ceramics with high Nb5+ amounts exhibit wide-temperature window, which is advantageous for preparing high-temperature ceramic capacitors.
The poled samples with x = 0, 0.01, and 0.02 show high d33 values between 110 and 138 pC/N at room temperature (Table 1). The x = 0.03 ceramic exhibits a low d33 of 13 pC/N. For the higher doping amounts of Nb5+, no d33 was observed at room temperature, implying depolarization behavior at room temperature. The curves of tanδ and εr of the poled samples are plotted in Fig. 6. Compared to the unpoled counterparts, a new dielectric anomaly appears on the curves of the poled samples with x ≤ 0.02. The new anomaly locates at the temperature denoted as Td. At Td, εr increases suddenly, and tanδ exhibits a sharp peak. Td is always used to determine depolarization temperature [26,27,28]. Below Td, long-range ferroelectric order exists in the ceramics due to the electric poling. As temperature rises above Td, the long-range order will be broken, corresponding to depolarization [28]. When temperature is lower than Td, the εr values of the poled samples are smaller than those of the unpoled counterparts at a given frequency and temperature. The phenomenon can be explained as follows. On one hand, the strong electric field during the poling process can reorient space charges, defect dipoles in the ceramics, and freeze them. The electric field for measuring dielectric spectra is weaker than that for poling the samples, which cannot activate the space charges and cannot reorient the defect dipoles. So, the decreased contribution from these factors causes decrease in dielectric constant [29]. On the other hand, compared to the unpoled samples, domain wall densities of the poled samples are reduced due to the electric poling, which also contribute to reduction in dielectric constant. The Td values of the ceramics BNBTN/0, BNBTN/0.01, and BNBTN/0.02 are 101 °C, 54 °C, and 41 °C, respectively. The doping of Nb5+ causes a decrease in depolarization temperature. For the ceramics with x ≥ 0.03, no Td anomaly was found, implying that it shifts below room temperature. The phenomenon is consistent with the ferroelectric behavior as shown later.
The loops of polarization (P), strain (S), and current density (J) under as a function of measurement electric fields (E) are exhibited in Fig. 7a–f. Here, the P–E loops were measured between 50 and 80 kV/cm; S–E loops and J–E loops were recorded at the maximum electric field of 80 kV/cm. For a given sample, the P–E loops become more saturated with increasing the measurement electric fields. It is found that the shape of P–E loops is strongly affected by the content of Nb5+. The ceramics with x = 0, 0.01, and 0.02 display typical P–E loops and butterfly-shaped S–E loops. Two peaks appear on the J–E loops, demonstrating ferroelectric character. With further increasing Nb5+ content, the P–E loops tend more pinched. The values of maximum polarization (Pm), remnant polarization (Pr), and coercive filed (Ec) of the ceramics under 80 kV/cm are shown in Fig. 7g. The polarization and Ec values decrease with increasing Nb5+ content, indicating destruction of long-range ferroelectric order with the doping of Nb5+. In the case of x ≥ 0.03, the Pr values are obviously decreased compared to the samples with x < 0.03. The S–E loops gradually change from butterfly shape to horn shape, accompanied by a decrease in negative strain. At the same time, there appear four peaks on the J–E curves. These characteristics suggest disorder of ferroelectric phase in the ceramics with the higher Nb5+ amounts caused by the Nb5+ doping and appearance of ergodic relaxation phase [30, 31], which is in good agreement with the change of Td in dielectric behavior.
