Abstract
Purpose
We prospectively investigated if oral enteric coating lactoferrin supplementation improves the reproductive outcomes in infertile women with a history of repeated implantation failure (RIF) and non-Lactobacillus-dominant (Lactobacillus rate < 90%) microbiota (NLDM) in vaginal secretions (VS)/endometrial fluid (EF).
Methods
Paired VS/EF samples were obtained from RIF women and control infertile women (non-RIF group) for microbiome analysis. Chronic endometritis (CE) was diagnosed histopathologically and hysteroscopically. In a pilot study, oral enteric coating lactoferrin (700 mg/day, at least 28 consecutive days) was administered to eligible patients with NLDM in VS/EF. Their reproductive outcomes in the subsequent vitrified-warmed embryo transfer cycles were followed up.
Results
While CE was more prevalent (OR 2.41, 95% CI 1.02–5.63, p = 0.042) in the RIF group (29.1%, n = 117) than in the non-RIF group (14.5%, n = 55), The NLDM rate was similar between the two groups (44.4 vs 52.7%). Lactoferrin supplementation improved NLDM in 43.2% of RIF women (n = 37). Within the RIF group, the live birth rate in the subsequent cycles was higher (OR 10.67, 95% CI 1.03 − 110.0, p = 0.046) in women with improved microbiota (57.1%, n = 14) than in those with unimproved microbiota (11.1%, n = 9).
Conclusion
Unlike CE, NLDM was not unique to RIF but was common in infertile women. Although the therapeutic effect of the oral lactoferrin supplementation on NLDM was limited in a pilot study, the reproductive outcomes were better in RIF women who overcame NLDM than in those who failed. Randomized controlled trials are required to confirm the results.
Trial registration number and date for prospectively registered trials
UMIN-CTR 000036990, June 7, 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Unlike chronic endometritis being prevalent in patients with a history of repeated implantation failure, genital tract dysbiosis seems to be more common in female infertility. Oral enteric coating lactoferrin supplementation may be a therapeutic option for genital tract dysbiosis in infertile women, although randomized controlled studies are required to confirm its effectiveness and to optimize the supplementation dose and duration. |
Introduction
The development of genomic science revealed the localization and distribution of the bacterial communities (microbiota) in the female genital tract. As it has been long believed, Lactobacillus was found to be the major bacterial genera in the vaginal cavity in healthy premenopausal women. Moreover, human vaginal bacterial communities were characterized as low diversity with the overwhelming predominance of four Lactobacillus species (L iners, L. crispatus, L. gasseri, and L. jensenii) [1]. These results suggest a pivotal role of lactic acid bacteria in the integrity and maintenance of the local environment against the invasion of the alien bacterial species and the homeostasis of the mucosal barrier functions [2]. Meanwhile, the microbiota in the human uterine cavity remains controversial. The comprehensive genome analysis demonstrated the inconsistent results between the studies, ranging from the predominance of Lactobacillus and Bacteroides to other species such as Acinetobacter, Pseudomonas, Cloacibacterium, and Comamonadaceae [3,4,5,6,7,8,9].
Recently, an increasing number of reports demonstrated an association between female genital tract dysbiosis and poor reproductive outcomes [5,6,7,8,9,10,11,12,13,14]. Moreno et al. [5], disclosed a positive association between Lactobacillus-dominant (90% or more) microbiota (LDM) in the vaginal secretions (VS)/endometrial fluid (EF) and successful pregnancy in the following embryo transfer (ET) cycles in infertile women. On the contrary, dysbiotic non-LDM (NLDM, 90% or less Lactobacillus species) in VS/EF is associated with recurrent pregnancy loss, preterm birth, and repeated implantation failure (RIF) which occurs in even 5% or more of infertile women undergoing three ET cycles of euploid blastocysts with morphologically normal endometrium [15].
