Abstract
Purpose
To evaluate the yearly prevalence and annual transition of multi-drug-resistant-chronic endometritis (MDR-CE) in infertile women with a history of repeated implantation failure (RIF) and to establish the third-line antibiotic treatment regimen against MDR-CE.
Methods
This retrospective/prospective cohort and pilot study included 3473 RIF women between April 2010 and September 2021. The endometrial stromal plasmacyte density index (ESPDI) was calculated in 3449 CD138-immunostained endometrial sections to evaluate CE. The microbiota in the vaginal secretions and endometrial fluid was compared between 17 patients with MDR-CE and 16 patients with antibiotics-sensitive CE. In a pilot study, oral moxifloxacin (400 mg/day, 10 days, n = 24) or azithromycin (500 mg/day, 3 days, n = 24) was administered to eligible patients with MDR-CE.
Results
From April 2010 to March 2020, CE was detected in 31.4% of RIF women and MDR was detected in 7.8% of CE. While the prevalence of CE was stable for a decade, MDR in CE increased steadily (OR 8.27, 95% CI 2.58–26.43, p trend < 0.001). The bacterial species/communities unique to MDR-CE were not found. The histopathologic cure rate of MDR-CE was similar between the moxifloxacin and azithromycin groups (79.2% vs 75.0%, OR 1.27, 95% CI 0.32–4.89, p value 0.73), as well as reproductive outcomes in subsequent embryo transfer cycles.
Conclusion
In RIF women, MDR in CE increased over the decade. As a third-line treatment for MDR-CE, azithromycin may have a clinical advantage due to its shorter time administration periods.
Clinical trial number
ClinicalTrials.gov Identifier: UMIN-CTR 000029449/000031909.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic endometritis (CE) is a localized inflammatory disease of the uterine lining, which is characterized by unusual plasmacyte infiltration in the endometrial stromal compartment and is asymptomatic/oligosymptomatic nature [1]. A growing body of evidence demonstrates that CE is identified in a substantial population of infertile women with repeated implantation failure (RIF), unknown etiology, and recurrent pregnancy loss, as well as some obstetric/neonatal complications such as preterm labor and periventricular leukomalacia/cerebral palsy in premature infants [2–21]. The mucosal expression of multiple genes such as ovarian steroid receptors, adhesion molecules, immunomodulators, and apoptosis are dysregulated in CE, suggesting the impaired endometrial receptivity, decidualization, and uterine contractility in this pathologic condition [11, 14, 15, 21].
The major cause of CE is intrauterine microbial infection triggered by a wide range of microorganisms such as common bacteria (Escherichia coli, Enterococcus faecalis, Streptococcus, and Staphylococcus), Mycoplasma, Ureaplasma, and Mycobacterium species [2, 3]. This finding is supported by the fact that antibiotic treatment against these microorganisms is effective to eradicate endometrial stromal plasmacytes in CE [3–12]. One of the most prescribed antibiotic agents against CE is doxycycline [3, 4, 6, 8, 22–24]. In a prospective study conducted between 2011 and 2014, we identified CE in 33.7% of endometrial biopsy specimens of RIF women. Oral doxycycline treatment (200 mg/day for 14 days) cured histopathologic CE in approximately 90% of the affected patients [3, 4]. In addition, the second-line treatment with a combination of oral metronidazole (500 mg/day) and ciprofloxacin (400 mg/day) for 14 days was effective for most of patients with doxycycline-resistant CE [3, 4]. Furthermore, the live birth rate in the immediate first embryo transfer (ET) cycle and cumulative three ET cycles in the RIF women who overcome CE (32.8% and 38.8%, respectively) was higher than in the RIF women without CE (22.1% and 27.9%, respectively). Favorable effects of antibiotic treatment against CE on the reproductive outcomes in the subsequent ET cycles were confirmed by a meta-analysis demonstrating that the ongoing pregnancy/live birth improved to the equivalent level to infertile women without RIF [10].
Antibiotic resistance is a global issue in the treatment of infectious diseases. CE is no exception [25–28]. In 2008, according to the results of the endometrial histopathology, culture, and antibiogram, Cicinelli et al. estimated that less than 20% of CE was resistant to single-course oral doxycycline treatment [2]. Seven years later, they reported that 24.6% of CE was untreatable with three courses of antibiotic administration, implying increment of multi-drug resistant chronic endometritis (MDR-CE) [7]. More recently, using a stringent criterion, Xiong et al. reported that 11.0% of CE was resistant to two courses of the combined antibiotic treatments for 14 days [29]. However, few studies so far tracked the prevalence of MDR-CE and its time transition in large and long-term settings. Moreover, there are no published clinical trials on the treatment strategies against MDR-CE.