The P–E loops measured at electric fields between 50 and 80 kV/cm, S–E loops and J–E loops measured at 80 kV/cm for the ceramics with x = 0 (a), x = 0.01 (b), x = 0.02 (c), x = 0.03 (d), x = 0.04 (e), x = 0.05 (f). The measurement frequency is 1 Hz. The values of Ec, Pm, and Pr measured at 80 kV/cm are shown in g
The formation of cation vacancies in the ceramics should also affect their dielectric and ferroelectric behaviors. As mentioned above, the doping of Nb5+ into the B-site will cause cation vacancies in the A-site (Eq. 2). In order to detect effect of cation vacancies, a typical composition of x = 0.01 was chosen, and excess (Bi0.5Na0.5)2+ ions were added into the composition to compensate cation vacancies. The excess (Bi0.5Na0.5)2+ amount was calculated to be δ = 0.015 according to the molecular formula (Bi0.5Na0.5)0.94-δBa0.06Ti1−xNbxO3. The obtained samples are noted as BNBTN/0.01 + δBN. The P–E loops of BNBTN/0.01 + δBN and BNBTN/0.01 recorded at 70 kV/cm are compared in Fig. 8. The ceramic BNBTN/0.01 + δBN shows the Pm, Pr, and Ec values of 24.3 μC/cm2, 18.6 μC/cm2, and 33.6 kV/cm, respectively. The corresponding values for BNBTN/0.01 are 39.6 μC/cm2, 32.8 μC/cm2, and 27.5 kV/cm, respectively. The addition of excessive (Na0.5Bi0.5)2+ reduces polarization and increases coercive field. The inset in Fig. 8 shows variation of εr and tanδ as a function of temperature measured at 1 kHz for the poled BNBTN/0.01 and BNBTN/0.01 + δBN ceramics. Via the dielectric spectra, it is found that the adding of excess (Na0.5Bi0.5)2+ ions increases depolarization temperature Td from 55 °C for BNBTN/0.01 to 65 °C for BNBTN/0.01 + δBN. As has been well known, cation vacancies always facilitate improving ferroelectric behavior, i.e., increasing polarization and decreasing coercive field [12]. In addition, depolarization temperatures for BNBT-based ceramics are related to point defects. Qiao et al. reported that point defects can affect depolarization temperature and cation vacancies facilitate decreasing Td [2]. Compared to BNBTN/0.01, BNBTN/0.01 + δBN exhibits increased Td, decreased polarization, and increased Ec, which are in accordance to the reduced amount of cation vacancies in BNBTN/0.01 + δBN due to the compensation of (Na0.5Bi0.5)2+. Via these results, it is reasonable to expect important effect of cation vacancies in the BNBTN/x ceramics on dielectric and ferroelectric properties.
Change of unipolar strain (Su) as a function of electric field is shown in Fig. 9a, and the unipolar strain values at Emax = 80 kV/cm (Smax) and corresponding piezoelectric strain constant d33* = Smax/Emax values are shown in Fig. 9b. It is found that Smax increases first and then decreases as the Nb5+ content increases. The ceramic BNBTN/0.03 exhibits the maximum unipolar strain 0.55%. The change of d33* with the increase in the Nb5+ amount is same as that of Smax. In general, strain in the ceramics is associated with various factors, such as lattice distortion, point defects, phase structure, destabilization of the ferroelectric phase, etc. As demonstrated via the XRD and Raman results, the doping of Nb5+ causes lattice distortion and variation in relative amount of the rhombohedral/tetragonal phase (Figs. 3, 4). Furthermore, the doping can also cause cation vacancies in the A-site (Eq. 1). In addition, the very slim P–E loops accompanied by significant reduction in polarization (Fig. 7) as well as no Td peak on the dielectric spectra (Fig. 6) for the ceramic with x = 0.03 suggests the disruption of the long-range ferroelectric order caused by the Nb5+ doping. It is known that the destabilization of the long-range ferroelectric order was always accompanied by enhancement in the strain behavior [32]. All of these factors should contribute to the change in strain and d33* as a function of Nb5+ amount. As is well known, superior strain response plays important role in actuators. Wang et al. showed that 0.715Bi0.5Na0.5TiO3–0.065BaTiO3–0.22SrTiO3 exhibits Smax = 0.2% and d33* = 490 pm/V [33]. Liu et al. reported that (1 − x)(0.8Na0.5Bi0.5TiO3–0.2K0.5Bi0.5TiO3)-xSrTiO3 has Smax = 0.3% and d33* = 500 pm/V [21]. Zhao et al. exhibited Smax = 0.13% and d33* = 448 pm/V for the (K,Na)NbO3-based ceramics [34]. Here, we found that BNBTN/0.03 shows the Smax as high as 0.55% and d33* as high as 675 pm/V, implying excellent strain performance.