Probiotics and prebiotics are being studied with growing interest as adjuncts to the standard therapies against inflammatory and infectious diseases. While probiotics are defined as products containing live microorganisms that provide a health benefit to the host when consumed, prebiotics are known as nondigestible nutritive substances acting as a substrate to stimulate the growth and metabolism of protective endogenous bacteria [16]. In the field of obstetrics and gynecology, vaginal probiotic application of Lactobacillus and Bifidobacterium against bacterial vaginosis and vulvovaginal candidiasis has been shown to improve the genital tract dysbiotic environments that are resistant to conventional antibiotic treatment. [17,18,19,20,21]. By contrast, it remains undetermined if probiotics/prebiotics administration restores LDM in VS and/or EF in infertile women and improves their reproductive outcomes.
Lactoferrin is a 703-amino acid glycoprotein primally isolated from bovine milk [22]. In humans, lactoferrin is produced by polymorphonuclear leukocytes and other endocrine cells, such as pancreatic acinar cells, and is released into a wide range of body fluids, including milk, plasma, saliva, tears, nasal secretions, and vagina [23]. The major roles of lactoferrin are antimicrobial iron-binding via iron adsorption and immune response regulation against a wide range of pathogens including bacteria, fungi, protozoa, and viruses, along with prebiotic activity, which draws attention from medical fields [22, 23].
This prospective study aimed to investigate if oral supplementation of enteric coating lactoferrin can restore the microbial integrity in the genital tract in infertile women with a history of RIF/NLDM. Furthermore, we followed up on their reproductive outcomes in the subsequent ET cycles following oral enteric coating lactoferrin supplementation.
Materials and methods
Study registration and design
The study was approved by the Ethical Committee of the Institutional Review Board (Approval Number 20191) on May 31, 2019, and registered on the University Hospital Medical Information Network-Clinical Trial Registration, Japan, on June 7, 2019 (UMIN-CTR 000036990). This prospective cohort study was conducted in accordance with the Declaration of Helsinki in a non-randomized setting. All patients provided written informed consent prior to participation in the study. The patients with suspicious endometrial hyperplasia/malignancy and who had taken any antibiotic agents and/or prebiotics/probiotics within three months were excluded from the study.
According to Gardner and Schoolcraft scoring system [24], good blastocysts were defined as 3BB or above on day 5. RIF was defined as continuously failed pregnancy tests (Tosoh Co., Shunan, Japan) despite five or more good blastocyst transfer cycles occurring in infertile women with the age less than 40 years old.
The endometrial curette biopsy was performed on the menstrual cycle day 6–12 in the natural cycle or hormone replacement cycle. The paired VS/EF samples were obtained carefully avoiding contamination from RIF women who desired examination on days 6–8 after luteinizing hormone surge in the natural cycle or on day 5 following initiation of luteal support in the hormone replacement cycle identical to CE testing, as described previously [10]. The VS and EF samples were separately solubilized into collection tubes.
Diagnosis of chronic endometritis
After being washed thoroughly, the specimens were subjected to overnight fixation in 4% paraformaldehyde (pH 7.3), paraffin embedding, and 4-μm thickness slicing. The sections mounted on slide glasses were dewaxed in limonen and rehydrated in a graded series of ethanol (in phosphate-buffered saline, pH 7.4). They were then microwaved in citrate buffer (pH 6.0) for 5 min to retrieve antigens and immersed in 3% hydrogen peroxide for 5 min for endogenous peroxidase inactivation. After being washed, the sections were soaked in 10% fetal calf serum (SAFC Biosciences, Lenexa, Kansas) for 10 min to block nonspecific antibody binding and then incubated with a stock solution of B-A38 (mouse monoclonal IgG against human plasmacyte marker CD138 (Nichirei, Tokyo) [25, 26] or control antibody. Immunostaining was visualized with an LSAB kit (Dako, Kyoto, Japan). After hematoxylin nuclear counterstaining, the sections were microscopically observed by an experienced gynecologic pathologist (400-fold magnification). The immunoreactive stromal cells with nucleic heterochromatin patterns were counted in 20 or more high power fields in the whole section. The endometrial stromal plasmacyte density index (ESPDI) was calculated as the sum of the stromal CD138 + cell counts divided by the number of the high power fields evaluated. Chronic endometritis (CE) was diagnosed as 0.25 or more ESPDI [25]. For the aid of histopathologic diagnosis of CE, office fluid hysteroscopy was performed using a 3.1-mm diameter flexible endoscope (Olympus, Tokyo, Japan or Pentax Ricoh Imaging, Tokyo, Japan) on days 6–12 of the menstrual cycle. The hysteroscopic images were preserved for later discussion using a consensus method by the International Working Group for Standardization of Chronic Endometritis Diagnosis [27, 28].