In this study, we aimed to evaluate the yearly prevalence and annual transition of MDR-CE over the last decade using the medical records of more than 3000 RIF women. To identify the bacterial species and communities associated with MDR in CE, the microbiota in the vaginal secretions (VS) and endometrial fluid (EF) was analyzed in some MDR-CE patients and compared with antibiotics-sensitive CE. Finally, we conducted a pilot study to compare the effectiveness and safety of two regimens of the third-line oral antibiotic administration against MDR-CE in RIF women. Following the confirmation of its histopathologic cure, we followed up their reproductive outcomes in the subsequent ET cycles.
Materials and methods
Study design
The study was approved by the Ethical Committee of the Institutional Review Board of Reproduction Clinic Osaka on September 20, 2017 (Approval Number 20172/20173). The yearly prevalence and annual transition of CE and MDR-CE in RIF women were analyzed retrospectively between April 2010 and September 2017 and prospectively from October 2017 to March 2021. The microbiota analysis was a part of an ongoing prospective case–control study registered on October 6, 2017 (UMIN-CTR 000029449), and conducted from October 2017. A pilot study comparing the effectiveness and safety of two regimens of oral antibiotic administration against MDR-CE was registered on March 26, 2018 (UMIN-CTR 000031909). We here chose two oral antibiotic agents, moxifloxacin and azithromycin, to treat RIF women with MDR-CE. Moxifloxacin is an extended-spectrum fluoroquinolone agent with improved activity against Gram-negative bacteria and anaerobes compared with older-generation fluoroquinolones and was reported to be superior to metronidazole in treatment against bacterial vaginosis-associated species, Atopobium vaginae and Gardnerella vaginalis [30, 31]. Meanwhile, few studies so far evaluated the effectiveness of oral azithromycin against CE. Azithromycin is an acid-stable macrolide structurally related to erythromycin, but with a broader spectrum of antimicrobial activity, and covers pelvic, genital, and/or sexually transmitted infectious diseases such as urethritis and cervicitis [32]. Under prospective non-randomized settings, these antibiotic agents were administrated to the eligible patients between April 2018 and September 2020 in accordance with the Declaration of Helsinki. All patients provided written informed consent before participation in the study.
Definition of RIF, CE, and MDR-CE
RIF was defined as serial negative pregnancy tests following transfer of three or more morphologically good cleavage-stage embryos (Veeck grade 1 or 2, seven-to-eight blastomere embryos on day 3 of cultivation) [33] and/or blastocysts (Gardner score 3BB or above on day 5) [34] over three or more transfer cycles. A total of 3473 infertile women with a history of RIF were included in the study. To avoid the double count of the same individual, we included only RIF women undergoing their primary screening for CE.
Proliferative phase (days 6–12 of the menstrual cycle) endometrial curette biopsy sections (4-μm thickness) obtained from RIF women and immunostained with mouse monoclonal IgG against a plasmacyte marker CD138 (B-A38, Nichirei, Tokyo) or mouse control IgG [35] were evaluated for CE. The sections with suspicious hyperplasia/malignancy were excluded. The whole sections were observed by an experienced gynecologic pathologist under a light microscope (× 400 magnification), and stromal CD138 + cells with nucleic heterochromatin patterns were enumerated in 20 or more high-power fields. The endometrial stromal plasmacyte density index (ESPDI) was calculated as the sum of the stromal CD138 + cell counts divided by the number of the high-power fields evaluated. CE was diagnosed as 0.25 or more ESPDI, as described previously [3]. MDR-CE was defined as treatment failure following the first-line oral doxycycline (Vibramycin, 200 mg/day, 14 days, Pfizer Inc., Tokyo, Japan) and second-line oral metronidazole (Asuzol, 500 mg/day, 14 days, Fuji Pharma, Inc., Tokyo, Japan)/ciprofloxacin (Ciproxan, 400 mg/day, 14 days, Bayer Healthcare Co., Osaka, Japan) administration, evaluated by ESPDI in the third endometrial biopsy specimens.