4 Conclusions
The dense BNBTN/x ceramics were successfully obtained by means of a conventional sintering method. The x < 0.05 ceramics show pure phase, while a secondary phase exists in the x = 0.05 sample. The relative amounts of the tetragonal phase are 45%, 48%, 50%, 52%, 54%, and 57% for the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05, respectively. For the ceramics with x = 0, 0.01, 0.02, 0.03, 0.04, and 0.05, the TRE values are 217 °C, 192 °C, 188 °C, 179 °C, 173 °C, and 171 °C, respectively, and the Tm values are 272 °C, 286 °C, 289 °C, 292 °C, 295 °C, and 297 °C, respectively. The εr values exhibit excellent temperature stability for the ceramics with the high Nb5+ amounts. The doping of Nb5+ causes a decrease in Td, which are 101 °C, 54 °C, and 41 °C for x = 0, 0.01, and 0.02, respectively. For the ceramics with x ≥ 0.03, Td decreased below room temperature. The P–E loops change from typical ferroelectric loops for BNBTN/0 to constricted P–E loops for the samples with x ≥ 0.03, indicating disruption of the long-range ferroelectric order caused by the Nb5+ doping. With the increase in Nb5+ amount from x = 0 to x = 0.05, the Ec value decreases from 37.2 kV/cm to 5.1 kV; the Pm value decreases from 41.1 to 28.9 μC/cm2; and the Pr value decreases from 35.1 to 3.1 μC/cm2. The BNBTN/0.03 ceramic shows the maximum unipolar strain Smax = 0.55% and d33* = 675 pm/V.
References
Z. Wang, J. Wang, X. Chao, L. Wei, B. Yang, D. Wang, Z. Yang, J. Mater. Sci. Mater. Electron. 27, 5047 (2016)
X.S. Qiao, X.M. Chen, H.L. Lian, W.T. Chen, J.P. Zhou, P. Liu, J. Am. Ceram. Soc. 99, 198 (2016)
F.F. Li, Y.F. Liu, Y.N. Lyu, Y.H. Qi, Z.L. Yu, C.G. Lu, Ceram. Int. 43, 106 (2017)
Y. Yang, H. Wang, L.N. Bi, Q.J. Zheng, G.F. Fan, W.J. Jie, D. Lin, J. Eur. Ceram. Soc. 39, 3051 (2019)
X.X. Wang, H.L.W. Chan, C.L. Choy, Appl. Phys. A 80, 333 (2005)
R.Y. Jing, X.M. Chen, H.L. Lian, X.S. Qiao, X.J. Shao, J.P. Zhou, J. Eur. Ceram. Soc. 38, 3111 (2018)
J. Wang, X.M. Chen, X.M. Zhao, X.X. Liang, X. Liu, P. Liu, J. Electroceram. 32, 332 (2014)
X.M. Chen, X.X. Gong, T.N. Li, Y. He, P. Liu, J. Alloys Compd. 507, 535 (2010)
T. Wang, X.M. Chen, Y.Z. Qiu, Ferroelectrics 510, 161 (2017)
T. Wang, X.M. Chen, Y.Z. Qiu, H.L. Lian, W.T. Chen, Mater. Chem. Phys. 186, 407 (2017)
R.D. Shannon, Acta. Crystallogr. A 32, 751 (1976)
V. Chauhan, S.K. Ghosh, A. Hussain, S.K. Rout, J. Alloys Compd. 674, 413 (2016)