Analysis of LDM/NLDM in vaginal secretions and endometrial fluid
Following treatment with proteinase K (Beckman Coulter Inc., Brea, CA, USA), 100 mg/mL lysozyme, and 100 mg/mL RNase A (Sigma-Aldrich, Darmstadt, Germany), the genomic DNA was extracted from VS and EF samples using an Agencourt Genfind v2 Blood & Serum DNA Isolation Kit (Beckman Coulter Inc.). After double-stranded DNA concentration measurement with a Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA), the hypervariable region 4 of the bacterial 16S rRNA was amplified with a primer pair (515f /806rB) along with Nextera XT (Illumina Inc., San Diego, CA, USA) adapter overhang sequences [29]. PCR was performed with 2.5 U of FastStart HiFi polymerase, 200 μmol/L 4-deoxynucleotide triphosphates, 400 nmol/L of each primer, 4% bovine serum albumin, 0.5 mol/L betaine, 25 ng DNA, and magnesium chloride buffer (Sigma-Aldrich). The amplicon mixture was purified using Agencourt AMPure XP (Beckman Coulter Inc.) and multiplexed using a dual-index approach according to the Illumina 16S Metagenomic Sequencing Library Preparation protocol. The indexing PCR was performed with a KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Boston, MA, USA) in 50 μL volume, and the products were purified with Agencourt AMPure XP beads. The final library was sequenced at 2 × 200 base pairs using a MiSeq Reagent Kit v3 on the Illumina MiSeq platform. The ZymoBIOMICS Microbial Community Standard (Zymo Research, Orange, CA, USA) containing a mixture of Pseudomonas, Escherichia, Salmonella, Lactobacillus, Enterococcus, Listeria, Bacillus, and two yeast species Saccharomyces and Cryptococcus was used as a positive control. UltraPure™ DNase/RNase-Free Distilled Water (Thermo Fisher Scientific Inc.) was used as a blank control.
Using EA-Utils fastq-join, a median 291-base pair merged sequence length was obtained. Quality control was performed using USEARCH v10.0.240 [30] to remove PhiX reads, truncate primer-binding sequences, and discard sequences with < 100 base pair and sequence quality < Q20. Quantitative Insights Into Microbial Ecology (QIIME) 1.9.1 [31] was utilized with default parameters for quality filtering, chimera check, sequence clustering into operational taxonomic units (OTUs), and taxonomy assignment. The sequence clustering was performed using the open-reference OTU picking strategy/UCLUST method based on 97% sequence identity. Ribosomal Database Project Classifier was used for taxonomy assignment with a 0.50 confidence threshold against the Greengenes database version 13_8 [32]. The following 15 bacterial taxa (Acidovorax, Acinetobacter, Chryseobacterium, Citrobacter, Elizabethkingia, Escherichia, Flavobacterium, Janthinobacterium, Leptothrix, Methylobacterium, Pseudomonas, Rhodococcus, Sphingomonas, Stenotrophomonas, and Yersinia), which are known as contaminants found in a blank control [17], were excluded from ES samples using QIIME.
Pilot study for oral enteric coating lactoferrin supplementation for RIF Women with NLDM and follow-up of reproductive outcomes
According to the previous study [5], LDM was defined as more than 90% Lactobacillus-dominant microbiota, and NLDM was defined as 90% or less Lactobacillus microbiota. For infertile women with NLDM who desired supplementation, we prescribed oral enteric coating lactoferrin (100 mg/capsule, 700 mg/day, Baby&Me™, Partners Inc, Yokohama, Japan) [33] and advised them to take it for at least consecutive 28 days. Following completion, the second pair of VS/EF samples were obtained in the next menstrual cycle and the microbiome analysis was performed as described above. Their reproductive outcomes in the subsequent ET cycle were compared between RIF women who overcame genital tract dysbiosis and those who failed it. The main outcome measures included clinical pregnancy (the presence of the intrauterine gestational sac and fetal heartbeat on transvaginal ultrasound) rate, miscarriage (pregnancy loss before 22 weeks of gestation) rate, and live birth rate.