Microbiota analysis in paired VS and EF in MDR-CE/RIF women
The microbiota analysis was performed for a total of 33 RIF women with MDR-CE (n = 17) and antibiotics-sensitive CE (n = 16) who desired and/or volunteered to the examination. The patients who self-reported the use of oral and/or vaginal prebiotics and/or probiotics were excluded. The paired VS and EF samples were obtained in the mid-secretory phase of the identical cycle to the endometrial biopsy within 2 months from the final antibiotic treatment. [35]. In brief, the perineum was cleansed with sterilized cotton balls soaked in benzalkonium chloride solution and a bivalve speculum was inserted into the vaginal cavity. VS was obtained from all directions using a sterilized swab. After removing the residual mucous, the vaginal cavity and cervix were cleaned using another benzalkonium chloride-soaked sterilized cotton balls. Avoiding contact with the speculum and vaginal wall, a pipette (MedGyn Products Inc., Addison, IL, USA) was inserted into the uterine cavity via the cervical ostium and EF was aspirated. VS and EF were solubilized into stabilizing liquid in separate collection tubes.
The genomic DNA was extracted following treatment with proteinase K/lysozyme/RNase A solution (Beckman Coulter Inc., Brea, CA, USA). The double-stranded DNA concentration was quantified using a Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). The variable 4 regions of the bacterial 16S rRNA gene were amplified by a modified primer pair 515f with Illumina Nextera XT (Illumina Inc., San Diego, CA, USA) adapter overhang sequences. Polymerase chain reaction cycles consisted of denaturation (94 °C, 2 min) followed by 30 cycles of denaturation (94 °C, 20 s), annealing (50 °C, 30 s), extension (72 °C, 1 min), and final extension (72 °C, 5 min). The amplicon mixture was purified using Agencourt AMPure XP beads (Beckman Coulter Inc.) and multiplexed using a dual-index approach with Nextera XT Index Kit v2 according to Illumina 16S Metagenomic Sequencing Library Preparation protocol. The indexing polymerase chain reaction was performed with a KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Boston, MA, USA). The final library was paired-end sequenced at 2 × 200 bp using a MiSeq Reagent Kit v3 on the Illumina MiSeq platform. The ZymoBIOMICS Microbial Community Standard (Zymo Research, Orange, CA, USA) containing a mixture of Pseudomonas, Escherichia, Salmonella, Lactobacillus, Enterococcus, Listeria, Bacillus, and two yeast species Saccharomyces and Cryptococcus, was used as a positive control [36]. UltraPure™ DNase/RNase-Free Distilled Water (Thermo Fisher Scientific Inc.) was used as a blank control. Using EA-Utils fastq-join [37], a median 291-base pair merged sequence length was obtained. The quality control was performed using USEARCH v10.0.240 to remove PhiX reads, truncate primer-binding sequences, and discard sequences with < 100-bp length and sequence quality Q < 20. Quantitative Insights Into Microbial Ecology 1.9.1 [38] was used with default parameters for quality filtering and chimera check. The sequences were clustered into operational taxonomic units using the UCLUST method based on 97% sequence identity. Taxonomy was assigned using Ribosomal Database Project Classifier [39] with a 0.50 confidence threshold against the Greengenes database version 13_8 [40]. Fifteen bacterial taxa (Acidovorax, Acinetobacter, Chryseobacterium, Citrobacter, Elizabethkingia, Escherichia, Flavobacterium, Janthinobacterium, Leptothrix, Methylobacterium, Pseudomonas, Rhodococcus, Sphingomonas, Stenotrophomonas, and Yersinia) known as contaminants found in a blank control [36], were excluded from EF samples.
Pilot study to compare the effectiveness and safety of two regimens of third-line oral antibiotic administration on histopathologic cure rate and subsequent reproductive outcomes of RIF women with MDR-CE
A pilot study was conducted to compare the effectiveness and safety of two regimens of the oral antibiotic administration for RIF women with MDR-CE under prospective non-randomized settings. The exclusion criteria were present illness/history of liver dysfunction, arrhythmia, hypokalemia, and hypersensitivity to quinolones and/or macrolides. The consecutive numbers from 1 to 48 were given to the eligible patients. Those with an odd number were allocated to the moxifloxacin (Avelox, 400 mg/day, 400 mg tablet once for 10 days, Bayer Healthcare Co, n = 24) group, and those with an even number were allocated to the azithromycin (Zithromac, 500 mg/day, 250 mg tablet twice for 3 days, Pfizer Inc, n = 24) group. The fourth endometrial biopsy was performed on days 6–12 of the subsequent menstrual cycle for reevaluation of CE. RIF women who overcome histopathological MDR-CE proceeded to subsequent ET cycles. Their reproductive outcomes were followed up until September 2021. The main outcome measures included the rate of the clinical pregnancy (the presence of the intrauterine gestational sac and fetal heartbeat on transvaginal ultrasound), miscarriage (pregnancy loss before 22 weeks of gestation), and ongoing pregnancy/live birth.