H.W. Zhang, H. Yan, X.B. Li, H.S. Luo, Scr. Mater. 156, 32 (2018)
N. Petnoi, P. Bomlai, S. Jiansirisomboon, A. Watcharapasorn, Ceram. Int. 39, S113 (2013)
Z.B. Shen, X.H. Wang, D.S. Song, L.T. Li, Adv. Appl. Ceram. 115, 435 (2016)
Z.D. Yu, X.M. Chen, H.L. Lian, Q. Zhang, W.X. Wu, J. Mater. Sci. Mater. Electron. 29, 19043 (2018)
X.M. Chen, H.Y. Ma, W.Y. Pan, M. Pang, P. Liu, J.P. Zhou, Mater. Chem. Phys. 132, 368 (2012)
N.S. Zhao, H.Q. Fan, X.H. Ren, S. Gao, J.W. Ma, Y.G. Shi, Ceram. Int. 44, 571 (2018)
R. Dittmer, K.G. Webber, E. Aulbach, W. Jo, X.L. Tian, J. Rödel, Acta Mater. 61, 1350 (2013)
Y.Z. Qiu, X.M. Chen, H.L. Lian, J.P. Ma, W.Q. Ouyang, Mater. Chem. Phys. 202, 197 (2017)
X. Liu, J.W. Zhai, B. Shen, F. Li, Y. Zhang, P. Li, B.H. Liu, J. Eur. Ceram. Soc. 37, 1437 (2017)
E. Aksel, J.S. Forrester, H.M. Foronda, R. Dittmer, J. Appl. Phys. 112, 054111 (2012)
J.D. Zang, M. Li, D.C. Sinclair, W. Jo, J. Am. Ceram. Soc. 97, 1523 (2014)
A. Khesro, D. Wang, F. Hussain, D.C. Sinclair, A. Feteira, I.M. Reaney, Appl. Phys. Lett. 109, 142907 (2016)
R.Y. Jing, X.M. Chen, J.P. Ma, H.L. Lian, W.T. Chen, J. Mater. Sci. 53, 274 (2018)
J.U. Rahman, A. Hussain, A. Maqbool, G.H. Ryu, T.K. Song, W.J. Kim, M.H. Kim, J. Alloys Compd. 593, 97 (2014)
J. Shi, W.C. Tian, X. Liu, H.Q. Fan, J. Am. Ceram. Soc. 100, 1080 (2016)
W. Jo, J. Daniels, D. Damjanovic, W. Kleemann, J. Rödel, Appl. Phys. Lett. 102, 192903 (2013)
X.S. Qiao, X.M. Chen, H.L. Lian, J.P. Zhou, P. Liu, J. Eur. Ceram. Soc. 36, 3995 (2016)
Y. Wu, G. Wang, Z. Jiao, Y. Fan, P. Peng, X. Dong, RSC Adv. 9, 21355 (2019)
T.Y. Li, X.J. Lou, X.Q. Ke, S.D. Cheng, S.B. Mi, X.J. Wang, J. Shi, X. Liu, G.Z. Dong, H.Q. Fan, Y.Z. Wang, X.L. Tan, Acta Mater. 128, 337 (2017)
A. Ullah, C.W. Ahn, A. Hussain, S.Y. Lee, I. W. Kim, J. Am. Ceram. Soc. 94, 3915 (2011)
F.F. Wang, M. Xu, Y.X. Tang, W. Tao, W.Z. Shi, C.M. Leung, J. Rödel, J. Am. Ceram. Soc. 95, 1995 (2012)
Y. Zhao, J. Du, Z.J. Xu, Mater. Sci. Eng. B 224, 110 (2017)
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (No. 51972202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lian, Hl., Shi, Jy., Qiu, Yz. et al. Dielectric and ferroelectric properties of (Bi0.5Na0.5)0.94-δBa0.06Ti1−xNbxO3 lead-free ceramics. J Mater Sci: Mater Electron 31, 21467–21477 (2020). https://doi.org/10.1007/s10854-020-04660-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04660-5