Statistics
The data sets were calculated for normal distribution using the chi-square goodness-of-fit test and compared using the Student t-test for continuous variables (age and body mass index). Categorical variables in patient demographics were compared using a nonparametric Mann–Whitney U test. The microbiota between the first and second VS/EF samples was compared using Wilcoxon rank-sum test. The contingency table and Fisher’s exact test were utilized for other comparisons. Pearson’s correlation coefficients were evaluated between the EF and VS microbiota within identical individuals. A p value less than 0.05 was regarded as statistically significant.
Results
Demographics of patients
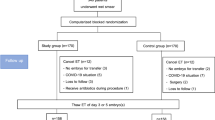
One hundred-seventeen RIF women (RIF group) and 55 infertile women without RIF (non-RIF group) who desired the analysis were enrolled in the study (Fig. 1). Their demographics are shown in Table 1. The age of the RIF group was lower [95% confidence interval (CI) − 2.90 to − 0.70, p = 0.0015] in the RIF group (mean ± SD, 36.4 ± 3.2 years old) than in the non-RIF group (38.2 ± 3.8 years old). About infertility etiology, the proportion of diminished ovarian reserve (OR 0.049, 95% CI 0.01–0.13, p < 0.001) was lower in the RIF group (6.0%) than in the non-RIF group (56.4%). By contrast, the proportion of polycystic ovarian syndrome [14.5% vs 1.8%, odds ratio (OR) 9.18, 95% CI 1.18–70.88, p = 0.033], tubal factor (27.4 vs 7.3%, OR 4.80, 95% CI 1.60–14.37, p = 0.005), and unexplained etiology (32.5 vs 12.7%, OR 3.30, 95% CI 1.36–7.98, p = 0.008) was higher in the RIF group than in the non-RIF group. The number of past ET cycles and the number of past embryos transferred were higher in the RIF group than in the non-RIF group (p < 0.0001, respectively).
Comparison of VS/EF microbiota between RIF and non-RIF infertile group
The prevalence of NLDM in the first VS samples was similar (OR 1.39, 95% CI 0.73–2.65, p = 0.16) between the RIF group (44.4%, 52/117) and non-RIF group (52.7%, 29/55) (Table 1). The prevalence of NLDM in the first EF samples was also similar (OR 1.10, 95% CI 0.57–2.10, p = 0.38) between the two groups (RIF group, 52.1%, 61/117 versus non-RIF group, 54.5%, 30/55). There were no statistical differences (p = 0.25) in the proportion of Lactobacillus in the VS microbiota between the RIF group (median 98.6%, range 0–100%) and non-RIF group (median 72.0%, range 0–100%) as well as EF microbiota (p = 0.25) between the RIF group (median 88.3%, range 0–100%) and non-RIF group (median 70.9%, range 0–100%). The mismatch between the VS and EF microbiota (the condition where one sample has LDM, the other has NLDM) was seen in 12.5% (13/117) of the first samples in the RIF group and 5.5% (3/55) of the first samples in the non-RIF group (OR 2.17, 95% CI 0.59–7.94, p = 0.12).