Statistics
Student t test and Tukey–Kramer test was used for continuous variables (Excel Statistics, SSRI, Tokyo, Japan). Mann–Whitney U test or Fisher’s exact test was used for categorical variables. The trend analysis was performed using a linear regression model and Cochran–Mantel–Haenszel test.
Results
Trends in prevalence of CE in RIF women and MDR-CE in whole CE cases in the last decade
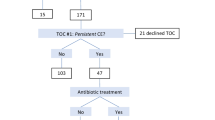
We evaluated the data of a total of 3473 RIF women between April 2010 and March 2020 (Table 1). The number of the referred RIF women increased steadily from the first year (n = 44, April 2010 to March 2011) to the last year (n = 464, April 2019 to March 2020) (p trend < 0.001). Of them, 24 patients whose clinical information was not fully available were excluded from the study. The remaining 3449 RIF patients were subjected to the trend analysis (Fig. 1). The relationships between the number of previously transferred morphologically good embryos and the number of RIF women are detailed in Table 2.
CE was detected in a total of 31.4% (1082/3449) in the first endometrial biopsy specimens of RIF women in the last decade. The prevalence of CE in the first biopsy was comparable between the first 5 years (30.2%, 237/785) and the last 5 years (31.7%, 845/2664) during the study period and steady throughout the 10 years (OR 1.07, 95% CI 0.90–1.28, p trend > 0.05). Of these 1082 CE patients, 1071 women completed the first-line oral doxycycline treatment, whereas 11 women declined or discontinued the medication due to the history or onset of the allergic episodes/adverse effects. In the second biopsy, the histopathologic cure of CE was confirmed in 78.8% (844/1071). Of the remaining 227 (21.2%) doxycycline-resistant CE patients, 224 patients completed the second-line ciprofloxacin/metronidazole treatment, whereas three patients dropped out. In the third biopsy, the histopathologic cure of CE was confirmed in 62.5% (140/224), resulting in 84 patients with MDR-CE. Thus, the total prevalence of MDR-CE in whole RIF/CE patients in the decade was 7.8% (84/1082), which markedly increased (OR 8.27, 95% CI 2.58–26.43, p trend < 0.001) from the first 5 years (1.3%, 3/237, between April 2010 and March 2015) to the last 5 years (9.6%, 81/845, between April 2015 and March 2020) (Table 3; Fig. 2). The age in the MDR-CE group was lower than that in the antibiotics-sensitive CE group (95% CI 0.12–2.08, p value 0.027) (Table 1). In addition, the number of the past transfer cycles (95% CI 0.04–0.35, p value 0.011) and embryos (95% CI 0.04–0.56, p value 0.024) were lower in the MDR-CE group than in the antibiotics-sensitive CE group.
Relationship between ESPDI and sensitivity/resistance to antibiotics in CE
We retrospectively assessed if the initial density of ESPC prior to the antibiotic treatment is associated with the occurrence of MDR-CE. Of 227 patients with doxycycline-resistant CE, 61 patients (26.9%) showed a remission (decreased in ESPDI but remained above the cutoff index 0.25) following doxycycline administration, whereas 166 patients (73.1%) had an increase in ESPDI. The mean ± SD ESPDI in the first biopsy specimens was higher (p value < 0.0001) in the MDR-CE group (n = 84, 19.3 ± 11.3, 95% CI 16.88–21.72) and doxycycline-resistant, ciprofloxacin/metronidazole-sensitive CE group (n = 140, 19.9 ± 10.2, 95% CI 18.23–21.57) than in the doxycycline-sensitive CE group (n = 844, 15.1 ± 9.0, 95% CI 14.47–15.73).
Of 84 patients with MDR-CE, 19 patients (22.6%) showed a remission following ciprofloxacin/metronidazole administration, whereas 65 patients (77.4%) had an increase in ESPDI. The mean ± SD ESPDI in the second biopsy specimens following doxycycline treatment was at a similar level (p value 0.51) between the MDR-CE group (n = 84, 20.1 ± 8.4, 95% CI 18.30–21.90) and the doxycycline-resistant, ciprofloxacin/metronidazole-sensitive CE group (n = 140, 19.3 ± 9.1, 95% CI 17.79–20.81).
VS and EF microbiota in RIF women with MDR-CE
Information on the local bacterial compositions was available in some RIF women with MDR-CE (n = 17) and antibiotics-sensitive CE who desired microbiota analysis in the paired VS and EF samples (n = 16). Sequencing was successful in each sample.
The VS microbiota in the MDR-CE group resembled that in the antibiotics-sensitive CE group (Supplemental Table). There was no difference in the detection rate of Lactobacillus, the representative bacterial genus that resides in the healthy vaginal cavity, between the MDR-CE group and antibiotics-sensitive CE group [88.2% (15/17) vs 93.8% (15/16), OR 0.50, 95% CI 0.04–6.13, p value 0.59]. Likewise, the rate of Lactobacillus-dominant (90% or more in the whole bacterial load) microbiota was similar between the MDR-CE group and antibiotics-sensitive CE group [77.8% (14/18) vs 76.5% (13/17), OR 1.08, 95% CI 0.22–5.22, p value 0.92]. There were no bacterial species that were uniquely detectable in each group. These results went for the EF microbiota.
Histopathologic cure rate of MDR-CE in RIF women following moxifloxacin versus azithromycin administration
Between April 2018 and March 2020, 48 MDR-CE/RIF women met the inclusion criteria for the pilot antibiotic administration study and agreed to participate. Under non-randomized settings, oral moxifloxacin was prescribed for 24 women, whereas oral azithromycin was for 24 women. There were no differences in the clinical characteristics including age, body mass index, cigarette smoking, and alcohol consumption. Mild abdominal discomfort was observed in one patient in the moxifloxacin group, and short-term diarrhea was observed in three patients in the azithromycin group. There were no reports on the serious adverse effects that required the discontinuation of the medication and/or additional treatments. The histopathologic cure rate of MDR-CE, which was evaluated in the fourth endometrial biopsy in the subsequent cycle, was similar (OR 1.27, 95% CI, 0.32–4.89, p value 0.73) between the moxifloxacin group [79.2% (19/24), the mean ± SD ESPDI 8.3 ± 2.1] and the azithromycin group [75.0% (18/24), the mean ± SD ESPDI 7.8 ± 4.6) (Table 3).
Reproductive outcomes in RIF women with moxifloxacin-sensitive MDR-CE versus azithromycin-sensitive MDR-CE
Following the confirmation of the histopathologic cure of MDR-CE, the reproductive outcome was followed up in patients undergoing the subsequent ET cycles. Five patients in the moxifloxacin group and six patients in the azithromycin group dropped out due to unsuccessful obtainment of transferrable embryos/blastocysts, resulting in enrollment of 19 women in the moxifloxacin group and 18 women in the azithromycin group. There was no difference in the follow-up duration between the moxifloxacin and azithromycin groups (mean ± SD; 12.1 ± 5.6 months vs 12.5 ± 6.8 months, 95% CI − 4.02–3.22, p value 0.82) as well as the number of the embryos transferred (mean ± SD; 1.87 ± 0.23 vs 2.03 ± 0.61, 95% CI − 0.44–0.12, p value 0.25). The live birth rate in the immediate first ET cycle was equivalent between the moxifloxacin and azithromycin groups [31.6% (6/19%) vs 33.3 (6/18), OR 0.92, 95% CI 0.23 − 3.66, p value 0.91] as well as other reproductive outcome measures (Table 4). The results in the cumulative three ET cycles per couple were also comparable between the two groups [moxifloxacin 57.9% (11/19) vs azithromycin 61.1% (11/18), OR 0.88, 95% CI 0.23–3.26, p value 0.84]. There were no patients who refused antibiotic treatment and/or proceeded to the next ET cycles without confirming the cure of CE.