Relationship between female genital tract dysbiosis and CE
The prevalence of CE was higher (OR 2.41, 95% CI 1.02–5.63, p = 0.042) in the RIF group (29.1%, 34/117) than in the non-RIF group (14.5%, 8/55) (Tables 1, 2). In the RIF group, there were no statistical differences (OR 1.92, 95% CI 0.85–4.31, p = 0.057) in the concomitance rate of CE/VS-NLDM (36.5%, 19/52) and CE/VS-LDM (23.1% 15/65). Likewise, the concomitance rate of CE/EF-NLDM (33.9%, 19/56) was similar (OR 1.38, 95% CI 0.64–2.98, p = 0.21) to CE/EF-LDM (24.6% 15/61). These results went for the non-RIF group (CE/VS-NLDM; 15.4%, 4/29 vs CE/VS-LDM; 13.5%, 4/26; OR 0.90, 95% CI 0.20–3.96, p = 0.44, and CE/EF-NLDM; 16.7%, 5/30 vs CE/EF-LDM; 12.0% 3/25; OR 1.39, 95% CI 0.30–6.40, p = 0.34). The concomitance rate of CE/VS-NLDM (RIF group, 16.2%, 19/117 vs non-RIF group, 7.3%, 4/55; OR 2.47, 95% CI 0.79–7.66, p = 0.16) and CE/EF-NLDM (RIF group, 16.2%, 19/117 vs non-RIF group, 9.1%, 5/55; OR 1.93, 95% CI 0.68–5.50, p = 0.21) was also similar between the two groups.
Effect of oral enteric coating lactoferrin supplementation on VS/EF microbiota in infertile non-CE/NLDM women
A total of 24 women (19 in the RIF group and five in the non-RIF group) with both CE /NLDM were excluded from the study, as they had undergone antibiotic treatments against CE which can be a potential bias. Thirty-seven women with non-CE/NLDM in the RIF group and 25 women with non-CE/NLDM in the non-RIF group agreed to oral enteric coating lactoferrin supplementation, whereas others did not desire it.
According to the self-report of patients, the supplementation duration ranged from 28 to 38 days. Following supplementation, the increase (10% or more) in Lactobacillus species in the VS microbiota was seen in 19 out of 37 (51.4%) non-CE/NLDM women in the RIF group and 8 out of 25 (32.0%) non-CE/NLDM women in the non-RIF group. There was no significant difference in the rate of change in Lactobacillus species between the RIF and non-RIF group (OR 2.24, 95% CI 0.77–6.47, p = 0.13). Similarly, the rate of women who improved to the VS-LDM level was at a comparable level between the two groups (RIF group, 32.7%, 12/37 and non-RIF group, 16.0%, 4/25; OR 2.52, 95% CI 0.70–8.99, p = 0.15).
Meanwhile, the increased proportion (10% or more) of Lactobacillus species in the EF microbiota was seen in 16 out of 37 (43.2%) non-CE/NLDM women in the RIF group and 6 out of 25 (24.0%) non-CE/NLDM women in the non-RIF group (OR 2.41, 95% CI 0.78–7.44, p = 0.12). Similarly, the rate of women who improved to the EF-LDM level was at a comparable level between the two groups (RIF group, 29.7%, 11/37 and non-RIF group, 16.0%, 4/25; OR 2.22, 95% CI 0.61–8.00, p = 0.22).
Reproductive outcomes in RIF women with improved VS/EF microbiota and unimproved VS/EF microbiota
Following oral enteric coating lactoferrin supplementation, 26 RIF women with NLDM and without CE proceeded to hormone replacement/vitrified-warmed blastocyst transfer in the immediate first menstrual cycle. RIF women who had failed to obtain transferrable blastocysts in the following in vitro fertilization cycles dropped out of the study. The number of the transferred blastocysts were at a similar level (95% CI − 0.55–0.36, p = 0.67) between 14 women with improved VS/EF microbiota (1.6 ± 0.5) and 9 women with unimproved VS/EF microbiota (1.7 ± 0.5) (Table 3). The clinical pregnancy rate was higher (OR 8.75, 95% CI 1.24 − 61.69, p = 0.029) in the improved VS/EF microbiota group (71.4%, 10/14) than in the unimproved VS/EF microbiota group (22.2%, 2/9). The miscarriage rate was similar (OR 0.25, 95% CI 0.01–5.99, p = 0.39) between the improved VS/EF microbiota group (9.1%, 1/11) and unimproved VS/EF microbiota group (50.0%, 1/2). Finally, the live birth rate was higher (OR 10.67, 95% CI 1.03 − 110.0, p = 0.046) in the improved VS/EF microbiota group (57.1%, 8/14) than in the unimproved VS/EF microbiota group (11.1%, 1/9).