Discussion
There have been no reports that followed up the prevalence of CE in large and long-term settings. In addition, only a few papers so far described MDR in CE. In this study, we evaluated the prevalence of histopathologic CE in RIF women for the last 10 years. CE was diagnosed in 31.4%, which was at a similar level to our previous report between 2011 and 2014 (33.7%) [3, 13]. MDR was diagnosed in 7.8% of whole CE cases in RIF women. In contrast to doxycycline resistance being found in 7.7% in our previous study between 2011 and 2014 [3], it was detected in 21.2% of CE in this study, suggesting the declining effectiveness of doxycycline as the first-line antibiotic agents against CE. While the prevalence of CE did not show a marked fluctuation over the decade, that of MDR in whole CE cases significantly rose from the first 5 years to the last 5 years. The mean age in the RIF women with MDR-CE group was lower than in those with antibiotics-sensitive CE group, although its significance remains unclear. Compared with non-RIF women, RIF women inevitably have more opportunities for antimicrobial prophylaxis with infertility-associated examinations and treatments. As with other medical fields [41], it is conceivable that antibiotic abuse is the main cause of MDR-CE. Additionally, these iatrogenic events, particularly intrauterine interventions, may potentially contribute to the development of MDR-CE [42]. Our results suggest the association between the ESPDI in the first endometrial biopsy specimens and the sensitivity/resistance to the first-line oral doxycycline treatment, whereas that in the second biopsy was not related to the sensitivity/resistance to the second-line oral ciprofloxacin/metronidazole treatment against doxycycline-resistant CE. To control the increase in MDR, individualized antibiogram-oriented antibiotic treatment may have the advantage of reducing the risk [2].
Several studies investigated the vaginal/endometrial microbiota in CE [42,43,44,45,46]. However, to our best knowledge, few reported local bacterial species and communities in MDR-CE. Using next-generation sequencing of bacterial 16S rRNA genes, we examined the microbiota in paired EF and VS samples in a fraction of RIF women with MDR-CE who agreed to the study. We were unable to identify unique microorganism(s) or characterize the local microbiota associated with MDR-CE. The detection status of Lactobacillus, the dominant genus in the healthy vaginal cavity, was comparable between MDR-CE and antibiotics-sensitive CE, suggesting the non-association between Lactobacillus-dominant/non-dominant microbiota and MDR-CE, although these results must be confirmed in larger samples with species-level analysis. In addition, there was no difference in the detection rate of Burkholderia, a proteobacteria uniquely found in a quarter of the EF microbiota in RIF women in our previous study [36]. Larger settings may be required to discover the microbiota associated with MDR-CE.
A wide range of antibiotic agents has been used against CE, but there are few pharmacological treatment strategies against MDR-CE. We sought for effective and safe regimens of oral antibiotic treatment against MDR-CE in a pilot study. We removed fluoroquinolone ofloxacin from the candidates due to the lower histopathologic CE cure rate in women with recurrent pregnancy loss compared with other antibiotic agents in a previous study [6]. In 2016, Bouet et al. hinted the adoption of the combination of 500 mg/day metronidazole and 400 mg/day moxifloxacin for the second-line antibiotic treatment against doxycycline-resistant CE but did not mention its cure rate and subsequent reproductive outcome [7].
Our results indicated that the histopathologic cure rate of MDR-CE was similar between moxifloxacin and azithromycin. In RIF women who overcome MDR-CE, the reproductive outcomes including the ongoing pregnancy/live birth rate were also comparable between the two regimens. These results are consistent with a recent study demonstrating that the efficacy and safety of moxifloxacin and azithromycin are equivalent in the doxycycline resistance–guided antibiotic treatment against sexually transmitted infection syndromes [47]. Although the validity and utility of this comparison await more studies, our findings suggest that azithromycin may have a clinical advantage over moxifloxacin in antibiotic treatment against MDR-CE due to its effectiveness following shorter-time administration periods.
The limitation of this study is as follows: (i) small sample size and the nonrandomized setting of the third-line antibiotic study, as we gave that up in the preliminary survey which disclosed that > 95% of RIF women did not desire to participate in randomization [3]; (ii) lack of preimplantation genetic testing for aneuploidy, as it has been prohibited in our nation since 1998 [48]; (iii) no patients who desired next embryo transfer without screening for CE; (iv) demographic variabilities (stages of transferred embryos and inclusion of RIF women only with primary screening).
In conclusion, this study disclosed the increase of MDR-CE in RIF women over the decade. Regarding the effectiveness and safety of the oral antibiotic administration against MDR-CE, the histopathologic cure rate and reproductive outcomes in the subsequent ET cycles were equivalent between moxifloxacin and azithromycin. Further studies are warranted to verify these findings.