Discussion
In this study, we found that there are no statistical differences in the prevalence of NLDM in VS/EF as well as the proportion of Lactobacillus in the VS/EF microbiota between RIF and non-RIF infertile women. We were unable to identify unique microorganisms or characterize the local microbiota associated with NLDM. Additionally, the relationship between NLDM and CE, a RIF-associated local inflammatory disease recognized as endometrial stromal plasmacyte infiltration, remained unclear [34, 35]. Many studies agree that the predominance of Lactobacillus in the VS microbiota is associated with a higher chance of live birth in subsequent ET cycles in RIF women [5, 13, 14, 36,37,38,39,40]. Meanwhile, the role of Lactobacillus in the EF (and endometrial) microbiota in embryo implantation remains controversial. Some studies suggest the beneficial effect of Lactobacillus in the uterine cavity for conception, whereas others do not [5, 13, 14, 36,37,38,39,40]. One potential explanation for these discrepancies is the contamination of VS microbiota into EF microbiota, of which bacterial loads are much lower (1/100–1/10000) compared with VS [7]. Another potential theory is the difference in the devices and routes used for sample collection [41, 42]. The technique and procedure are needed to be optimized in future studies [43].
Lactoferrin has been known to be secreted into the human female reproductive tract [44]. For example, in the fallopian tube, lactoferrin is capable of binding zona pellucida of oocytes and spermatozoa and inhibits gamete interaction in vitro [45]. The role of lactoferrin in human reproduction, however, remains largely unknown. While some studies suggest a positive correlation of follicular fluid lactoferrin concentration with fertilization rate and embryo quality [46], others implicate the association between lower cervical fluid lactoferrin concentration and better in vitro fertilization outcomes [47]. Several randomized controlled studies demonstrated that short-term oral and/or vaginal lactoferrin supplementation improved the symptoms and findings of bacterial vaginosis, the most common cause of vaginal discomfort characterized by vaginal dysbiosis with depletion of Lactobacilli and predominance of anaerobic microorganisms, such as Gardnerella vaginalis and Atopobium vaginae, in the affected women [48, 49].
However, there have been no reports regarding the effectiveness of lactoferrin supplementation on genital tract dysbiosis and reproductive outcomes in infertile women. In a small sample study, 700 mg/day of oral enteric coating lactoferrin supplementation was found to restore vaginal LDM in all six premenopausal women examined with a history of late miscarriages, very early preterm delivery, and chorioamnionitis, along with refractory vaginosis to conventional treatment [33]. Following this study, we set the supplementation dose at 700 mg/day. At least 28 consecutive days of supplementation improved the genital tract dysbiosis, defined as NLDM in VS/EF, in 43% of infertile women with a history of RIF, which was at a similar level to non-RIF infertile women. The results indicate that NLDM is not unique to RIF, but is common in infertile women.
The therapeutic effect of the supplementation on NLDM was limited, as more than half of women failed to increase the proportion of Lactobacillus in VS/EF microbiota. However, we here first demonstrate that the reproductive outcomes in the immediate subsequent vitrified-warmed blastocyst transfer cycle in RIF women who overcame NLDM following supplementation were higher compared with those with the unimproved local microbiota. These results suggest that oral enteric coating lactoferrin supplementation may be a potential therapeutic option to increase the chance of the live birth rate in infertile women suffering from RIF with genital tract dysbiosis, although cautions should be taken for the interpretation of the results, particularly for the live birth rate which is statistically underpowered.
The bias and limitation of this study are (i) the difference in age between RIF and non-RIF women, which potentially brought about some differences in infertility etiology and the history of the past ET cycles between the two groups, (ii) the lack of information on embryonic (blastocyst) chromosomal normalcy/aberrations, of which examinations (preimplantation genetic testing for aneuploidy) is not yet authorized in our nation, and (iii) non-randomized controlled trial with small sample size and without a strict supplementation duration (ranging from 28 to 38 days), where stratification was impossible to adjust the confounding factors including transferred embryos and supplementation duration.