References
Kitaya K, Takeuchi T, Mizuta S, Matsubayashi H, Ishikawa T. Endometritis: new time, new concepts. Fertil Steril. 2018;110:344–50.
Cicinelli E, De Ziegler D, Nicoletti R, Colafiglio G, Saliani N, Resta L, Rizzi D, De Vito D. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89:677–84.
Kitaya K, Matsubayashi H, Takaya Y, Nishiyama R, Yamaguchi K, Takeuchi T, Ishikawa T. Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol. 2017;78:e12719.
Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93:437–41.
Kitaya K, Tada Y, Taguchi S, Funabiki M, Hayashi T, Nakamura Y. Local mononuclear cell infiltrates in infertile patients with endometrial macropolyps versus micropolyps. Hum Reprod. 2012;27:3474–80.
McQueen DB, Bernardi LA, Stephenson MD. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil Steril. 2014;101:1026–30.
Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, Marrocchella S, Greco P, Resta L. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30:323–30.
Bouet PE, El Hachem H, Monceau E, Gariépy G, Kadoch IJ, Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105:106–10.
Cicinelli E, Matteo M, Trojano G, Mitola PC, Tinelli R, Vitagliano A, Crupano FM, Lepera A, Miragliotta G, Resta L. Chronic endometritis in patients with unexplained infertility: prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. 2018;79:e12782.
Vitagliano A, Saccardi C, Noventa M, Di Spiezio SA, Saccone G, Cicinelli E, Pizzi S, Andrisani A, Litta PS. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil Steril. 2018;110:103-12.e1.
Kitaya K, Yasuo T. Aberrant expression of selectin E, CXCL1, and CXCL13 in chronic endometritis. Mod Pathol. 2010;23:1136–46.
Song D, He Y, Wang Y, Liu Z, Xia E, Huang X, Xiao Y, Li TC. Impact of antibiotic therapy on the rate of negative test results for chronic endometritis: a prospective randomized control trial. Fertil Steril. 2021;115:1549–56.
Kitaya K. Prevalence of chronic endometritis in recurrent miscarriages. Fertil Steril. 2011;95:1156–8.
Di Pietro C, Cicinelli E, Guglielmino MR, Ragusa M, Farina M, Palumbo MA, Cianci A. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2013;69:509–17.
Wu D, Kimura F, Zheng L, Ishida M, Niwa Y, Hirata K, et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod Biol Endocrinol. 2017;15:16.
Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53-69.
Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta Obstet Gynecol Scand. 2005;84:547–50.
Gundogan F, Bianchi DW, Scherjon SA, Roberts DJ. Placental pathology in egg donor pregnancies. Fertil Steril. 2010;93:397–404.
Edmondson N, Bocking A, Machin G, Rizek R, Watson C, Keating S. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol. 2009;12:16–21.
Maleki Z, Bailis AJ, Argani CH, Askin FB, Graham EM. Periventricular leukomalacia and placental histopathologic abnormalities. Obstet Gynecol. 2009;114:1115–20.
Buzzaccarini G, Vitagliano A, Andrisani A, Santarsiero CM, Cicinelli R, Nardelli C, et al. Chronic endometritis and altered embryo implantation: a unified pathophysiological theory from a literature systematic review. J Assist Reprod Genet. 2020;37:2897–911.
Volodarsky-Perel A, Badeghiesh A, Shrem G, Steiner N, Tulandi T. Chronic endometritis in fertile and infertile women who underwent hysteroscopic polypectomy. J Minim Invasive Gynecol. 2020;27:1112–8.
Elder S, Bortoletto P, Romanski PA, Spandorfer S. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol. 2021;86:e13410.
Cicinelli E, Resta L, Loizzi V, Pinto V, Santarsiero C, Cicinelli R, et al. Antibiotic therapy versus no treatment for chronic endometritis: a case-control study. Fertil Steril. 2021;115:1541–8.
Janjua S, Mathioudakis AG, Fortescue R, Walker RA, Sharif S, Threapleton CJ, et al. Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;1:CD013198.
Gasparrini AJ, Markley JL, Kumar H, Wang B, Fang L, Irum S, et al. Tetracycline-inactivating enzymes from environmental, human commensal, and pathogenic bacteria cause broad-spectrum tetracycline resistance. Commun Biol. 2020;3:241.