In conclusion, our results indicate that oral enteric coating lactoferrin supplementation is a promising therapeutic option for RIF women with genital tract dysbiosis. Well-designed randomized controlled studies are required to confirm its effectiveness and to optimize the supplementation dose and duration.
References
Ravel J, Gajer P, Abdo Z et al (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):4680–4687
Chee WJY, Chew SY, Than LT (2020) Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact 19(1):203
Mitchell CM, Haick A, Nkwopara E et al (2015) Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol 212(5):611.e1–9
Franasiak JM, Werner MD, Juneau CR et al (2016) Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J Assist Reprod Genet 33(1):129–136
Moreno I, Codoñer FM, Vilella F et al (2016) Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 215(6):684–703
Verstraelen H, Vilchez-Vargas R, Desimpel F et al (2016) Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1–2 region of the 16S rRNA gene. Peer J 4:e1602
Chen C, Song X, Wei W et al (2017) The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 8(1):875
Winters AD, Romero R, Gervasi MT et al (2019) Does the endometrial cavity have a molecular microbial signature? Sci Rep 9(1):9905
Molina NM, Sola-Leyva A, Saez-Lara MJ et al (2020) New opportunities for endometrial health by modifying uterine microbial composition: present or future? Biomolecules 10(4):593
Kitaya K, Nagai Y, Arai W et al (2019) Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediators Inflamm 2019:4893437
Garcia-Grau I, Perez-Villaroya D, Bau D et al (2019) Taxonomical and functional assessment of the endometrial microbiota in a context of recurrent reproductive failure: a case report. Pathogens 8(4):205
Kadogami D, Nakaoka Y, Morimoto Y (2020) Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod Biol 20(3):307–314
Fu M, Zhang X, Liang Y et al (2020) Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. MBio 11(3):e03242-19
Ichiyama T, Kuroda K, Nagai Y et al (2021) Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod Med Biol 20(3):334–344
Pirtea P, Scott RT Jr, de Ziegler D et al (2021) Recurrent implantation failure: how common is it? Curr Opin Obstet Gynecol 33(3):207–212
Li Y, Tan Y, Xia G et al (2021) Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1951155
Oerlemans EFM, Bellen G, Claes I et al (2020) Impact of a Lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci Rep 10(1):7976
Mastromarino P, Macchia S, Meggiorini L et al (2009) Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 15(1):67–74
Bradshaw CS, Pirotta M, De Guingand D et al (2012) Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PLoS ONE 7(4):e34540
Gilboa Y, Bar-Hava I, Fisch B et al (2005) Does intravaginal probiotic supplementation increase the pregnancy rate in IVF-embryo transfer cycles? Reprod Biomed Online 11(1):71–75
López-Moreno A, Aguilera M (2021) Vaginal probiotics for reproductive health and related dysbiosis: systematic review and meta-analysis. J Clin Med 10(7):1461
Sorensen M, Sorensen SPL (1940) The proteins in whey. Compte Rendu Des Trav Lab Carlsberg Ser Chim 23(7):55–99
Zhang Y, Lu C, Zhang J (2021) Lactoferrin and its detection methods: a review. Nutrients 13(8):2492
Gardner DK, Lane M, Stevens J et al (2000) Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 73(6):1155–1158
Bayer-Garner IB, Korourian S (2001) Plasma cells in chronic endometritis are easily identified when stained with syndecan-1. Mod Pathol 14(9):877–879
Kitaya K, Matsubayashi H, Takaya Y et al (2017) Live birth rate following oral antibiotic treatment for chronic endometritis in infertile women with repeated implantation failure. Am J Reprod Immunol 78(5):e12719
Kitaya K, Tada Y, Taguchi S et al (2012) Local mononuclear cell infiltrates in infertile patients with endometrial macropolyps versus micropolyps. Hum Reprod 27(12):3474–3480
Cicinelli E, Vitagliano A, Kumar A, International Working Group for Standardization of Chronic Endometritis Diagnosis et al (2019) Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: proposal and reliability evaluation through an international randomized-controlled observer study. Fertil Steril 112(1):162–173