Alauzet C, Lozniewski A, Marchandin H. Metronidazole resistance and nim genes in anaerobes: A review. Anaerobe. 2019;55:40–53.
Stapleton AE, Wagenlehner FME, Mulgirigama A, Twynholm M. Escherichia coli resistance to fluoroquinolones in community-acquired uncomplicated urinary tract infection in women: a systematic review. Antimicrob Agents Chemother. 2020;64:e00862-e920.
Xiong Y, Chen Q, Chen C, Tan J, Wang Z, Gu F, et al. Impact of oral antibiotic treatment for chronic endometritis on pregnancy outcomes in the following frozen-thawed embryo transfer cycles of infertile women: a cohort study of 640 embryo transfer cycles. Fertil Steril. 2021;116:413–21.
Balfour JA, Wiseman LR. Moxifloxacin. Drugs. 1999;57:363–73.
Petrina MAB, Cosentino LA, Wiesenfeld HC, Darville T, Hillier SL. Susceptibility of endometrial isolates recovered from women with clinical pelvic inflammatory disease or histological endometritis to antimicrobial agents. Anaerobe. 2019;56:61–5.
Peters DH, Friedel HA, McTavish D. Azithromycin. A review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1992;44:750–99.
Veeck LL. Preembryo Grading. Baltimore: Williams and Wilkins; 1991. p. 121–49.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8.
Bayer-Garner IB, Korourian S. Plasma cells in chronic endometritis are easily identified when stained with syndecan-1. Mod Pathol. 2001;14:877–9.
Kitaya K, Nagai Y, Arai W, Sakuraba Y, Ishikawa T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediators Inflamm. 2019;2019:4893437.
Aronesty E. Comparison of sequencing utility programs. Open Bioinformatics J. 2013;7:1–8.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–8.
Morris S, Cerceo E. Trends, epidemiology, and management of multi-drug resistant Gram-negative bacterial infections in the hospitalized setting. Antibiotics. 2020;9:196.
Tanaka SE, Sakuraba Y, Kitaya K, Ishikawa T. Differential vaginal microbiota profiling in lactic-acid-producing bacteria between infertile women with and without chronic endometritis. Diagnostics. 2022;12:878.
Moreno I, Cicinelli E, Garcia-Grau I, Gonzalez-Monfort M, Bau D, Vilella F, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218:602.
Liu Y, Ko EY, Wong KK, Chen X, Cheung WC, Law TS, et al. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil Steril. 2019;112:707–17.
Lozano FM, Bernabeu A, Lledo B, Morales R, Diaz M, Aranda FI, et al. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur J Obstet Gynecol Reprod Biol. 2021;263:25–32.
Takeda E, Sugiura-Ogasawara M, Ebara T, Kitaori T, Goto S, Yoshihara H, et al. Attitudes toward preimplantation genetic testing for aneuploidy among patients with recurrent pregnancy loss in Japan. J Obstet Gynaecol Res. 2020;46:567–74.
Chen W, Wei K, He X, Wei J, Yang L, Li L, et al. Identification of uterine microbiota in infertile women receiving in vitro fertilization with and without chronic endometritis. Front Cell Dev Biol. 2021;9:693267.
Durukan D, Read TRH, Murray G, Doyle M, Chow EPF, Vodstrcil LA, et al. Resistance-guided antimicrobial therapy using doxycycline-moxifloxacin and doxycycline-2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: efficacy and tolerability. Clin Infect Dis. 2020;71:1461–8.
Acknowledgements
The authors sincerely thank Dr. Yoko Nagai who passed away in Summer of 2021 for her significant contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10815_2022_2528_MOESM1_ESM.xls
Supplementary file1 Microbiota Analysis in paired VS and EF in RIF Women with MDR-CE (n = 17) and antibiotics-sensitive CE (n = 16). The proportion of each bacterium in the microbiota are listed. Totals are not 100% as the bacteria comprising less than 0.1% and/or of unclassified species are not shown. (XLS 123 KB)
Rights and permissions
About this article
Cite this article
Kitaya, K., Tanaka, S.E., Sakuraba, Y. et al. Multi-drug-resistant chronic endometritis in infertile women with repeated implantation failure: trend over the decade and pilot study for third-line oral antibiotic treatment. J Assist Reprod Genet 39, 1839–1848 (2022). https://doi.org/10.1007/s10815-022-02528-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02528-7