Aronesty E (2013) Comparison of sequencing utility programs. The Open Bioinformatics J 7:1–8
Edgar RC, Haas BJ, Clemente JC et al (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
McDonald D, Price MN, Goodrich J et al (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6(3):610–618
Otsuki K, Imai N (2017) Effects of lactoferrin in 6 patients with refractory bacterial vaginosis. Biochem Cell Biol 95(1):31–33
Kitaya K, Takeuchi T, Mizuta S et al (2018) Endometritis: new time, new concepts. Fertil Steril 110(3):344–350
Vitagliano A, Saccardi C, Noventa M et al (2018) Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil Steril 110(1):103-112.e1
Koedooder R, Singer M, Schoenmakers S et al (2019) The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 34(6):1042–1054
Saxtorph MH, Hallager T, Persson G et al (2020) Assessing endometrial receptivity after recurrent implantation failure: a prospective controlled cohort study. Reprod Biomed Online 41(6):998–1006
Sola-Leyva A, Andrés-León E, Molina NM et al (2021) Mapping the entire functionally active endometrial microbiota. Hum Reprod 36(4):1021–1031
Lüll K, Saare M, Peter M et al (2022) Differences in microbial profile of endometrial fluid and tissue samples in women with in vitro fertilization failure are driven by Lactobacillus abundance. Acta Obstet Gynecol Scand 101(2):212–220
Moreno I, Garcia-Grau I, Perez-Villaroya D et al (2022) Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 10(1):1
Vitale SG, Ferrari F, Ciebiera M et al (2021) The role of genital tract microbiome in fertility: a systematic review. Int J Mol Sci 23(1):180
Tanaka SE, Sakuraba Y, Kitaya K et al (2022) Differential vaginal microbiota profiling in lactic-acid-producing bacteria between infertile women with and without chronic endometritis. Diagnostics 12(4):878
Molina NM, Sola-Leyva A, Haahr T et al (2021) Analysing endometrial microbiome: methodological considerations and recommendations for good practice. Hum Reprod 36(4):859–879
Wyatt KA, Filby CE, Davies-Tuck ML et al (2021) Menstrual fluid endometrial stem/progenitor cell and supernatant protein content: cyclical variation and indicative range. Hum Reprod 36(8):2215–2229
Zumoffen CM, Gil R, Caille AM et al (2013) A protein isolated from human oviductal tissue in vitro secretion, identified as human lactoferrin, interacts with spermatozoa and oocytes and modulates gamete interaction. Hum Reprod 28(5):1297–1308
Yanaihara A, Mitsukawa K, Iwasaki S et al (2007) High concentrations of lactoferrin in the follicular fluid correlate with embryo quality during in vitro fertilization cycles. Fertil Steril 87(2):279–282
Massa E, Pelusa F, Lo Celso A et al (2021) Lactoferrin levels in cervical fluid from in vitro fertilization (IVF) patients - correlation with IVF parameters. Biochem Cell Biol 99(1):91–96
Pino A, Giunta G, Randazzo CL et al (2017) Bacterial biota of women with bacterial vaginosis treated with lactoferrin: an open prospective randomized trial. Microb Ecol Health Dis 28(1):1357417
Russo R, Karadja E, De Seta F (2019) Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: a double blind, placebo controlled, randomised clinical trial. Benef Microbes 10(1):19–26
Acknowledgements
We thank Dr. Suguru E Tanaka, Ph.D., Dr. Yoshiyuki Sakuraba, Ph.D., and the late Dr. Yoko Nagai, Ph.D. for their assistance in microbiome analysis.
Funding
There is no funding sourcing.
Author information
Authors and Affiliations
Contributions
KK: protocol/project development, data collection, data analysis, manuscript writing/editing. TI: manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no financial interests and/or competing interests in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kitaya, K., Ishikawa, T. Genital tract dysbiosis in infertile women with a history of repeated implantation failure and pilot study for reproductive outcomes following oral enteric coating lactoferrin supplementation. Arch Gynecol Obstet 306, 1761–1769 (2022). https://doi.org/10.1007/s00404-022-06755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06755-2